Review Article - Imaging in Medicine (2012) Volume 4, Issue 1
Na18F PET in oncology
Monica Celli* and Stefano FantiNuclear Medicine Department, University Hospital S.Orsola-Malpighi, Bologna, Italy
- *Corresponding Author:
- Monica Celli
Nuclear Medicine Department University Hospital S.Orsola-Malpighi Bologna, Italy
E-mail: moki.celli@gmail.com
Abstract
Primary tumors of the skeleton are rare, but metastatic involvement from solid neoplasms, such as prostate, breast and lung, are unfortunately frequent. Skeletal metastases are clinically significant because of associated symptoms and complications (refractory pain, pathological fractures, spinal cord compression, hypercalcemia). Early detection and accurate evaluation of the extent of skeletal involvement is pivotal for treatment planning and prognosis. Na18F resurgence as an osteotropic agent for whole-body imaging of the skeleton has been made possible by the fast and wide diffusion of PET and PET/CT, which offer higher spatial resolution and sensitivity than conventional g‑cameras used in planar scintigraphy or SPECT. The article reviews the published literature reporting on Na18F PET and PET/CT diagnostic accuracy in the evaluation of osteosarcoma and bone metastases from different nonosseous solid tumors. A brief overview on current recommendations for bone metastasis imaging, Na18F general aspects and kinetics, PET scanning technical aspects, and radiation dosimetry are included.
Keywords
bone metastases; breast cancer; fluoride; lung cancer; ; a18F; primary bone tumors; prostate cancer; skeletal PET; skeletal PET/CT
The rationale for diagnostic imaging in primary and metastatic bone malignancies is to identify bone involvement early, to determine its full extent in order to appropriately guide patient therapy and prevent skeletal-related events (e.g., fractures, cord compression), reason for severe morbidity and mortality in oncologic population.
For over four decades 99mtechnetium diphosphonates (99mTc‑DP) bone scintigraphy (BS) has served this purpose, imaging areas of increased osteogenic activity throughout the whole skeleton with high diagnostic accuracy (sensitivity 62–100%; specificity 78–100%; evidence level II–III) [1] and at reasonable costs. Nevertheless, the nontumor-specific nature of 99mTc‑DP uptake limits BS specificity, whereas planar imaging combined with a relatively low spatial resolution reduces sensitivity. The availability of SPECT and SPECT/CT studies significantly increases 99mTc‑DP BS accuracy in differentiating malignant from benign lesions in the axial skeleton [2], the most affected area for both solitary and multiple metastases due to its abundant vascularity and red marrow microenvironment (SPECT sensitivity for the diagnosis of bone metastases [BM] 87–92%; specificity 91–93%; evidence level II–III) [1]. Spinal SPECT has proven particularly accurate in detecting transcortical and subcortical metastases. However, this time-consuming technique is limited to suspicious conditions encountered at planar BS and it can fail to image small, predominantly lytic, metastases, especially when they are located in the bone marrow [3].
As a consequence the diagnostic strategy for imaging BM often relies on a multimodality approach where scintigraphic equivocal findings or negative feedback of a clinical suspicion advocate morphological confirmation by means of planar x‑ray and, if that is not diagnostic, high-resolution CT, targeted MRI, or even biopsy [4]. High-resolution CT provides highquality morphological detail of bone and bone marrow densities (high-resolution CT sensitivity for the diagnosis of BM 71–100%; evidence level II–III) [1]. It is recommended in the confirmation of suspected lesions at BS, the assessment of BM-related incipient fractures or collapses, surgical planning, and guiding bone biopsies [5]. MRI is suggested if scintigraphically doubtful findings are located in bones with large marrow cavities (e.g., vertebrae) [6]. Furthermore, MRI is advocated in case BS and planar x‑ray are negative but vertebral involvement is clinically suspected; it is also the method of choice for the study of spinal cord compression (diagnostic sensitivity of skeletal MRI 82–100%; specificity 73–100%; evidence level II–III) [1]. Conversely, MRI is inadequate in assessing cortical involvement and the thoracic cage owing to respiratory artifacts.
The use of such a composite approach in the diagnosis of BM can result in an expensive and time-consuming process. With this regard a recent literature review by Talbot et al. summarized and commented on results from more than 140 comparative studies casting light on the strengths and limitations of each available diagnostic technique in staging and restaging a broad spectrum of neoplasms [7].
In this elaborate scenario a promising contribution could result from PET and especially from hybrid PET/CT imaging. Indeed both nonspecific ([18F]FDG, [18F]/[11C]‑choline) and specific ([68Ga]‑DOTATOC) oncotropic tracers (radiopharmaceuticals tracing tumor metabolic features) have proven highly accurate in detecting both skeletal and extraskeletal localizations in several clinical conditions, and the advent of hybrid PET/CT systems has provided tomographic metabolic maps with a morphological characterization and an anatomic localization resulting in an increased specificity and diagnostic accuracy.
With regard to skeletal metastases [18F]FDG PET imaging, increasingly used in staging and restaging of multiple solid tumors, has proven more accurate than 99mTc‑DP BS in detecting early bone marrow-based and lytic metastases, obviating in such cases the need for BS (sensitivity of [18F]FDG PET for detecting bone metastasis 62–100%; specificity 96–100%; evidence level II–III) [1,8].
In spite of a relatively higher specificity compared with 99mTc‑DP BS, [18F]FDG PET/CT has proven less sensitive in detecting sclerotic metastases [9]. Indeed, as a positive tracer of glycolytic metabolism [18F]FDG may fail to image sclerotic BM that are often characterized by poor and less aggressive cellularity, not prone to hypoxia. Pertaining to [18F]FDG PET/CT other insidious conditions include on the one hand tumors with high mucin content, low proliferation rates and necrosis, which are likely to show low [18F]FDG avidity.
Thus, favored by the wider availability of PET and especially hybrid PET/CT systems and the recent worldwide 99molybdenum/99mTc supply shortage, the interest directed towards Na18F, a PET radiopharmaceutical able to define with high sensitivity areas of increased osteogenic activity. Its translation into clinical practice aims to replace 99mTc‑DP BS in its staging and restaging indications. First published experiences and comparative studies with Na18F PET claim a higher diagnostic accuracy than 99mTechnetium medronate (99mTc‑MDP) BS (including SPECT) and suggest Na18F PET as a complementary survey to oncotropic PET in the skeletal assessment of several solid malignancies [10–15]. The same papers, however, considered heterogeneous oncological populations, and had different study designs and statistical analyses of effectiveness and non-negligible methodological flaws, hence our aim is not to provide the reader with a meta-analysis or a systematic review on Na18F PET imaging diagnostic accuracy, but rather we aim to convey a descriptive overview of these preliminary experiences (hematological malignancies are beyond the scope of this review). A table with essential information and major methodological issues from each included study is also provided (Table 1).
General aspects
First introduced by Blau et al. in 1962 as the standard bone-seeking agent for conventional BS, Na18F was approved by the US FDA in 1972 but soon abandoned for market reasons in 1975 when the wide availability of 99molybdenum/99mTc generators allowed for more suitable solutions for g‑based bone imaging (i.e., 99mTc‑DP) [16].
With the dramatic development of PET imaging technology and the consequent improvement of logistics for the delivery of [18F]‑radiopharmaceuticals, Na18F has been reconsidered for bone imaging so much that in December 2008 the US National Cancer Institute (NCI, MD, USA) filed a new drug application for a different potency and dose from the Na18F original new drug application. On 1 February 2011, as the new drug application for Na18F was declared acceptable from a clinical pharmacological perspective, the FDA finally approved Na18F use in PET bone scans. As far as the EU situation is concerned, Na18F has an established monograph in the European Pharmacopoeia [17], which defines its standards for production, radioisotopic and radiochemical purity. Despite this, its clinical use is also subjected to national regulatory authorities, which are variable across the EU countries, therefore its use is not extensively accepted. Where accepted, clinical use of Na18F must comply with Good Manufacturing Practice guidelines; Na18F can also be purchased through nationally approved industrial suppliers that comply with national directives and Good Manufacturing Practice guidelines.
The revived interest towards Na18F in PET imaging rely on its short half-life (t1/2 = 109.7 min) positron-emission combined with the desirable characteristics of a rapid and high accumulation in bone and fast clearance from the circulation allowing for a high bone:background ratio in a short time.
