Research Article - International Journal of Clinical Rheumatology (2020) Volume 15, Issue 4
Nail-bed infarctions as a paradoxical effect of abatacept in rheumatoid arthritis
- Corresponding Author:
- Sahar Mahfouz Abdel Galil
Department of Rheumatology and Rehabilitation
Faculty of Medicine, Zagazig University, Egypt
E-mail: dr_saharmahfouz@yahoo.com
Abstract
Objective: Evaluating a group of Rheumatoid Arthritis (RA) patients under abatacept therapy regarding the risk of developing vasculitis. Methods: An exploratory study was conducted on RA patients with ongoing combined methotrexate/ abatacept therapy and reporting a case who developed small-vessel vasculitis. Follow-up visits with thorough clinical examination and routine laboratory investigations were done every 3-6 months. Disease activity was assessed by the Clinical Disease Activity Index (CDAI). Functional assessment was done by Health Assessment Questionnaire (HAQ) score. Results: Sixteen biologic-naïve female patients with newly diagnosed moderate-to-severe RA under abatacept therapy (mean age, 44 ± 10.86 years) were enrolled in this study. The mean disease duration was 4.63 ± 2.20, mean duration of methotrexate/abatacept therapy was 22.75 ± 9.40 months. All over the follow-up periods, patients were clinically improved with low disease activity score and within normal laboratory investigations apart from rising titers of Sedimentation Rate (ESR) and C-Reactive Protein (CRP). One patient was presented by nail-bed infarctions after 15 months follow-up of abatacept therapy. Abatacept–induced small-vessel vasculitis was the most likely diagnosis. The remaining 15 patients still clinically free of any manifestations suggesting vasculitis. Conclusion: Abatacept can induce vasculitis in RA patients having risk factors for RV. Combination therapy of methotrexate/abatacept may be a delaying or inhibiting factor in the appearance of RV manifestations. Persistently elevated acute phase reactants despite clinical and laboratory improvement on early aggressive treatment of RA is an alarm for a possibly imminent vasculitis.
Keywords
rheumatoid arthritis • rheumatoid vasculitis • small-vessel vasculitis • Abatacept
Introduction
Rheumatoid Vasculitis (RV) represents an inflammatory reaction affecting multiple vascular beds (small- and medium-sized vessels) that occurs in about 1-5% of patients with established rheumatoid arthritis [1,2]. Rheumatoid Arthritis (RA) complicated by small-vessel vasculitis manifests by nail fold infarctions, petechiae or purpuric eruption [1]. The less common medium-vessel vasculitis can occur with digital tip ischemia and/or gangrene, leg ulcers [3] and peripheral neuropathy (mainly; mononeuritis multiplex) [1].
Due to lack of specific signs and symptoms, the RV is diagnosed clinically by exclusion of other diseases associated with vasculitis such as atherosclerosis, diabetes mellitus, infections, malignancies, drug hypersensitivity or other vasculitides [4], but ideally it is diagnosed by visualization of necrotizing vasculitis in histopathological examinations [1], although not always possible [3].
A minority of patients with cutaneous RV develop systemic vasculitis affecting major organs that can be treated successfully with high-dose glucocorticoids, cytotoxic drugs as cyclophosphamide, azathioprine and sometimes with rituximab [5].
Abatacept (ABA) is a recombinant fully human fusion protein. It is composed of the extracellular domain of human cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and the Fc domain of human immunoglobulin G (IgG) [6]. It inhibits T-cell activation and proliferation by binding to CD80 and CD86 on antigen-presenting cells and prevents binding to CD28 on T-cells [7].
Abatacept is FDA approved to treat adult RA, juvenile idiopathic arthritis, and psoriatic arthritis [3]. Because there are no specific guidelines for the management of RV [8], ABA was considered as an alternative to standard immunosuppressant/glucocorticoid therapies and was successfully used for treatment of RV in previous case reports [5,9].
