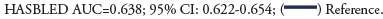Research Article - Interventional Cardiology (2022) Volume 14, Issue 5
National trends, outcomes and readmissions secondary to major bleeding in patients with intracardiac thrombus
- Corresponding Author:
- Tanveer Mir
Department of Internal Medicine,
Wayne State University,
Detroit Medical Center,
Michigan,
USA
E-mail: gr6723@wayne.edu
Received date: 16-Sep-2022, Manuscript No. FMIC-22-74977; Editor assigned: 19-Sep-2022, PreQC No. FMIC-22-74977 (PQ); Reviewed date: 03-Oct-2022, QC No. FMIC-22-74977; Revised date: 10-Oct-2022, Manuscript No. FMIC-22-74977 (R); Published date: 17-Oct-2022, DOI: 10.37532/1755- 5310.2022.14(5).573
Abstract
Background: Bleeding risk in intracardiac thrombus has not been examined previously.
Aim: To evaluate the predictors and bleeding outcome of patients with intracardiac thrombus.
Methods: A national representative cohort of 214,476 intracardiac thrombus patients were included for the years 2010–2017 from the national readmission database. We examined predictors and trends of major bleeding in patients that were diagnosed with intracardiac thrombus. We examined the association of major bleeding with mortality and readmission. We also evaluated the association of HAS-BLED score with readmission.
Results: Out of 214,476 patients with intracardiac thrombus, 38,545 (18%) suffered from major bleeding patients during the index hospitalization. Of the total 204,432 discharged alive within the first 11 months of the year, 4079 (2.0%) had readmissions due to major bleeding. During index hospitalization, the commonest form of major bleeding was gastrointestinal 10808 (28%). Higher mortality rates were observed in the major bleeding group (9.2% vs. 3.7%) with increasing trends over the years 2010– 2017 (p trend=0.03). Major bleeding was associated with increased risk of mortality (OR: 2.40; 95% CI: 2.20–2.62, p<0.001). Among predictors for major bleeding, malignancy (OR 1.49, p<0.001), atrial fibrillation (OR: 1.14, p<0.001), peripheral arterial disease (OR: 1.27, p<0.001) were the predominant ones. The predictors of readmission with major bleeding included female sex (HR: 1.26, p<0.001), malignancy (HR 2.13, p<0.001), peripheral vascular disease (HR 1.37, p<0.001), chronic heart failure (HR: 1.81, p<0.001) and HAS-BLED score (HR: 1.23, p<0.001). HAS-BLED score improved the predictability of readmission significantly over and above the conventional bleeding risk factors (p=0.004).
Conclusion: Major bleeding is common in patients with intracardiac thrombus. Malignancy was one of the predominant risk factors for major bleeding. HAS-BLED even though was associated with major bleeding readmission however was found to have mild improvement in prediction in addition to other risk factors. Up-trending mortality was observed in major bleeding patients which suggests more research for better risk scales.
Keywords
Intracardiac thrombus . HAS-BLED score . Major bleeding . Predictors
Introduction
The annual major bleeding risk in patients treated with antithrombotic medications was reported up to 6.4 per 1000 events in males and 1.5-5.0 per 1000 in females [1]. A prospective study of 842 patients reported an incidence of 4.9% major bleeding, over a period of 12 months, in patients who were on vitamin K antagonists [2]. Patients with cardiomyopathy and atrial fibrillation are at higher risk of intracardiac thrombus, and hence thromboembolism [3-5]. These patients face a particularly challenging aspect of management because they are usually not healthy and are also at increased risk of bleeding [6]. There is a dearth of information regarding the patients with intracardiac thrombus and their risk of major bleeding. In addition, current risk factors’ scores such as HAS-BLED have not been well studied in this group of patients [7-9]. Learning about the risk of major bleeding in this group of patients and the predictability of HASBLED will help physicians make informed decision about the use of anticoagulation and hence may prevent morbidity and mortality. Therefore, our aim of this analysis is to 1) evaluate the national trends and clinical profile of intracardiac thrombus patients that develop major bleeding 2) analyze the association of major bleeding with mortality 3) examine the association of HAS-BLED score with readmissions and major bleeding specific readmissions for the years 2010-2017 in the United States.