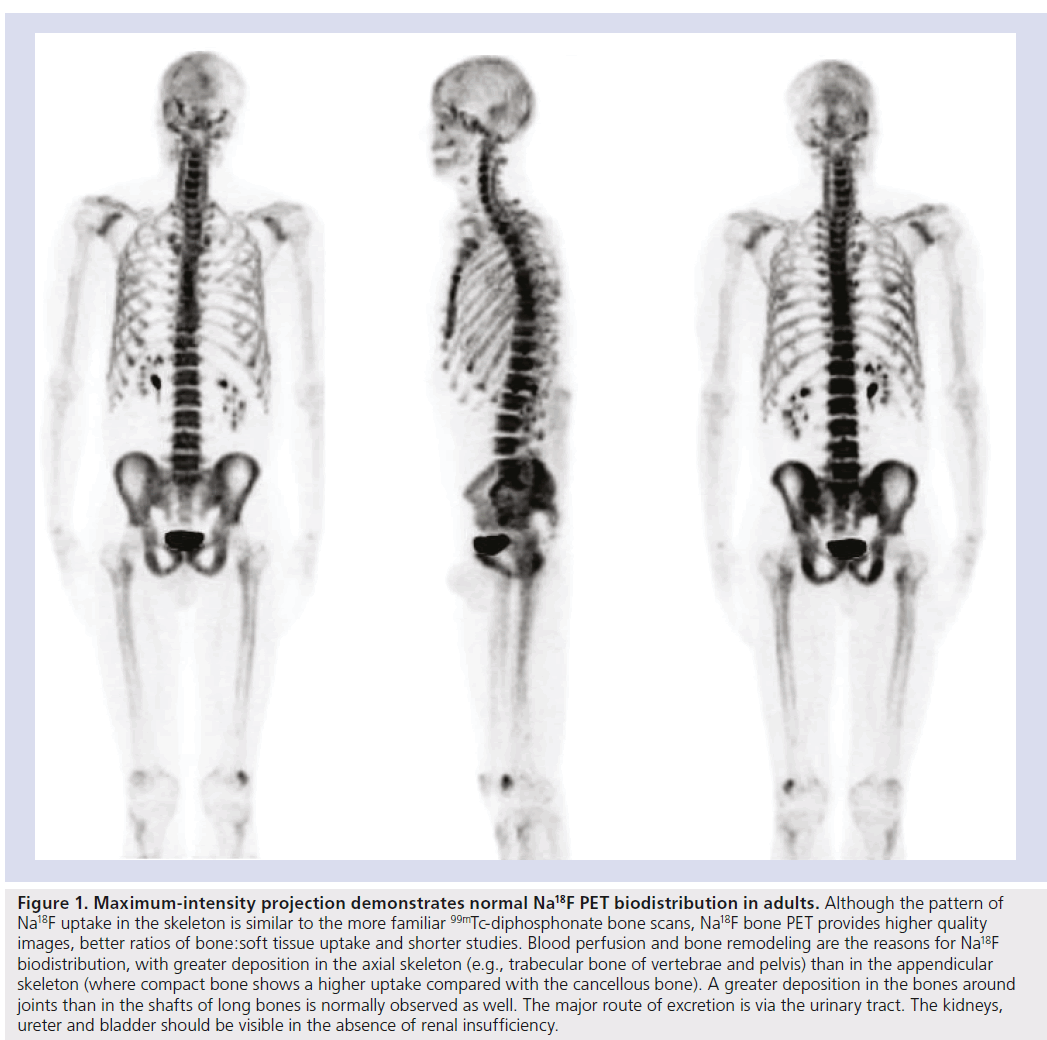
Its distribution and uptake are conditional on two limiting steps. Initial Na18F distribution reflects blood perfusion that varies among different bones [18]. Around 30% of the injected Na18F is present in erythrocytes, but this fact does not hamper 18F- ions exchange in bone because Na18F is freely diffusible across membranes [19]. Contrary to what happens with 99mTc‑DP, 18F- ions do not bind to plasma proteins either and clear out of the circulation twofold faster. Essentially all the Na18F that is delivered to bone by the blood flow is retained in the bone (Figure 1) [20]. Tracer retention is a two-phase process [21]. In the first phase, 18F- ions diffuse through capillaries into bone extracellular fluid and are chemisorbed onto bone surface by exchanging with hydroxyl groups in hydroxyapatite crystal of bone to form fluoroapatite [22]. In the second phase, the 18F- ion migrates into the crystalline matrix of bone, where it is retained until the bone is remodeled. At 1 h after Na18F administration approximately 10% of the injected dose remains in the blood [23].
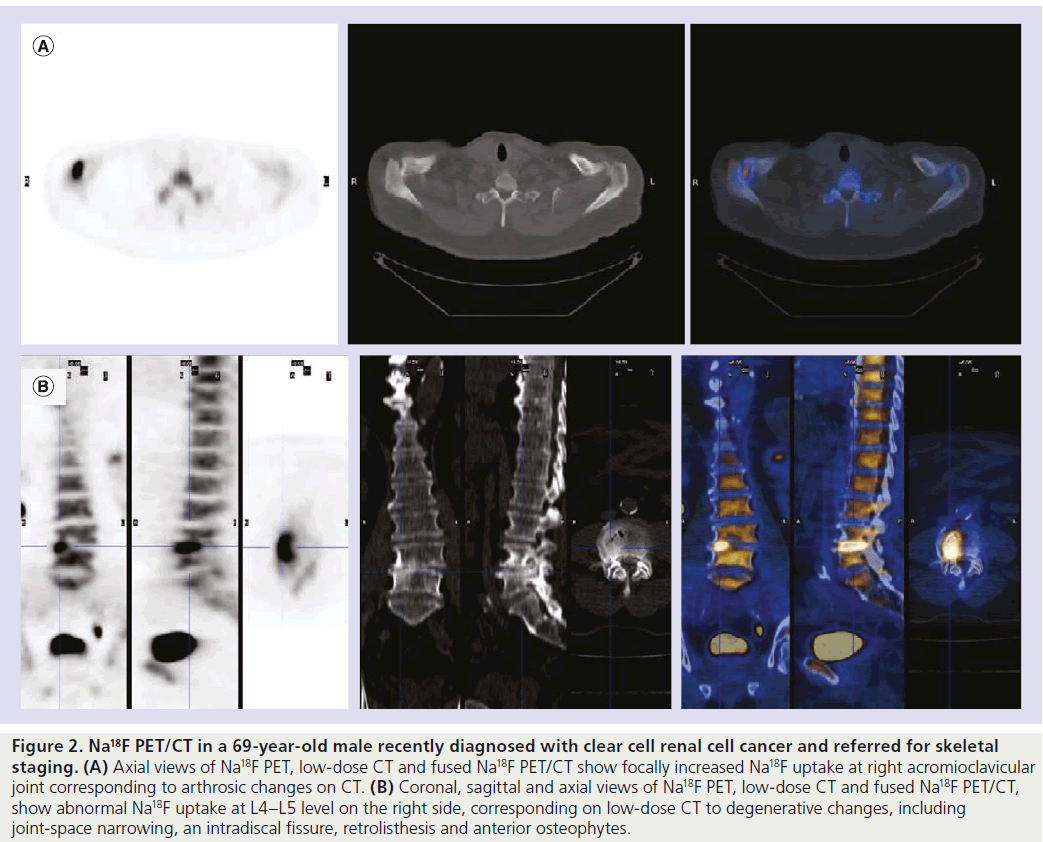
Thus, similarly to 99mTc‑DP, Na18F distribution reflects blood perfusion and osteogenic activity; it is not tumor specific (Figure 2) [24], therefore it accumulates not only in malignant processes but also when nonmalignant causes for altered blood flow and increased deposition of osteoid matrix occur (e.g., fracture, arthrosis, arthritis, osteomyelitis and benign bone tumors).