Inflammatory markers (ESR and CRP) are widely used for the diagnosis and follow-up of disease activity of different Rheumatic Diseases (RD). Commonly, elevated ESR and CRP levels may due to newly diagnosed RD, flaring of an existing disease, infection, or malignancy with a very low specificity in differentiating between these conditions. Thus, highly elevated inflammatory markers in RD necessitates ruling out other nonrheumatic conditions such as infection or malignancy before concluding with rheumatic etiologies such as disease reactivation or resistance to therapy [10].
Here, we evaluated a group of RA patients under ABA therapy in whom clinical improvement was associated with progressively rising titers of both ESR and CRP and one of them (the index case) was clinically presented with nail-bed infarctions (smallvessel vasculitis). Development of vaculitis in that case directed our attention towards exploring the hypothesis that sustained elevation of acute phase reactants may be an alarm for imminent vaculitis in well-controlled RA patients under ABA therapy.
Patients and methods
Patients selection, setting and study duration
From April 2017 to October 2019, sixteen adult female patients were consecutively diagnosed RA according the ACR/EULAR 2010 criteria [11] and treated by a combination of MTX/ABA therapy. They were longitudinally followed-up in the outpatient clinic of Rheumatology, with recording all relevant data presented in the current study in King Faisal Hospital, Makkah, Saudi Arabia. Patients were biologic-naïve and were included in this study after completing at least 6 months of MTX/ABA continuous therapy with persistently elevated ESR and CRP despite improvement of the clinical activity of the disease.
Study design and sample size
It is an exploratory study included the number of patients who were suffering from the same problem under study, without intending previous sample calculation.
Baseline and follow-up evaluation
Demographic, clinical, and laboratory data were collected before beginning of therapy and every 3-6 months all through the period of biological treatment, (for up to 24 months). Basically, all patients were subjected to full history taking, physical examination and routine diagnostic baseline investigations, including complete blood count with differential (CBC), erythrocyte sedimentation rate (ESR, by the modified Westergren method), C-reactive protein (CRP, by the nephelometric method), rheumatoid factor (RF), anticyclic citrullinated peptide (anti-CCP), antinuclear antibodies (ANA), liver and kidney function tests (LFT and KFT), viral hepatitis markers and tuberculin (TB) skin test. Plain radiography of the affected joints and the chest were also done at the time of diagnosis.
Follow up visits were routinely done every 3-6 months, where thorough clinical examination and routine laboratory investigations (CBC, ESR, CRP, LFT, KFT) were repeated.
Assessment of disease activity and drug response Baseline disease activity was assessed by the disease activity-28 joints (DAS-28) score [12], then clinical disease activity index (CDAI) [13], was preferred to assess the disease activity at all the subsequent followup visits because DAS-28 depends on either ESR or CRP in its calculation, which were found to be always elevated along the entire follow-up period for this group of patients. Health Assessment Questionnaire (HAQ) for functional disability [14] was estimated at baseline and every 6 months.
Ethical approval
The study was approved by the ethical committee of our institute and an informed written consent was obtained from all participants for publication and related images prior to their inclusion in the study.
Statistical analysis
All data of frequent follow-up evaluations were given as mean ± standard deviation (mean ± SD) applied to SPSS software program version 21, USA.
Results
Baseline evaluation results
The demographic and clinical characteristics of the 16 patients were presented in Table 1. The mean age ± SD of all patients was 44 ± 10.86 years, RA disease duration ± SD was 4.63 ± 2.20 years. All patients were females with moderate-to-severe disease activity (at the start of diagnosis) as measured by DAS-28 score (6.68 ± 0.37). The mean duration of abatacept therapy was 22.75 ± 9.40 months. None of the patients had a history of RV or another type of vasculitides. All were biologicnaïve then put under a combination therapy of abatacept 125 mg subcutaneous (SC) ampoule, methotrxate 15 mg and folic acid 5 mg orally, once weekly doses. The laboratory outcomes of all patients, before and after abatacept therapy, are summarized in Table 2.