Materials and Methods
Study population and design
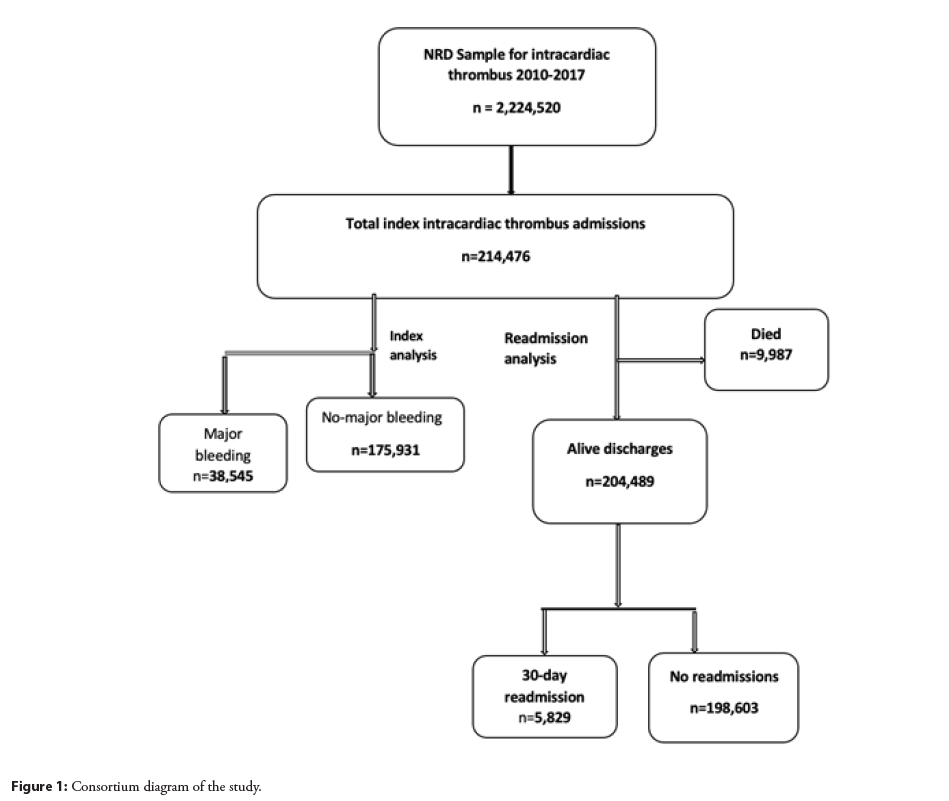
This is a cohort study of a nationally representative cohort of hospitalized admissions that were admitted from January 1, 2010 to December 31, 2017. The database was obtained from the Agency for Healthcare Research and Quality’s (AHRQ) Healthcare Cost and Utilization Project (HCUP) National Readmission (NRD) dataset files between 2010–2017. The NRD is the largest publicly available all-payer inpatient care database in the United States and contains discharge-level data that participate in the HCUP project. It provides approximately 20% of the stratified sample of all hospitals in the United States, representing more than 95% of the national population. The database provides de-identified information about the patients’ demographics and hospital-based information. Besides, it provides information about the days to readmission and readmission status. Since a publicly available database was used, the study was considered exempt from obtaining permission from the institutional review board.
We used the International Classification of Diseases, Ninth Edition, (ICD-9) and ICD-10 diagnostic codes to identify patients with intracardiac thrombus ((ICD: 9; 429.79 and 429.89) and (ICD: 10; I51.3 and I23.6)) and major bleeding defined as gastrointestinal bleeding, acute blood loss anemia, intracranial hemorrhage, blood transfusion, and genitourinary bleeding (Supplementary file). All adult patients ≥ 18 years were included in the study. Patients were excluded if they were discharged during December to ensure at least 30-day follow up. Based on this exclusion we identified 214,476 patients with intracardiac thrombus patients, and 38,595 major bleed patients were identified with a national estimate of 18%.