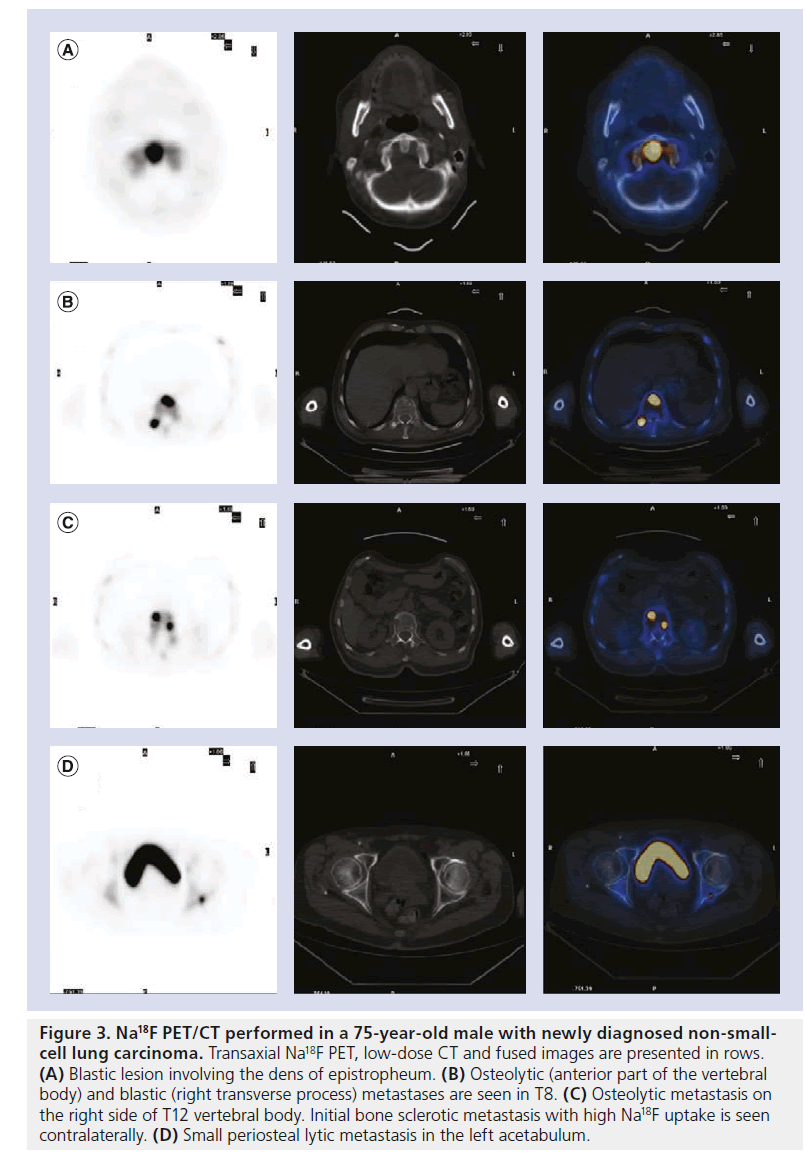
Both primary bone tumors and metastatic bone lesions are often characterized by an increased regional blood flow and bone turnover. With respect to osteogenic activity not only sclerotic metastases are easily imaged by Na18F PET but also predominantly lytic lesions, as they prompt, to some extent, a reactive new bone deposition (Figure 3). Na18F uptake in BM is fast and it is threefold higher compared with that of normal bone resulting in an optimal tumor:normal-bone tissue ratio. Combined with the higher resolution of PET scanners and morphological and anatomical characterization provided by PET/CT hybrid systems Na18F has proved accurate in detecting even small lesions with minimal osteoblastic activity and/or normal CT patterns [10,11,25–28].
To conclude, no safety issues concerning the clinical use of Na18F have been reported so far. The impact of treatments, such as bisphosphonates, antihormonal therapy, chemotherapy and radiotherapy, on the uptake of 18F- ions are yet to be determined.
Figure 1. Maximum-intensity projection demonstrates normal Na18F PET biodistribution in adults. Although the pattern of Na18F uptake in the skeleton is similar to the more familiar 99mTc-diphosphonate bone scans, Na18F bone PET provides higher quality images, better ratios of bone:soft tissue uptake and shorter studies. Blood perfusion and bone remodeling are the reasons for Na18F biodistribution, with greater deposition in the axial skeleton (e.g., trabecular bone of vertebrae and pelvis) than in the appendicular skeleton (where compact bone shows a higher uptake compared with the cancellous bone). A greater deposition in the bones around joints than in the shafts of long bones is normally observed as well. The major route of excretion is via the urinary tract. The kidneys, ureter and bladder should be visible in the absence of renal insufficiency.
Technical aspects of Na18F PET scanning
Patients undergoing Na18F PET/CT scanning do not require specific preparation, however, they should be well hydrated. The Na18F activity recommended for adults is 185–370 MBq, reserving a highest dose (444 MBq) for obese patients. Pediatric activity should be weight based (2.1 MBq/kg), using a range of 19–148 MBq [29]. In patients with a normal renal function whole-body images can be acquired 1 h after Na18F administration, preferably in 3D mode because the higher count rates compensate for the shorter acquisition times required for imaging a large area.
With regard to tumors predominantly displaying a retrograde venous metastatic dissemination (e.g., prostate and breast cancers) and no clinical suspicion of acral involvement, the overall PET field of view can be limited between the cranial vault and the proximal tibiae epiphyses resulting in a reduced effective dose and time saving. Conversely, neoplasms likely to metastasize via arterial embolization (e.g., lung and kidney carcinomas) as well as clinical suspicion of peripheral skeletal involvement prompt a complete scan to rule out peripheral localizations.
For a more comprehensive treatment of technical aspects of Na18F PET/CT scanning the reader is invited to consult Segall et al. [30].
Radiation dosimetry
In adults the effective dose for Na18F is 0.027 mSv/MBq. For a typical activity of 370 MBq, the effective dose is nearly 10 mSv. With regard to hybrid systems an additional dose from the low-dose CT portion should be considered. For a whole-body, low-dose CT scan the effective dose is at least 3.2 mSv (CT parameters: voltage of 120 keV, current of 30 mA, rotation of 0.5 s, pitch of 1).
Conversely the effective dose for 99mTc‑MDP is 0.0057 mSv/MBq. Thus a typical adult activity of 740 MBq would result in an effective dose of 4.2 mSv. Compared with planar 99mTc‑MDP BS the radiation dose to patients is approximately twofold higher using Na18F PET and threefold higher using Na18F PET/CT. The highest absorbed doses extrapolated to patients are in the bone surface, bone red marrow and bladder walls for both modalities.
The effective dose for children is significantly higher. Considering a 15‑year-old patient weighing 55 kg, the extrapolated effective doses would be 0.034 mSv/MBq for Na18F (Na18F dose: 116 MBq; overall effective dose: 4 mSv) and 0.0070 mSv/MBq for 99mTc‑MDP (99mTc activity: 407 MBq; overall effective dose: 2.9 mSv) [31,32].
Na18F PET imaging in oncology
Na18F PET in osteosarcoma
Figure 2. Na18F PET/CT in a 69-year-old male recently diagnosed with clear cell renal cell cancer and referred for skeletal staging. (A) Axial views of Na18F PET, low-dose CT and fused Na18F PET/CT show focally increased Na18F uptake at right acromioclavicular joint corresponding to arthrosic changes on CT. (B) Coronal, sagittal and axial views of Na18F PET, low-dose CT and fused Na18F PET/CT, show abnormal Na18F uptake at L4–L5 level on the right side, corresponding on low-dose CT to degenerative changes, including joint-space narrowing, an intradiscal fissure, retrolisthesis and anterior osteophytes.
Figure 3. Na18F PET/CT performed in a 75-year-old male with newly diagnosed non-smallcell lung carcinoma. Transaxial Na18F PET, low-dose CT and fused images are presented in rows. (A) Blastic lesion involving the dens of epistropheum. (B) Osteolytic (anterior part of the vertebral body) and blastic (right transverse process) metastases are seen in T8. (C) Osteolytic metastasis on the right side of T12 vertebral body. Initial bone sclerotic metastasis with high Na18F uptake is seen contralaterally. (D) Small periosteal lytic metastasis in the left acetabulum.