Table 1. Demographic and clinical characteristics of all patients.
| Clinical Characteristics | |
|---|---|
| Age; (years) Mean ± SD | 44 ± 10.86 |
| RA duration; (years) Mean ± SD | 4.63 ± 2.20 |
| Treatment duration; (months) Mean ± SD | 22.75 ± 9.40 |
| Follow up duration; (months) Mean ± SD | 28.75 ± 10.94 |
| DAS-28 score, Mean ± SD | 6.68 ± 0.37 |
| RF-positivity; N (%) | 9(62.5 %) |
| Ant-CCP positivity; N (%) | 11(68.75 %) |
| Combined ACPA and RF positive; N (%) | 9(62.5 %) |
| ANA- positivity; N (%) | 12(75 %) |
| Smoking; N (%) | 1(6.25 %) |
| Extraarticular manifestations | |
| Rheumatoid nodules | 3 (18.75%) |
| Erosions | 4 (25%) |
| Deformities | 4(25%) |
| Other co-morbidities; N (%) | |
| Diabetes mellitus | 4(25%) |
| Hypertension | 2(12.5%) |
DAS-28: Disease Activity Score-28 joints; RF: Rheumatoid Factor;
Anti-CCP: Anti-Cyclic Citrullinated Peptide antibody; ANA: Antinuclear Antibody
Table 2. Laboratory characteristics of patients before and after abatacept treatment (mean ± SD).
| Case | ESR (mm/H) Before-Therapy | ESR (mm/H) After-Therapy | CRP (mg/dl) Before-Therapy | CRP (mg/dl) After-Therapy | CDAI Before-Therapy | CDAI After-Therapy | HAQ Before-Therapy | HAQ After-Therapy |
|---|---|---|---|---|---|---|---|---|
| Case-1 (index case) | 95 | 72.01 ± 16.60 | 22 | 14 ± 7.01 | 49 | 4.63 ± 3.40 | 2.8 | 1.71 ± 0.47 |
| Case-2 | 94 | 76.77 ± 24.41 | 14 | 19.77 ± 5.86 | 64 | 6.78 ± 2.68 | 2.6 | 1.78 ± 0.30 |
| Case-3 | 58 | 64.9 ± 25.1 | 16 | 6.6 ± 1.89 | 62 | 7.90 ± 2.38 | 2.5 | 1.54 ± 0.36 |
| Case-4 | 48 | 55.77 ± 10.21 | 14 | 11.77 ± 5.78 | 45 | 7.35 ± 3.32 | 2.4 | 1.6 ± 0.46 |
| Case-5 | 49 | 53.2 ± 8.16 | 12 | 11.2 ± 5.01 | 70 | 8.01 ± 3.24 | 2.3 | 1.52 ± 0.33 |
| Case-6 | 91 | 77.66 ± 6.62 | 24 | 20.66 ± 5.31 | 40 | 6.16 ± 3.31 | 2.5 | 1.68 ± 0.60 |
| Case-7 | 100 | 75.01 ± 14.14 | 12 | 15 ± 7.37 | 62 | 7.25 ± 3.30 | 2.7 | 1.95 ± 0.44 |
| Case-8 | 50 | 48.1 ± 34.9 | 10 | 13 ± 2.0 | 52 | 8.25 ± 3.30 | 2.4 | 1.67 ± 0.43 |
| Case-9 | 88 | 65.6 ± 14.75 | 24 | 13.12 ± 576 | 45 | 6.38 ± 3.46 | 2.5 | 1.67 ± 0.42 |
| Case-10 | 91 | 68.5 ± 16.37 | 22 | 18.44 ± 4.97 | 58 | 5.66 ± 2.55 | 2.7 | 1.76 ± 0.28 |
| Case-11 | 62 | 56.3 ± 14.90 | 12 | 7 ± 1.94 | 54 | 5.70 ± 2.75 | 2.3 | 1.53 ± 0.45 |
| Case-12 | 68 | 53.5 ± 8.80 | 18 | 11.6 ± 5.29 | 50 | 6.44 ± 3.13 | 2.1 | 1.55 ± 0.44 |
| Case-13 | 71 | 47.2 ± 4.14 | 20 | 12.4 ± 4.09 | 58 | 5.60 ± 2.40 | 2.5 | 1.60 ± 0.43 |
| Case-14 | 81 | 56.8 ± 8.84 | 24 | 16.33 ± 3.20 | 60 | 6.50 ± 2.73 | 2.6 | 1.72 ± 0.58 |
| Case-15 | 74 | 53.7 ± 9.83 | 18 | 13.75 ± 3.77 | 56 | 6.50 ± 3.10 | 2.4 | 1.82 ± 0.49 |
| Case-16 | 52 | 36.3 ± 11.08 | 16 | 10.75 ± 4.27 | 46 | 6.25 ± 2.98 | 2.3 | 1.55 ± 0.34 |
ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; DAS-28: Disease Activity Score-28 joints; CDAI: Clinical Disease Activity Index; HAQ, Health Assessment Questionnaire
Follow-up evaluation results
The mean follow-up duration ± SD after RA diagnosis was 28.75 ± 10.94 months. All patients clinically improved on the applied treatments, presented by lower CDAI after and all over the duration of therapy. Conversely, all patients showed persistently elevated ESR and CRP (mean ± SD of each variable for each patient is presented in Table 2). Only One patient (the index case) was presented by nail-bed infarcts (small-vessel vasculitis) after 15 months of ABA therapy while the others did not represent by any clinical manifestations throughout the period of their follow-up. Laboratory outcomes of patients are presented in Table 2.