Patient and hospital characteristics
Baseline patients’ demographic characteristics (age, sex, ethnicity and insurance payer) were extracted. Diagnostic codes were used to identify hypertension, diabetes mellitus, hyperlipidemia, obesity, congestive heart failure, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, malignancy, chronic kidney disease, atrial fibrillation, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior myocardial infarction, dementia, and Intensive Care Unit (ICU) admissions (Table 1). Charlson’s comorbidity index was also calculated [10].
| Variables | Major bleeding | ||
|---|---|---|---|
| Present (n=38,545) | Absent (n=175,931) | p-value | |
| Age | 63.1 ± 17.0 | 63.0 ± 16.9 | <0.001 |
| Female | 16,084 (41.7%) | 66,989 (38.1%) | <0.001 |
| Primary payer | |||
| Medicare | 23,820 (61.9%) | 98,070 (55.8%) | <0.001 |
| Medicaid | 4,627 (12.0%) | 26,177 (14.9%) | <0.001 |
| Private | 7,789 (20.2%) | 37,069 (21.1%) | <0.001 |
| Self | 1,151 (2.99%) | 7,779 (4.43%) | <0.001 |
| Hypertension | 20,371 (52.8%) | 98,288 (55.9%) | <0.001 |
| Diabetes | 13,523 (35.1%) | 63,015 (35.8%) | 0.12 |
| Hyperlipidemia | 15,623 (40.5%) | 75,230 (42.8%) | <0.001 |
| Smoker | 11,026 (28.6%) | 59,459 (33.8%) | <0.001 |
| Alcohol | 1,621 (4.21%) | 7,244 (4.12%) | 0.65 |
| Obesity | 5,503 (14.3%) | 28,155 (16%) | <0.001 |
| Chronic obstructive pulmonary disease | 14,951 (38.8%) | 68,543 (38.9%) | 0.82 |
| Acute myocardial infarction | 12,946 (33.6%) | 60,431 (34.3%) | 0.16 |
| Congestive heart failure | 24,954 (64.7%) | 123,111 (70%) | <0.001 |
| Systolic heart failure | 9,660 (25.1%) | 57,128 (32.5%) | <0.001 |
| Cerebrovascular disease | 4,501 (11.7%) | 20,958 (11.9%) | 0.5 |
| Peripheral vascular disease | 10,029 (26.1%) | 37,436 (21.3%) | <0.001 |
| Renal disease | 13,770 (35.7%) | 56,407 (32.1%) | <0.001 |
| Prior myocardial infarction | 5,363 (13.9%) | 29,151 (16.6%) | <0.001 |
| Atrial fibrillation | 16,876 (43.8%) | 70,608 (40.1%) | <0.001 |
| Malignancy | 5,151 (13.4%) | 15,573 (8.85%) | <0.001 |
| Dementia | 1,720 (4.47%) | 7,560 (4.3%) | 0.39 |
| Prior percutaneous coronary intervention | 4,062 (10.6%) | 22,394 (12.7%) | <0.001 |
| Prior coronary artery bypass graft | 3,267 (8.49%) | 18,125 (10.3%) | <0.001 |
| Chronic liver disease | 3,344 (8.67%) | 13,255 (7.53%) | <0.001 |
| Intensive care unit admission | 9,426 (24.5%) | 16,072 (9.14%) | <0.001 |
| Teaching hospital | 29,336 (76.1%) | 122,440 (69.6%) | <0.001 |
| Urban hospital | 30,871 (80.1%) | 133,329 (75.8%) | 0.001 |
Table 1: Baseline characteristics of patients with intracardiac thrombus admitted with and without major bleeding.
Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included 30-day readmission secondary to major bleeding and no bleeding.