A heterogeneous mesenchymal malignancy osteosarcoma is one of the most common pediatric cancers. Frequently affecting long bones, such as the femur, tibia and humerus, it is associated with the production of extracellular osteoid matrix and early hematogenous spread, mainly to the lungs and bone. Currently, staging of primary tumors and synchronous regional metastases (skip metastases) mainly relies on planar x‑ray, CT and MRI, whereas lung metastases and distant bone localizations are screened by means of CT and 99mTc‑MDP BS, respectively. Despite all these diagnostic measures, only 15% of patients harboring metastases will be correctly assessed. An accurate evaluation of both regional and distant metastases is, however, crucial for surgical planning and prognostic stratification. In the first published report on the use of Na18F for skeletal PET, Hoh et al. enrolled 13 patients with primary and metastatic bone lesions including four cases with osteosarcoma [33]. All of them showed increased Na18F uptake in the primary tumor site and in one case Na18F PET also imaged CT-proven lung metastases. Three patients with untreated osteosarcoma at the time of Na18F PET scan, showed the highest tumor:normal-bone uptake ratios compared with other malignant bone lesions, whereas one patient, referred for Na18F PET imaging after treatment with chemotherapy and immunotherapy, had a tumor uptake ratio clearly reduced when compared with untreated cases, therefore suggesting a Na18F PET semiquantitative approach for monitoring therapy response. A case report by Tse et al. on a patient with a history of congenital polyostotic fibrous dysplasia, metastatic osteosarcoma and a breast mass described abnormal Na18F uptake in lung nodules supporting the diagnosis of osteosarcoma metastases [34].
Brenner et al. reviewed the potential applications of PET imaging in osteosarcoma and suggested that Na18F PET could be useful in staging and restaging of distant lung and BM thus replacing conventional 99mTc‑MDP BS and assisting thoracic CT assessment and prognostic stratification [35]. Given the exceptional dual nature of Na18F in osteosarcoma (oncotropic and osteotropic agent at once) its application in therapy response monitoring has been suggested but it is still speculative and beyond the scope of this review.
Skeletal Na18F PET & PET/CT in
heterogeneous oncologic populations
In the attempt to select a population with a similar prevalence of lytic and sclerotic BM Schirrmeister et al. [12] prospectively included 44 patients affected by prostate (n = 20), thyroid (n = 19) and lung cancer (n = 5). Their aim was to estimate the sensitivity of 99mTc‑MDP BS in detecting both BM patterns and describe how their anatomic localization inf luenced 99mTc‑MDP BS detection rate by direct intrapatient comparison with Na18F PET. Reference standard included a composite panel of imaging techniques and clinical follow-up. On a lesionbased analysis the receiver operating characteristic (ROC) curve was 0.99 for Na18F PET and 0.64 for 99mTc‑MDP BS. Indeed, Na18F PET yielded a significantly higher detection rate regardless of BM pattern and localization, whereas 99mTc‑MDP scan detected half of osteoblastic and osteolytic lesions. Furthermore, 99mTc‑MDP BS sensitivity varied according to the anatomic location of the lesion, confirming a lower sensitivity in the spine and pelvis. Na18F PET had a limited number of equivocal findings and was found to be more accurate than 99mTc‑MDP BS in discriminating benign from malignant findings. In a patient-based analysis, two patients (4.5%) with undetectable BM on 99mTc‑MDP BS (positive at Na18F PET) were later proven false negative, and the extent of BM was underestimated in eight patients (18.2%). Conversely, Na18F PET accurately assessed the extent of disease in all 15 true positive patients.
Considering the downside of aspecific Na18F uptake, Even-Sapir et al. evaluated the added value of low-dose CT morphological characterization offered by hybrid PET/CT systems compared to PET alone in assessing malignant osseous involvement and in differentiating malignant from benign findings in an heterogeneous oncologic population [36]. Reference methods for final diagnosis were histopathology, imaging and clinical follow-up. In a lesion-based analysis, the sensitivity of PET alone in differentiating benign from malignant bone lesions ranged from 72 to 90%, whether inconclusive lesions (Na18F positive, no CT abnormalities) were considered false negative or true positive. On the other hand, PET/CT yielded an overall sensitivity of 99% for tumor detection when inconclusive findings were considered as true positive. Furthermore, PET/CT specificity was significantly higher than that of PET alone (97 vs 72%; p < 0.001). Noteworthy among the 12 patients referred for Na18F assessment because of bone pain despite negative findings on 99mTc‑MDP BS, Na18F PET/CT suggested malignant bone involvement in all four patients with proven skeletal metastases.
Recently Withofs et al. have prospectively studied 34 patients with breast (n = 24) and prostate cancer (n = 10) at high risk of BM to evaluate Na18F PET/CT diagnostic accuracy compared with 99mTc‑MDP BS completed with SPECT/CT [25]. Both examinations were obtained for all 34 patients and the results were compared with a radiological gold standard (MRI or thin-slice CT). The overall sensitivity, specificity and accuracy of Na18F PET/CT were 76.0, 84.2 and 80.0%, respectively. For BS, they were 44.8, 79.2 and 60.0%, respectively (sensitivity significantly decreased for lytic lesions). They also reported that low-dose CT scanning did not improve specificity of PET compared with BS, but greatly improved lesion localization. PET/CT imaging with Na18F correctly modified the BS results in 12.1% (four patients). On the basis of their results Na18F PET/CT was suggested as an alternative for staging high-risk patients.
A meta-analysis performed by Tateishi on 11 eligible studies (overall including 425 patients) aimed to evaluate the diagnostic accuracy of Na18F PET, Na18F PET/CT, 99mTc‑MDP BS and 99mTc‑MDP SPECT in detecting bone metastatic involvement [37]. The patient-based sensitivity and specificity obtained for Na18F PET or Na18F PET/CT were 96 and 99%, respectively. Conversely 99mTc‑MDP BS, even when completed with a SPECT study, showed a sensitivity of 81 and a specificity of 99%. On a lesion-based analysis, sensitivity and specificity were 97 and 98%, respectively, for Na18F PET or PET/CT, and 56 and 96% for 99mTc‑MDP BS or SPECT, respectively.
Na18F PET in BM from prostate cancer
Prostate cancer is the most common neoplasm in men in the western world [26,38], and, although it is not always lethal, it accounts for approximately 27,000 deaths per year in the USA, making it the second leading cause of cancer-related deaths in men [39,40]. Prostate cancer is a heterogeneous disease; it ranges from asymptomatic slowgrowing forms to rapidly progressive systemic malignancy, the skeleton being the most affected distant organ. As the major cause of morbidity and mortality the presence of BM is related to a poor prognosis. Indeed prostate BM are predominantly sclerotic but are associated with an increased osteolysis as well, causing destruction of normal bone and formation of abnormally woven bone generated by osteoblastic hyperstimulation [41]. Consequently patients are at risk of vertebral deformity or collapse, spinal cord compression and fractures. Approximately 20% of patients with BM will develop pathologic fractures typically in load-bearing sites, and approximately 30% will have bone pain requiring palliative radiation therapy [42]. Economically, the lack of an early detection method for these complications will understandably imply increased healthcare costs. Therefore, a thorough evaluation of BM is pivotal both in staging as it will lead to the choice of the optimal therapeutic strategy, and restaging when evocative symptoms or biochemical recurrence occur after radical prostatectomy or radiotherapy to assess the true extent of skeletal disease at an earlier stage and prevent skeletalrelated events. With this regard 99mTc‑DP BS is currently indicated in the asymptomatic patient staging if the risk of metastatic disease is deemed high [43,44] (i.e., prostate-specific antigen [PSA] higher than 10 ng/ml; Gleason score >7; stage T3 or higher); after radical prostatectomy or radiation therapy in case of clinical or biochemical recurrence (suggested by a PSA at least higher than 10 ng/dl or by a PSA doubling time shorter than 6 months). Nevertheless several studies comparing the sensitivity of planar BS with that of MRI have shown that planar BS is less sensitive than previously accepted [45–47]. Current clinical indications for oncotropic PET agents such as 18F‑choline and 11C‑choline in prostate cancer include preoperative lymph nodal staging for intermediate and high-risk selected patients as they perform better than clinical nomograms [48]. 18F/11C‑choline is also indicated for the early detection of locoregional and/or distant recurrence after radical prostatectomy and radiation therapy [49–51] even with small increases of serum PSA levels. Considering skeletal metastases, a comparative study by Beheshti et al. examined 38 patients with prostate cancer by means of 18F‑choline and Na18F PET/CT [13]. Inclusion criteria comprised preoperative high-risk (high Gleason score and/or elevated PSA) and a postoperative clinical or radiological suspect of bone recurrence. Their results documented a sensitivity, specificity and accuracy for detection of BM respectively of 74, 99 and 85% for 18F‑choline PET/CT, respectively, and 81, 93 and 86% for Na18F PET/CT, respectively. Lytic lesions showed more intense uptake than sclerotic lesions using both imaging modalities. Although on a patient basis both procedures had a close concordance (k = 0.76), on a lesion basis they coincided in 80% of lesions (k = 0.57). Na18F PET/CT documented a higher number of BM in some patients, with these findings not affecting their clinical management. 