Confirmatory work-up
Sustained elevations of inflammatory markers (ESR and CRP) necessitated other laboratory investigations for ruling out other differential diagnosis. Confirmatory investigations included blood film, 24-hour urine protein, glycosylate hemoglobin, viral serology, cryoglobulins, anti-neutrophil cytoplasmic antibodies (ANCA profile), anti-nuclear and antiphospholipid antibodies, Complement (C3 and C4). Pelvi-abdominal ultrasound and computerized tomography of the chest for exclusion of hidden malignancy. Nerve conduction studies and electromyography for exclusion of mononeuritis multiplex and carpal tunnel syndrome.
Index case presentation
A 37-year-old female patient, single, smoker diagnosed with RA since 2011. She received low dose steroids for a long duration with irregular courses and was maintained on prednisolone tablets 5 mg/day and hydroxychloroquine tablets 200 mg twice daily for about three years. In November 2017, she came to the outpatient clinic of Rheumatology, complaining from flaring of her disease. She was presented with morning stiffness for more than two hours with active arthritis affecting both hands (wrists, metacarpophalangeal joints (MCPs), proximal interphalangeal joints (PIPs)) and elbows and affecting both feet (metacarpophalangeal joints (MTPs)) and ankles. She also complained from recurrent subcutaneous nodules on her elbows and swan–neck deformity of the left index and little fingers. Review of other systems was unremarkable. Baseline investigations were done, including CBC, ESR, CRP, RF, anti-CCP, ANA, liver and kidney function tests, viral hepatitis markers and tuberculin (TB) skin test. Both RF and anti-CCP antibodies were positive, ANA was negative. Both ESR and CRP were elevated (95 mm/hour and 22 mg/dl, respectively). The CBC showed iron deficiency anemia and thrombocytosis (545 × 109/L). Other laboratory investigations were all within normal. Plain radiography of the affected joints showed narrow joint spaces in the wrists and PIPs with erosions and periarticular osteopenia while chest x-ray revealed no abnormalities.
The diagnosis was confirmed as a sero-positive RA. DAS-28 score was 6.75 denoting high activity. Oral methotrexate (MTX)15 mg once weekly and folic acid 5mg once weekly oral dose were added to hydroxychloroquine and prednisolone. Vitamin D and calcium were added as an adjuvant therapy.
In January 2018, she showed slight improvement of arthritis (DAS-28 became 6.5) while ESR and CRP still elevated (60 mm/H and 12mg/dl, respectively). Abatacept 125 mg subcutaneous ampoules once weekly was added to previous treatments. Initial improvement of RA activity occurred after one month of ABA injections. Then hydroxychloroquine was stopped (due to left eye amblyopia, discovered during follow-up eye examination) and gradual tapering of prednisolone till complete withdrawal was done to avoid complications as recommended by the 2019 updated EULAR RA management recommendations [15]. In May 2018, she was clinically free with no any complaints, no arthritis and no morning stiffness. All investigations were within normal except for sustained elevated ESR and CRP (72 mm/H and 16 mg/dl, respectively).