18F‑choline PET/CT on the other hand led to a change in management in two of 38 patients in preoperative evaluation owing to early detection of BM; in both patients, CT and Na18F PET scans were negative, but malignancy was confirmed in follow-up examinations. These findings were interpreted as bone marrow metastases without significant bone remodeling, suggesting that 18F‑choline PET/CT has an advantage in the early detection of BM. Conversely, for discordant Na18F positive/18F‑choline negative findings observed exclusively in patients under hormone therapy, the hypothesis of reactive bone replacing a no longer metabolically active lesion was put forward. This assumption was supported by the evidence of increasing bone mineralization observed on CT in view of a progressive decrease in 18F‑choline uptake expressing positive response to therapy. Indeed, what is known under the name of ‘flare phenomenon’ in conventional BS, was also reported by Wade et al. using Na18F PET/CT [14]. The same trend, metabolically negative and sclerotic at CT, was also observed with Na18F PET/CT, but in a later phase of hormonal therapy and with an even higher level of density at CT (mean HU, 1148 ± 364), likely to express the completion of reparative bone deposition. A prospective study by Even- Sapir et al. compared the diagnostic accuracy of planar 99mTc‑DP BS, SPECT, Na18F PET and Na18F PET/CT in patients with either newly diagnosed, localized, high-risk prostate cancer or suspected recurrence/disease progression [52]. The sensitivity and specificity for detection of BM was 70 and 57% for planar BS, respectively, and 92 and 82% for bone SPECT, respectively. As far as PET imaging was concerned, sensitivity and specificity, when equivocal lesions were characterized as malignant, yielded 100 and 62% for Na18F PET, respectively, and 100 and 100% for Na18F PET/CT, respectively. Of the 23 patients with proven BM (on biopsy or follow-up) Na18F PET/CT correctly identified 20 patients with corresponding sclerotic pattern on CT, whereas findings in three patients were classified as equivocal given the radiologically normal bone appearance. Overall Na18F imaging caused a change of treatment in seven patients (15.9%): in three of the 11 newly diagnosed cases with bone metastatic spread Na18F PET/CT detected early bone involvement, otherwise overlooked on 99mTc‑DP BS, leading to the choice of a systemic therapy. Among the 19 patients with suspected recurrence or disease progression Na18F PET/CT made two patients shift to chemotherapy and two others modify their androgen withdrawal therapy.
Na18F PET in BM from breast cancer
Breast carcinoma is the most prevalent cancer in women. BM affect approximately 5–10% of breast cancer patients at early stages and are found in up to 70% of advanced stages. The skeleton represents the most common site of distant recurrence; the first site of recurrence in 25–50% of relapsed patients. At diagnosis, risk factors for skeletal involvement are a primary tumor size greater than 2 cm (T2 or more) and/or more than three axillary nodes and/or an estrogen receptor-positive status [53–55].
However, a skeletal-confined breast carcinoma is associated with a more indolent clinical course compared with visceral involvement. Its distribution has proven a prognostic factor itself. Yamashita et al. [27] found that patients who had BM exclusively located superiorly to the lumbosacral junction had a significantly longer survival than patients with BM in the pelvis and the lower limbs [56].
BM from breast cancer are predominantly lytic (50%) or mixed (40%), being sclerotic in approximately 10% of cases. Nevertheless in a retrospective analysis of patients presenting with neoplastic bone involvement from breast cancer Quattrocchi et al. described an increased prevalence of sclerotic lesions in patients under zoledronic acid treatment, suggesting diphosphonates as a possible cause for this change [57]. No significant correlation between the histotype of breast cancer and radiological appearance of BM have been found.
99mTc‑DP BS has a low diagnostic yield in early stages and it is currently recommended in staging patients with positive axillary nodes (N+), large tumors (T3) or clinical signs, symptoms, or laboratory values that suggest a metastatic involvement. It is also indicated to rule out a bone involvement if a neoadjuvant therapy is planned [58]. During follow-up 99mTc‑DP BS is indicated if patients are clinically symptomatic, with negative planar x‑ray and/or show elevated bone or tumor markers (alkaline phosphatase, carcinoembryonic antigen, CA 15.3) [59,60].
Currently [18F]FDG PET is complementary to BS in surveying the skeleton for metastatic involvement as it has proven superior in detecting lytic and intramedullary metastases, but unable to demonstrate sclerotic lesions [61].
Indeed, a recent meta-analysis comparing diagnostic accuracies of [18F]FDG PET, MRI and 99mTc‑DP BS in detecting BM in patients with breast cancer pointed out the superiority of MRI, but also described a significantly higher lesion-based sensitivity for 99mTc‑DP BS compared with [18F]FDG PET, the latter resulting more specific (99mTc‑DP BS sensitivity and specificity of 87.8 and 96.1%, respectively; [18F] FDG PET sensitivity and specificity of 52.7 and 99.6%, respectively) [62].
As specificity is the main limitation of 99mTc‑DP BS an additional potential pitfall must be taken into account when restaging breast cancer patients who also underwent local-regional radiotherapy. Park et al. reviewed bone scans from 294 such patients and described hot spots inside the irradiated field of the bony thoracic cage in 30 patients (cumulative incidence at 5 years = 12.9%) [63]. These findings, benign in nature but misleading at interpretation, were more common in postmenopausal patients who weighed less than 60 kg and whose field of irradiation included the supraclavicular area.
Currently, only a few studies have evaluated the ability of Na18F PET to detect BM in breast cancer patients. In a case series including five patients with multiple skeletal metastases from breast cancer, Pétren-Mallmin et al. reported a high tracer uptake in both sclerotic and lytic BM [64]. Schirrmeister et al. compared Na18F PET with BS in 34 patients with high-risk breast cancer and clinical or biological suspect of skeletal involvement [10]. The gold standard was represented by MRI, CT and planar x‑ray. On a lesionbased analysis Na18F PET detected 64 metastatic lesions in 17 patients, whereas BS only detected 29 metastases in 11 patients. The reported ROC area was 0.99 for Na18F PET and 0.74 for BS. Overall Na18F skeletal PET changed the clinical management of four patients (11.8%).
Na18F PET in BM from lung cancer
Unlike prostate and breast cancer, lung neoplasms are often diagnosed at advanced stages and 30–50% have distant metastases at the time of presentation, the skeleton being one of the most common sites of distant metastases [11,65]. The extent of disease is the most important prognostic factor, suffice it to say that nonsmall- cell lung cancer (NSCLC) without distant metastases is potentially curable, whereas small-cell lung cancer, which accounts for approximately 25% of lung cancers, has a high propensity for the early systemic spread so that 70% patients already have distant metastases at the time of diagnosis [66]. Lung cancer metastases normally appear purely lytic, with poor margination, no matrix and cortical destruction. Regarding the limited survival prospect after diagnosis of BM and the high costs of thoracic surgery, preoperative exclusion of BM is crucial. As a consequence current protocols include the routine use of [18F]FDG PET for assessing both lymphonodal and distant metastatic involvement. With respect to skeletal disease [18F]FDG PET showed a sensitivity similar to BS, but a higher specificity (98 vs 61%) proving very useful in staging patients eligible for radical surgery, even where there is a lack of symptoms and signs of BM. As Cook et al. suggested, [18F]FDG might be generally less sensitive in detecting osteoblastic metastases but more sensitive in detecting osteolytic lesions [66]. Conversely, Na18F PET has been shown to be highly sensitive in detecting both osteolytic and osteoblastic lesions (Figure 3). Schirrmeister et al. prospectively studied 53 patients affected by small-cell lung cancer and locally advanced NSCLC in order to evaluate the clinical impact of BS, SPECT and Na18F PET [67]. MRI, FDG PET, spiral CT and follow-up were used as reference methods. All 12 patients who harbored BM were correctly identified by Na18F PET, whereas one was missed at SPECT. BS failed to prove BM in six patients. The area under the ROC curve was then 0.779 for BS, 0.944 for SPECT and 0.993 for Na18F PET. As a result of Na18F PET imaging, clinical management was changed in six patients (11%). Another study by the group from Ulm, primarily designed to assess Na18F PET accuracy and cost–effectiveness compared with BS and SPECT in skeletal staging of NSCLC, evaluated 103 patients, of whom 33 had BM [15]. Na18F PET correctly staged 31 BM patients, proving to be more accurate with a significantly superior area under the ROC curve (Na18F PET = 0.989 vs BS = 0.771; BS and SPECT = 0.875). Thirteen patients were falsely negative at BS, four at SPECT and one at 18F PET. Owing to the superior diagnostic accuracy of Na18F PET imaging, clinical management was changed in 9.7% of cases either because curative surgery was cancelled or because radiation therapy was omitted. Of note, in the same study [18F]FDG PET/CT was carried out in 41 patients, correctly indicating BM in eight out of ten patients and strongly underestimating the extent of skeletal spread in four patients. To conclude, in a series of 126 NSCLC patients studied by means of [18F]FDG PET/CT Krüger et al. assessed its diagnostic accuracy compared with BS (in 58 patients) and to Na18F PET (in 68 patients) [68]. Na18F PET proved to be at least as sensitive as [18F]FDG PET/CT. Krüger et al. concordantly diagnosed BM in 13 out of 18 patients. On a patient-based analysis Na18F PET correctly identified four patients with BM and a negative [18F]FDG PET/CT. Noteworthy a patient with one osteolytic BM resulted positively true at [18F]FDG PET/CT but falsely negative at Na18F PET. On a lesion basis [18F]FDG PET/CT identified a higher number of BM compared with Na18F PET (53 malignant lesions vs 40).
Na18F PET in BM from other cancers Well-differentiated thyroid cancer
BM is a frequent complication of well-differentiated thyroid carcinoma that severely reduces a patient’s quality of life and decreases their 10‑year survival by 50% [69]. Indeed, it has been demonstrated that patients with BM have a worse prognosis than those with iodine-avid lung lesions. The skeletal distribution of thyroid metastases presents a lower percentage of vertebral localizations as compared with other malignancies and the number of patients with one single metastasis is higher [28]. The onset of bone pain or an increasing trend of thyroglobulin serum levels in thyroidectomized patients justifies a whole-body imaging assessment in order to localize and evaluate the extent of skeletal involvement. Since thyroid BM often maintain the ability to concentrate iodine and have a predominantly lytic pattern with a poor osteosclerotic reaction whole-body iodine scintigraphy (131I WBS) proved to be more accurate in identifying bone (and soft tissue) lesions than conventional 99mTc‑DP BS. 131I WBS proved more accurate when performed after the administration of therapeutic doses. On the other hand 131I WBS carried out with diagnostic doses yields lower sensitivity, and is burdened with a stunning effect. Experience with Na18F PET is limited. In a prospective study carried out on 35 patients with suspected thyroid BM, Schirrmeister et al. used Na18F PET as a gold standard procedure to evaluate results of visual interpretation of planar 99mTc‑MDP BS with and without 131I WBS [28]. At this juncture Na18F PET could detect 21 previously unknown BM, 13 of which had very low sclerotic activity that was undetectable on BS, confirming the high sensitivity and resolution of the PET procedure.
Hepatocellular carcinoma
The prognosis for patients with extrahepatic metastases of hepatocellular carcinoma (HCC) is poor with a 1‑year survival rate of approximately 21.7%. The skeleton is the third most frequent target-organ, after the lungs and lymph nodes. Although most HCC patients with extrahepatic metastases should undergo treatment for the intrahepatic HCC mainly, treatment of extrahepatic metastases in selected HCC patients who have good hepatic reserve, low intrahepatic tumor stage and are free of portal venous invasion may improve survival [70]. Yen et al. compared the diagnostic accuracy of Na18F PET/CT and BS in 34 HCC patients with a suspect skeletal involvement. Both procedures were performed within 1 month for each patient. Pathology and clinical follow-up were the standard of reference [71]. Once again Na18F PET/CT demonstrated significantly higher accuracy than BS (95.7 vs 75.4%; p = 0.0001). They also reported a significant correlation between the presence of Na18F PET/CT positive bone lesions and the survival time of HCC patients, which was not observed with BS.
Neuroendocrine tumors
The incidence of BM in neuroendocrine patients, typically sclerotic or mixed, varies between 7 and 17% [72]. As a predictor of poor prognosis and a contraindication to extended surgical resection skeletal involvement must be accurately evaluated. Putzer et al. compared the diagnostic accuracy of CT and 68Ga‑DOTATOC PET in the detection of BM in a cohort of 51 patients affected by NET tumors, and included Na18F PET among the standards of reference [73]. A subset of 19 patients were evaluated by means of 68Ga‑DOTATOC, [18F]FDG and Na18F PET. In this subset Na18F revealed 245 secondary lesions versus 218 disclosed by 68Ga‑DOTATOC and 80 observed with [18F]FDG. Despite the higher sensitivity of Na18F, 68Ga‑DOTATOC was reported superior in the initial detection of still unknown BM, thus having a greater impact on therapeutic management [73].
Renal clear cell carcinoma
Renal clear cell carcinoma accounts for 80–90% of all renal malignancies and the overall 5‑year survival rate is approximately 45%. BM present in 20–25% of renal clear cell carcinoma cases, and are highly osteolytic and are particularly destructive. Their number and localization are established prognostic factors. Szendroi et al. reported that in case of solitary resectable metastasis 1-year-survival was 75.0% whereas at 5 years only 35.5% of patients survived. [74]. If multiple metastases were present, no patient survived at 5 years. Palliative treatments include surgery to prevent or stabilize pathological fractures, antiresorptive drugs, painkillers, radionuclide therapy and local irradiation to relieve pain, thus impacting on patients’ quality of life [74]. Na18F PET experience in this contest is limited. Bhargava et al. reported the case of a symptomatic 59‑year-old patient with metastatic renal clear cell carcinoma and documented a higher sensitivity of Na18F PET/CT compared with CT [75].
Conclusion & future perspective
Imaging BM often results in a complex and multimodal process primarily influenced by the patient’s underlying tumor, clinical situation and expected change in clinical management. Nevertheless when a whole-body skeletal assessment is specifically advocated 99mTc‑DP BS is still the recommended modality for the majority of primary solid tumors and for osteosarcoma. However, with the advent of high-resolution modalities such as CT, PET and MRI, 99mTc‑DP BS sensitivity is no longer perceived as highly as it was in the past decades. On the one hand, given the low specificity of 99mTc‑DP uptake, BS can result in equivocal or falsely positive findings. On the other hand 99mTc‑DP BS can fail to image purely lytic and early intramedullary BM as well as lesions whose dimensions are below BS spatial resolution. Although 99mTc‑DP BS completed with a SPECT or SPECT/CT study may partially obviate these limitations, resulting at once as the most cost-effective approach in the assessment of BM, its routine use in clinical practice is strongly hampered by the prolonged examination time. On the contrary the favorable biochemical kinetics of Na18F allows for a faster whole-body acquisition resulting in a more efficient workflow and improved patient compliance. Na18F PET reproducibility is not an issue either, since recent official guidelines are available to recommend doses and scanning protocols. As far as skeletal staging and restaging indications are concerned, Na18F PET and PET/CT have proven undisputedly more sensitive and accurate than 99mTc‑DP BS and SPECT in a variety of malignancies. Indeed Na18F can image sclerotic, mixed and lytic lesions with poor and/or radiologically undetectable margination. Besides, PET spatial resolution allows for the detection of a higher number of small metastases when compared with 99mTc‑DP BS and SPECT. Conversely, a dramatic limitation seems to emerge from the few comparative studies testing diagnostic accuracy of Na18F and oncotropic PET agents, as the former displays a low sensitivity in imaging bone marrow-based BM that is the early phase of metastatic dissemination. Since Na18F is not tumor specific, the reviewer should also be aware of the different causes for benign Na18F uptake and seek them out in patient’s anamnesis (Box 1). In general, distinction between malignant and benign lesions of the skeleton is not insidious owing to new hybrid PET/low-dose CT systems that provide anatomical and morphological characterization of PET findings, further improving the specificity and overall accuracy of this imaging modality (Figure 2). If intermodality discrepancies are encountered (i.e., PET positive/CT negative or PET negative/ CT positive or other multimodality fusion imaging when available), their interpretation, as suggested by Paycha et al., should prompt an integrative reading aimed at maximizing the chances to correctly classify benign and malignant skeletal lesions [76]. In fact, such discrepant combinations should convey clues to achieve the highest possible levels of expertise. In spite of the increasing availability of PET scanners and the improved logistics for the delivery of 18F‑derived radiopharmaceuticals, Na18F PET imaging has not widely entered clinical practice yet. This delay is mainly due to the higher costs and lack of insurance coverage with Na18F PET, as its cost–effectiveness has not been systematically demonstrated yet. Indeed most of the cited studies had heterogeneous inclusion criteria and designs, and primary outcomes mostly addressed diagnostic accuracy rather than Na18F PET impact on therapeutic management. For this purpose, starting from 2010, the US Centers for Medicare and Medicaid Services (CMS) initiated an Evidence Development Program under whose aegis prospective, well-controlled clinical trials are being financially covered. Their aim is to produce sufficient evidence on the real cost–effectiveness of Na18F PET and PET/CT, especially in assisting the primary therapeutic strategy or guide subsequent therapies by the identification, location and quantification of BM in patients in whom metastases are strongly suspected, based on clinical symptoms or the results of other diagnostic studies. Compared with 99mTc‑DP BS and oncotropic PET/CT the greatest diagnostic gain by means of Na18F PET/CT would be reasonably expected for specific subsets of patients. With regards to prostate cancer, Na18F PET imaging could complement radiolabeled- choline PET/CT in staging high-risk patients (PSA >10 ng/ml; T3; Gleason score >7; N+) with no evidence of skeletal involvement or when equivocal findings are encountered (typically sclerotic, noncholine-avid lesions) to better characterize them. Na18F PET/CT could be equally indicated in a restaging scenario if skeletal metastases are not detectable at radiolabeled- choline PET/CT and conventional imaging but signs of recurrence are present (i.e., PSA doubling time <6 months). In locally advanced NSCLC a whole-body Na18F PET/CT could assist [18F]FDG PET imaging in preoperative staging if no BM have been detected, but the risk is deemed high on a clinical or biochemical basis. Moreover, Na18F PET/CT would be particularly useful in bronchioloalveolar carcinoma since it is often characterized by mild or no [18F]FDG uptake. In small-cell lung cancer, Na18F PET/CT could be indicated at staging for confirmation of limited disease.