With periodic follow-up every 3 months (clinical and laboratory evaluations), she remains clinically improved without any complaints. Clinical disease activity index (CDAI) was used to assess activity of her disease as DAS- 28 depends on ESR and CRP that were persistently elevated. CDAI was 49 at initial diagnosis, 38 at the start of ABA treatment with a mean ± SD of 4.63 ± 3.40 all over the period of follow-up, denoting significant improved RA activity and within normal follow-up investigations revealing good drug response.
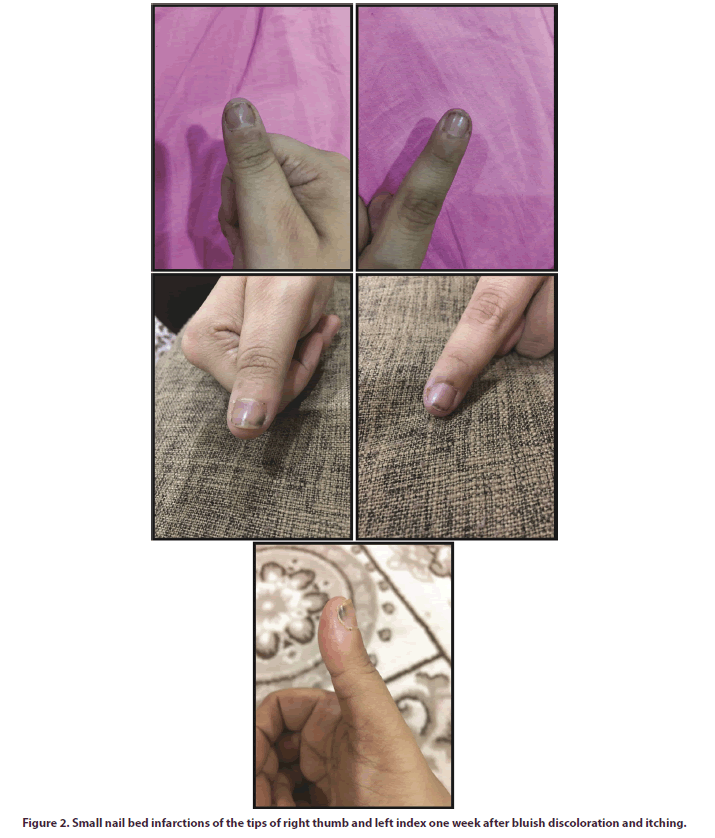
In April 2019 (after 15 months of ABA therapy) she complained from redness, pricking pain and itching in the tips of the right thumb and left index fingers. One week later, both fingers showed increased pain with paresthesia and bluish discoloration (Figure 1), without any motor deficit. Another week later, she presented with superadded small nail-bed infarcts under the periphery of the affected nails (Figure 2). There were no manifestations denoting other organ involvement. Follow-up investigations were within normal, but again showed more elevations of ESR and CRP (100 mm/H, and 24 mg/dl, respectively). Other diagnostic investigations for exclusion of other causes, as explained above, were done, and all were negative.
Blood culture and sensitivity of aerobic and anaerobic organisms were negative. Pelvi-abdominal ultrasound and computerized tomography of the chest revealed no abnormalities. Mononeuritis multiplex and carpal tunnel syndrome was excluded by nerve conduction studies and electromyography, of both the median and ulnar nerves. The patient refused to do a tissue biopsy of the affected fingers due to the severity of pain.
As she had no other co-morbidities that can cause vasculitis and had no evidence of current RA activity, we diagnosed her as abatacept-induced cutaneous (smallvessel) vasculitis, with no systemic involvement.
Abatacept was stopped and she was treated with oral prednisolone 45 mg/day with gradual descending course over about one month till reaching 15 mg daily which is the maintenance dose, along with the same doses of MTX and folic acid. Three months later, vasculitis resolved; nail-bed infarcts regenerated and cyanotic colors turn normal with improved parasthesia. Now, she is maintained on oral MTX 15 mg and folic acid 5mg weekly, prednisolone 15 mg daily. She improved completely and all new investigations, including ESR and CRP were within normal.