As far as breast cancer is concerned potential indications to Na18F PET imaging could be preoperative staging of high-risk patients with a locally advanced tumor and a [18F]FDG PET/CT negative for distant metastases. During follow-up Na18F PET/CT might be equally indicated if patients are clinically symptomatic, with negative [18F]FDG PET and conventional imaging and elevated bone remodeling or tumor markers.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: *of interest * of considerable interest
- Hamaoka T, Madewell JE, Podoloff DA et al. Bone imaging in metastatic breast cancer. J. Clin. Oncol. 22(14), 2942–2295 (2004).
- Savelli G, Chiti A, Grasselli G et al. The role of bone SPET study in diagnosis of single vertebral metastases. Anticancer Res. 20(2B), 31115–31120 (2000).
- Taoka T, Mayr N, Lee HJ et al. Factors influencing visualization of vertebral metastases on MR imaging versus bone scintigraphy. Am. J. Roentgenol. 176, 1525–1530 (2001).
- Scutellari PN, Antinolfi G, Galeotti R et al. [Metastatic bone disease. Strategies for imaging]. Minerva Med. 94(2), 77–90 (2003).
- Ghanem N, Uhl M, Brink I et al. Diagnostic value of MRI in comparison to scintigraphy, PET, MS-CT and PET/CT for the detection of metastases of bone. Eur. J. Radiol. 55, 41–55 (2005).
- Gold RI, Seeger LL, Bassett LW et al. An integrated approach to the evaluation of metastatic bone disease. Radiol. Clin. North Am. 28, 471–483 (1990).
- Talbot JN, Paycha F, Balogova S. Diagnosis of bone metastasis: recent comparative studies of imaging modalities. Q. J. Nucl. Med. Mol. Imaging 55(4), 374–410 (2011). & A complete and well-developed review addressing the current state-of-the-art of diagnostic imaging of bone metastases using a comparative approach.
- Nakai T, Okuyama C, Kubota T et al. Pitfalls of FDG-PET for the diagnosis of osteoblastic bone metastases in patients with breast cancer. Eur. J. Nucl. Med. Mol. Imaging 32, 1253–1258 (2005).
- Du Y, Cullum I, Illidge TM et al. Fusion of metabolic function and morphology: sequential 18F fluorodeoxygluoce positron-emission tomography/computed tomography studies yeld new insights into the natural history of bone metastases in breast cancer. J. Clin. Oncol. 25, 3440–3447 (2007).
- Schirrmeister H, Guhlmann A, Kotzerke J et al. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J. Clin. Oncol. 17, 2381–2389 (1999).
- Vaporciyan AA, Nesbitt JC, Lee JS. Cancer of the lung. In: Cancer Medicine (2nd Edition). Holland JF, Frei E (Eds). BC Decker, London, UK, 1227–1292 (2001).
- Schirrmeister H, Guhlmann A, Elsner K et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J. Nucl. Med. 40(10), 1623–1629 (1999).
- Beheshti M, Vali R, Waldenberger P et al. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET–CT: a comparative study. Eur. J. Nucl. Med. Mol. Imaging 35, 1766–1774 (2008). & This prospective study compared 18F-fluorocholine and 18F-fluoride PET/CT for the detection of bone metastases from prostate cancer.
- Wade AA, Scott JA, Kuter I, Fischman AJ. Flare response in 18F-fluoride ion PET bone scanning. Am. J. Roentgenol. 186(6), 1783–1786 (2006).
- Hetzel M, Arslandemir C, König HH et al. 18NaF PET for detection of bone metastases in lung cancer: accuracy, cost–effectiveness, and impact on patient management. J. Bone Miner. Res. 18(12), 2206–2214 (2003). & Prospective study analyzing Na18F PET cost–effectiveness compared to 99mtechnetium diphosphonate bone scintigraphy and 99mtechnetium diphosphonate SPECT in staging bone metastases from lung cancer.
- Blau M, Ganatra R, Bender M. 18F-fluoride for bone imaging. Semin. Nucl. Med. 2, 31–37 (1972).
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia 2011 (7th Edition).Directorate for the Quality of Medicines and HealthCare of the Council of Europe, Strasbourg, France (2010).
- Frost ML, Blake GM, Cook GJ, Marsden PK, Fogelman I. Differences in regional bone perfusion and turnover between lumbar spine and distal humerus: 18F-fluoride PET study of treatment-naive and treated postmenopausal women. Bone 45, 942–948 (2009).
- Carlson CH, ArmstrongWD, Singer L. Distribution, migration and binding of whole blood fluoride evaluated with radiofluoride. Am. J. Physiol. 199, 187–189 (1960).
- Wootton R, Dore C. The single-passage extraction of 18F in rabbit bone. Clin. Phys. Physiol. Meas. 7, 333–343 (1986).
- Costeas A, Woodard HQ, Laughlin JS. Depletion of 18F from blood flowing through bone. J. Nucl. Med. 11, 43–45 (1970).
- Blake GM, Park-Holohan S, Cook GJR, Fogelman I. Quantitative studies of bone with the use of 18F-fluoride and 99mTc-methylene diphosphonate. Semin. Nucl. Med. 31(1), 28–49 (2001).
- Blau M, Ganatra R, Bender MA. 18F-fluoride for bone imaging. Semin. Nucl. Med. 2, 31–37 (1972).
- Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J. Nucl. Med. 51, 1826–1829 (2010).
- Withofs N, Grayet B, Tancredi T et al. 18F-fluoride PET/CT for assessing bone involvement in prostate and breast cancers. Nucl. Med. Commun. 32(3), 168–176 (2011).
- Damber JE, Aus G. Prostate cancer. Lancet 371, 1710–1721 (2008). n An excellent review on prostate cancer.
- Yamashita K, Koyama H, Inaji H. Prognostic significance of bone metastasis from breast cancer. Clin. Orthop. (312), 89–94 (1995).
- Schirrmeister H, Buck A, Guhlmann A, Reske SN. Anatomical distribution and sclerotic activity of bone metastases from thyroid cancer assessed with F-18 sodium fluoride positron emission tomography. Thyroid 11, 677–683 (2001).
- Ruth L, Fahey FH, Drubach LA et al. Early experience with fluorine-18 sodium fluoride bone PET in young patients with back pain. J. Pediatr. Orthop. 27(3), 277–282 (2007).
- Segall G, Delbeke D, Stabin MG et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J. Nucl. Med. 51(11), 1813–1820 (2010). & Society of Nuclear Medicine practice guidelines for the use of 18F-fluoride PET/CT.
- No authors listed. Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53). Ann. ICRP 28(3), 1–126 (1998).
- ICRP. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP Publication 53. ICRP Publication 106. Approved by the Commission in October 2007. Ann. ICRP 38(1–2), 1–197 (2008).
- Hoh CK, Hawkins RA, Dahlbom M et al. Whole body skeletal imaging with 18F fluoride ion and PET. J. Comput. Assist. Tomogr. 17(1), 34–41 (1993).
- Tse N, Hoh C, Hawkins R, Phelps M, Glaspy J. Positron emission tomography diagnosis of pulmonary metastases in osteogenic sarcoma. Am. J. Clin. Oncol. 17, 22–25 (1994).
- Brenner W, Bohuslavizki KH, Eary JF. PET imaging of osteosarcoma. J. Nucl. Med. 44(6), 930–942 (2003).
- Even-Sapir E, Metser U, Flusser G et al. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET-CT. J. Nucl. Med. 45(2), 272–278 (2004).
- Tateishi U, Morita S, Taguri M et al. A meta-analysis of 18F-fluoride positron emission tomography for assessment of metastatic bone tumours. Ann. Nucl. Med. 24, 523–531 (2010).
- Jemal A, Siegel R, Ward E et al. Cancer statistics. CA Cancer J. Clin. 59, 225–249 (2009).
- Trump DL. Prostate cancer. In: Encyclopedia of Cancer (2nd Edition). Bertino JR (Ed.). Academic Press, CA, USA, vIII, 463 (2002).
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann. Oncol. 16, 481–488 (2005).
- Ibrahim T, Flamini E, Mercatali L et al. Pathogenesis of osteoblastic bone metastases from prostate cancer. Cancer 116(6), 1406–1418 (2010).
- Garnero P, Buchs N, Zekri J et al. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br. J. Cancer 82, 858–864 (2000).
- Kelloff GJ, Choyke P, Coffey DS. Challenges in clinical prostate cancer: role of imaging. Am. J. Roentgenol. 192, 1455–1470 (2009). & Results from a recent workshop on prostate cancer and imaging technology are reviewed. The workshop was arranged by the Cancer Imaging Program of the National Cancer Institute, NIH, Bethesda, MD, USA.
- Briganti A, Passoni N, Ferrari M et al. When to perform bone scan in patients with newly diagnosed prostate cancer: external validation of the currently available guidelines and proposal of a novel risk stratification tool. Eur. Urol. 57(4), 551–558 (2010).
- Hochstenbag MM, Snoep G, Cobben NA et al. Detection of bone marrow metastases in small cell lung cancer: comparison of magnetic resonance imaging with standard methods. Eur. J. Cancer (32A), 779–782 (1996).
- Frank JA, Ling A, Patronas NJ et al. Detection of malignant bone tumors: MR imaging vs. scintigraphy. Am. J. Roentgenol. 55, 1043–1048 (1990).
- Horvath LJ, Burtness BA, McCarthy S, Johnson KM. Total-body echo-planar MR imaging in the staging of breast cancer: comparison with conventional methods – early experience. Radiology 211, 119–128 (1999).
- Schiavina R, Scattoni V, Castellucci P et al. 11C-choline positron emission tomography/ computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur. Urol. 54(2), 392–401 (2008).
- Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology 243(1), 28–53 (2007).
- Picchio M, Messa C, Landoni C et al. Value of 11C choline-positron emission tomography for re-staging prostate cancer: a comparison with 18F fluorodeoxyglucose-positron emission tomography. J. Urol. 169, 1337–1340 (2003).
- Picchio M, Giovannini E, Messa C. The role of PET/computed tomography scan in the management of prostate cancer. Curr. Opin. Urol. 21(3), 230–236 (2011).
- Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J. Nucl. Med. 47(2), 287–297 (2006).
- Coleman RE, Smith P, Rubens RD. Clinical course and prognostic factors following bone recurrence from breast cancer. Br. J. Cancer 77(2), 336–340 (1998).
- Manders K, van de Poll-Franse LV, Creemers GJ et al. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer 6, 179 (2006).
- Colleoni M, O’Neill A, Goldhirsch A et al. Identifying breast cancer patients at high risk for bone metastases. J. Clin. Oncol. 18, 3925–3935 (2000).
- Buck AK, Schirrmeister H, Mattfeldt T, Reske SN. Biological characterisation of breast cancer by means of PET. Eur. J. Nucl. Med. Mol. Imaging 31(Suppl. 1), S80–S87 (2004).
- Quattrocchi CC, Piciucchi S, Sammarra M et al. Bone metastases in breast cancer: higher prevalence of osteosclerotic lesions. Radiol. Med. 112(7), 1049–1059 (2007).
- Aebi S, Davidson T, Gruber G et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 22(Suppl. 6), vi12–vi24 (2011).
- Rybak LD, Rosenthal RI. Radiological imaging for the diagnosis of bone metastases. Q. J. Nucl. Med. 45, 53–64 (2001).
- Bares R. Skeletal scintigraphy in breast cancer management. Q. J. Nucl. Med. 42(1), 43–48 (1998).
- Rosen EL, Eubank WB, Mankoff DA. 18F FDG PET, PET/CT and breast cancer imaging. Radiographics 27, S215–S229 (2007).
- Liu T, Cheng T, Xu W et al. A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol. 40(5), 523–531 (2011).
- Park W, Huh SJ, Kim HY et al. The implication of hot spots on bone scans within the irradiated field of breast cancer patients treated with mastectomy followed by radiotherapy. Ann. Nucl. Med. 22, 685–691 (2008).
- Petrén-Mallmin M, Andréasson I, Ljunggren Ö et al. Skeletal metastases from breast cancer: uptake of 18F-fluoride measured with positron emission tomography in correlation with CT. Skeletal Radiol. 27(2), 72–76 (1998).
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 27, 165–176 (2001).
- Cook GJR, Fogelman I. The role of positron emission tomography in the management of bone metastases. Cancer 88(Suppl. 12), 2927–2933 (2000).
- Schirrmeister H, Glatting G, Hetzel J et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and 18F-labeled NaF PET in newly diagnosed lung cancer. J. Nucl. Med. 42(12), 1800–1804 (2001).
- Krüger S, Buck AK, Mottaghy FM et al. Detection of bone metastases in patients with lung cancer: 99mTc-MDP planar bone scintigraphy, 18F-fluoride PET or 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 36, 1807–1812 (2009).
- Schlumberger M, Tubiana M, De Vathaire F et al. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J. Clin. Endocrin. Metab. 63, 960–967 (1986).
- Uka K, Aikata H, Takaki S et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J. Gastroenterol. 13(3), 414–420 (2007).
- Yen RF, Chen CY, Cheng MF et al. The diagnostic and prognostic effectiveness of F-18 sodium fluoride PET-CT in detecting bone metastases for hepatocellular carcinoma patients. Nucl. Med. Commun. 31(7), 637–645 (2010).
- Kim SJ, Kim JW, Han SW et al. Biological characteristics and treatment outcomes of metastatic or recurrent neuroendocrine tumors: tumor grade and metastatic site are important for treatment strategy. BMC Cancer 10, 448 (2010).
- Putzer D, Gabriel M, Henninger B et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3- octreotide PET in comparison to CT and bone scintigraphy. J. Nucl. Med. 50(8), 1214–1221 (2009).
- Szendroi A, Dinya E, Kardos M et al. Prognostic factors and survival of renal clear cell carcinoma patients with bone metastases. Pathol. Oncol. Res. 16(1), 29–38 (2010).
- Bhargava P, Hanif M, Nash C. Whole-body F-18 sodium fluoride PET-CT in a patient with renal cell carcinoma. Clin. Nucl. Med. 33(12), 894–895 (2008).
- Paycha F, Girma A. Pattern-oriented approach in hybrid imaging (bisphosphonates-(99mTc) SPECT/CT and fluoride-(18F) PET/CT) according to bone abnormality phenotype: the sclerotic/osteoblastic lesion and the osteolytic/osteoclastic lesion. Méd. Nucl. 35, 332–335 (2011).