Discussion
Rheumatoid vasculitis is a multi-organ affection with heterogeneous clinical presentation. The skin and peripheral nerves are most commonly (80%) affected [1]. Involvement of major organs as the heart, kidneys, gastrointestinal tract or the central nervous system is much less common, but associated with significant morbidity and mortality as described in previous case reports [16]. Up till now, there is no universally accepted definition of systemic RV, while possible criteria were formalized [1,17]. Clinically, the RV is considered when established RA patients present with clinical manifestations of vasculitis, that cannot be explained by other conditions or other types of systemic vasculitis [4,9].
Here, we describe the clinical presentation and followup results of a group of RA patients who were clinically improved under MTX/ABA combination therapy, but showed persistently elevated acute phase reactants (ESR and CRP) and one case of them developed nail-bed infarctions after 15 months of ABA therapy.
Cases included in this study (except the index case) are nonsmoker females, few of them (4 cases) have erosions, hand deformities or subcutaneous rheumatoid nodules as well as most of them (12 cases) started combination therapy of MTX/ABA early in their disease course. However, all of them were still having elevated acute phase reactants although clinically proved to have low CDAI and HAQ scores.
It is well known that these elevated acute phase reactants indicate either ongoing active inflammation either due to flaring of the disease or malignant conditions or severe infections. However, none of our patients were represented by any clinical manifestations or even any abnormal results of laboratory investigations performed for differential diagnosis of such cases. The development of nail-bed infarctions in one of this group of patients directed our attention to the imminent drug-induced vasculitis caused by ABA therapy.
The diagnosis of abatacept-induced small-vessel vasculitis is most likely the underlying mechanism in the case presented here. First, the acute phase reactants (ESR and CRP) did not return to normal all over the duration of ABA treatment in spite of improved arthritis (proved by low disease activity) and no morning stiffness. Second, improvement of symptoms of vasculitis and nail-bed infarctions was noted after stoppage of ABA and administration of high dose oral prednisolone. In addition, drug-induced vasculitis was confirmed to be present with isolated cutaneous manifestations [18], as happened in this patient.
We had reviewed the literature and found a similar case described by Alegria et al (2016). A long-standing RA case developed vasculitis in spite of ongoing ABA treatment that was explained by the inability of ABA to protect from the development of RV in some patients although it is effective in treating some other cases [18]. Another male patient of psoriasis and psoriatic arthritis, presented with multiple leg ulcers after 3 months of ABA treatment, proved to be small- and medium-vessel vasculitis that likely caused by ABA administration and there was no other trigger identified, and rapid clinical improvement was noted after discontinuation of ABA and administration of prednisone [3].
As vasculitis can be a complication of RA, we cannot ignore this possibility. This patient has multiple risk factors to develop RV such as sero-positivity for both RF and Anti-CCP [19-21], chronic tobaccosmoking, untreated RA for a long disease duration and subcutaneous rheumatoid nodules early in the course of her disease [17,22]. However, the absence of other clinical manifestations of active RA such as morning stiffness, active arthritis, recent subcutaneous nodules or active extra-articular disease [23], and the absence of manifestations of other systemic vasculitides along with not fulfilling any of the criteria proposed by the Scott and Bacon’s [24] for the diagnosis of systemic RV, this patient cannot be diagnosed as RV. In addition, ANCA negative testing in that RA case excludes the possibility of RV, where atypical perinuclear ANCA was found positive in 48% of patients with RV [25].
Notably, according to the EULAR remission criteria explained in the 2019 updated EULAR RA management recommendations [15], the underlying disease (i.e., RA) of this case as well as all the group of patients, was well managed and controlled medically as determined by the frequently done clinical evaluation proving low CADI scores and decreased HAQ scores all over the period of follow-up in conjunction with unremarkable follow-up laboratory investigations apart from the elevated ESR and CRP.
Ideally, RV should be confirmed by histopathological examination of the affected tissues [3,26]. But it was refused by our case, as it is painful, difficult, requires multiple step sections of adequate samples into subcutis [3]. Abatacept is FDA approved to treat moderateto- severe RA not responding to disease-modifying anti-rheumatic drugs (DMARDs) and tumor necrosis factor alpha (TNF-α) antagonists [27]. Review of the literature also revealed that, abatacept efficacy in treating RV has been previously reported in two patients [2,9], but it failed to protect from the development of RV in other patients [3,18,28,29]. In the present case, vasculitis developed despite ongoing treatment with abatacept, which is considered as a paradoxical effect of the drug. There may be a potential role for individual genetic susceptibility, which would explain why some biologic agents are successful in treating vasculitis, while paradoxically trigger it in other patients. The exact cause of this paradoxical reaction is still unknown, possibly there may be an imbalance in cytokine production or imbalance between effector and regulatory T-cells [29,30]. Abatacept has been found to enhance regulatory T-cell function but reduce the number of circulating regulatory T-cells [29].
Methotrexate is well-studied in the treatment of RA and some reports support its efficacy in treating RV [31,32]. In this group of patients, combined methotrexate therapy with ABA could be the factor that delaying their clinical presentation with RV in spite of previously explained risk factors. The question now, why ESR and CRP are still high in spite of clinical improvement and can they be alarming factors for imminent vasculitis or an indication for discontinuation of ABA therapy in cases of RA before developing vasculitis.
Conclusion
ABA can induce or at least can't protect from developing vasculitis in RA patients who have predisposing risk factors that should be considered during ABA therapy to reduce morbidity. Combination therapy of MTX/ABA acts as a delaying or inhibiting factor for the clinical presentation of rheumatoid vasculitis in RA patients. Further prospective studies on large numbers of RA patients comparing ABA monotherapy with combined MTX/ABA therapy are recommended.
Disclosures
None.
Financial support and sponsorship
None.
References
- Genta MS, Genta RM, Gabay C. Systemic Rheumatoid Vasculitis: A Review. Semin. Arthritis. Rheum. 36(2), 88–98 (2006).
- Makol A, Crowson CS, Wetter DA et al. Vasculitis associated with rheumatoid arthritis: a case-control study. Rheumatol. Oxf. Engl. 53(5), 890–899 (2014).
- Holt MH, Liu V, Fairley J. Medium-vessel vasculitis presenting as multiple leg ulcers after treatment with abatacept. JAAD. Case. Rep. 4(8), 811–813 (2018).
- Vollertsen RS, Conn DL. Vasculitis associated with rheumatoid arthritis. Rheum. Dis. Clin. North. Am. 16, 445–461 (1990).
- Korkosz M, Kro´lczyk J, Telesicska-Jasio´wka D et al. Successful Treatment of Digital Tip Necrosis in Rheumatoid Vasculitis with Anti-CD20 Antibody Rituximab. J. Clin. Rheumatol. 19(1), 43–45 (2013).
- Scott DG, Bacon PA. Intravenous cyclophosphamide plus methylprednisolone in treatment of systemic rheumatoid vasculitis. Am. J. Med. 76(3), 377–384 (1984).
- Ruderman EM, Pope RM. Drug insight: abatacept for the treatment of rheumatoid arthritis. Eur. J. Dermatol. 23(5), 738–739 (2006).
- Gaujoux-Viala C, Gossec L, Cantagrel A et al. Recommendations of the French Society for rheumatology for managing rheumatoid arthritis. Joint. Bone. Spine. 81(4), 287–297 (2014).
- Fujii W, Kohno M, Ishino H et al. The rapid efficacy of abatacept in a patient with rheumatoid vasculitis. Mod. Rheumatol. 22(4), 630–634 (2012).
- Bitik B, Mercan R, Tufan A et al. Differential diagnosis of elevated erythrocyte sedimentation rate and C-reactive protein levels: a rheumatology perspective. Eur. J. Rheumatol. 2(4), 131–134 (2015)
- Aletaha D, Neogi T, Silman AJ et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheum. 62(9), 2569-2581 (2010).
- Prevoo ML, van 't Hof MA, Kuper HH et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis. Rheum. 38(1), 44–48 (1995).
- Smolen JS, Breedveld FC, Schiff MH et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 42(2), 244–257 (2003).
- Fries JF, Spitz PW, Kraines RG et al. Measurement of patient outcome in arthritis. Arthritis. Rheum. 23(2), 137–145 (1980).
- Smolen JS, Landewé RBM, Bijlsma JWJ et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 1–15 (2020).
- Bartels CM, Bridges AJ. Rheumatoid Vasculitis: Vanishing Menace or Target for New Treatments? Curr. Rheumatol. Rep. 12(6), 414–419 (2010).
- Turesson C, Jacobsson LT. Epidemiology of extra-articular manifestations in rheumatoid arthritis. Scand. J. Rheumatol. 33(2), 65–72 (2004).
- Alegria GC, Uguen A, Genestet S et al. New onset of rheumatoidvasculitis during abatacept therapy and subsequent improvement after rituximab. Joint. Bone. Spine. 83(5), 605–606 (2016).
- Scott DG, Bacon PA, Allen C et al. IgG rheumatoid factor, complement and immune complexes in rheumatoid synovitis and vasculitis: comparative and serial studies during cytotoxic therapy. Clin. Exp. Immunol. 43, 54–63 (1981).
- Veys EM, Gabriel PA, Coigne E et al. Rheumatoid factor and serum IgG, IgM and IgA levels in rheumatoid arthritis with vasculitis. Scand. J. Rheumatol. 5, 1–6 (1976).
- Westedt ML, Herbrink P, Molenaar JL et al. Rheumatoid factors in rheumatoid arthritis and vasculitis. Rheumatol. Int. 5(5), 209–214 (1985).
- Voskuyl AE, Zwinderman AH, Westedt ML et al. Factors associated with the development of vasculitis in rheumatoid arthritis: results of a case-control study. Ann. Rheum. Dis. 55(3), 190–192 (1996).
- Guignard S, Gossec L, Bandinelli F et al. Comparison of the clinical characteristics of vasculitis occurring during antitumor necrosis factor treatment or not in rheumatoid arthritis patients: a systematic review of 2707 patients, 18 vasculitis. Clin. Exp. Rheumatol. 26(Suppl 49), S23–S29 (2008).
- Scott DG, Bacon PA, Tribe CR. Systemic rheumatoid vasculitis: a clinical and laboratory study of 50 cases. Medicine. 60(4), 288–297 (1981).
- Coremans IE, Hagen EC, Daha MR et al. Antilactoferrin antibodies in patients with rheumatoid arthritis are associated with vasculitis. Arthritis. Rheum. 35(12), 1466–1475 (1992).
- Laskari K, Ahmadi-Simab K, Lamken M et al. Are anti-cyclic citrullinated peptide autoantibodies seromarkers for rheumatoid vasculitis in a cohort of patients with systemic vasculitis? Ann. Rheum. Dis. 69(2), 469–471 (2010).
- Genant HK, Peterfy CG, Westhovens R et al. Abatacept inhibits progression of structural damage in rheumatoid arthritis: result from the long-term extension of the AIM trial. Ann. Rheum. Dis. 67(8), 1084–1089 (2008).
- Makol A, Matteson EL, Warrington KJ. Rheumatoid vasculitis: an update. Curr. Opin. Rheumatol. 27(1), 63–70 (2015).
- Diamanti PA, Rosado MM, Scarsella M et al. Abatacept (cytotoxic T lymphocyte antigen 4-immunoglobulin) improves B cell function and Treg inhibitory capacity in rheumatoid arthritis patients non responding to anti-TNF-alpha agents. Clin. Exp. Immunol. 177(3), 630–640 (2014).
- Álvarez-Quiroga C, Abud-Mendoza C, Doníz-Padilla L et al. CTLA-4-Ig therapy diminishes the frequency but enhances the function of Treg cells in patients with rheumatoid arthritis. J. Clin. Immunol. 31(4), 588–595 (2011).
- Espinoza LR, Espinoza CG, Vasey FB et al. Oral methotrexate therapy for chronic rheumatoid arthritis ulcerations. J. Am. Acad. Dermatol. 15(3), 508–512 (1986).
- Upchurch KS, Heller K, Bress NM. Low-dose methotrexate therapy for cutaneous vasculitis of rheumatoid arthritis. J. Am. Acad. Dermatol. 17(2), 355–359 (1987).