Statistical analysis
Baseline characteristics were compared by major bleeding status. Categorical variables were reported as frequency and percentages, and continuous variables were expressed as mean ± standard deviation for variables with normal distribution and as median with Interquartile Range (IQR) for non-normally distributed variables. These were compared with the Chi-square test and analysis of variance where appropriate for patients with and without major bleeding. We calculated HAS-BLED score by combining hypertension, renal disease, liver disease, stroke history and alcohol use.
For primary outcome, we examined association of major bleeding with mortality in multivariable logistic regression models adjusted for model 1 and model 2 variables. We then added HAS-BLED score to the model to evaluate the predictivity of mortality in these patients. Similarly, we examined the association of 30- day readmission and 30-day readmission with major bleeding in Cox proportional hazard models adjusted for model 1 and model 2 variables. Model 1 included age, sex, diabetes mellitus, hyperlipidemia, obesity, congestive heart failure, peripheral vascular disease, chronic obstructive pulmonary disease, atrial fibrillation, urban vs. rural hospital location, teaching hospital status, insurance status. Model 2 included model 1 variables and HAS-BLED score. Area under the curve for major bleeding readmissions was also calculated for model 1 and then model 2 variables. Both areas were compared and p-value was reported. Weighted analyses were used for all statistical calculations. Statistical analysis was performed using STATA version 14.2 (College Station, Texas). All p values were 2 sided, with a significance threshold of p=0.05.
Results
Among 214,476 index hospitalizations (mean age 63.3 ± 17.0, 38.7% females) with a diagnosis of an intracardiac thrombus in the NRD database during the years of 2010–2017, there were 38,545 (18%) patients developed major bleeding. Of those, 204,432 (95.3%) were discharged alive from January until November that was used for readmission analysis (Figure 1). The baseline characteristics of the patients with index admission with a diagnosis of the intracardiac thrombus are given in Table 1. Patients with major bleeding were more likely to be elderly, males, and had higher prevalence of peripheral vascular disease, renal disease, atrial fibrillation, chronic liver disease, ICU admission, and were treated at urban and teaching hospitals. In terms of major bleeding, 4262 (11.1%) patients had genitourinary bleeding, 10,808 (28%) had gastrointestinal bleeding and 2917 (7.6%) had intracranial bleeding.
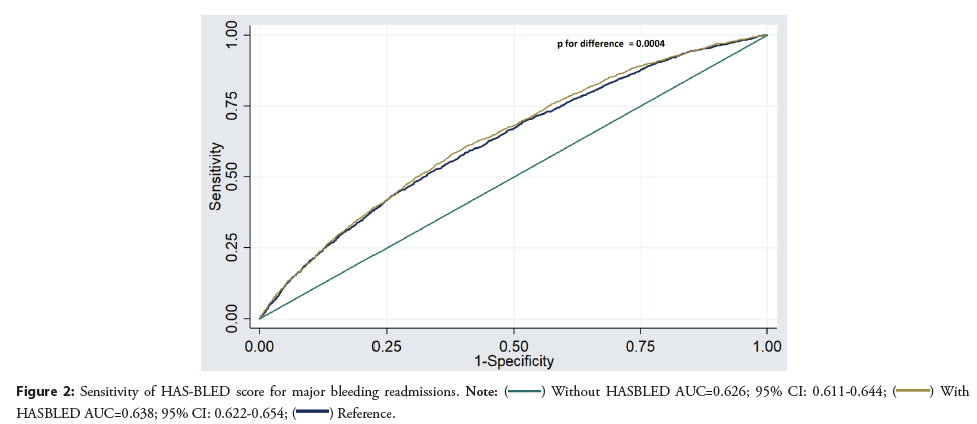
There were 3525 (9.1%) deaths in the patients that had major bleeding and 6462 (3.7%) in the group without major bleeding. In multivariable logistic regression model adjusted for model 1 variables, major bleeding was significantly associated with mortality (OR: 2.40; 95% CI: 2.20–2.62, p<0.001). When HASBLED score was added to the analysis, major bleeding remained significantly associated with mortality (OR: 2.40; 95% CI: 2.20– 2.62, p<0.001) (Figure 2).
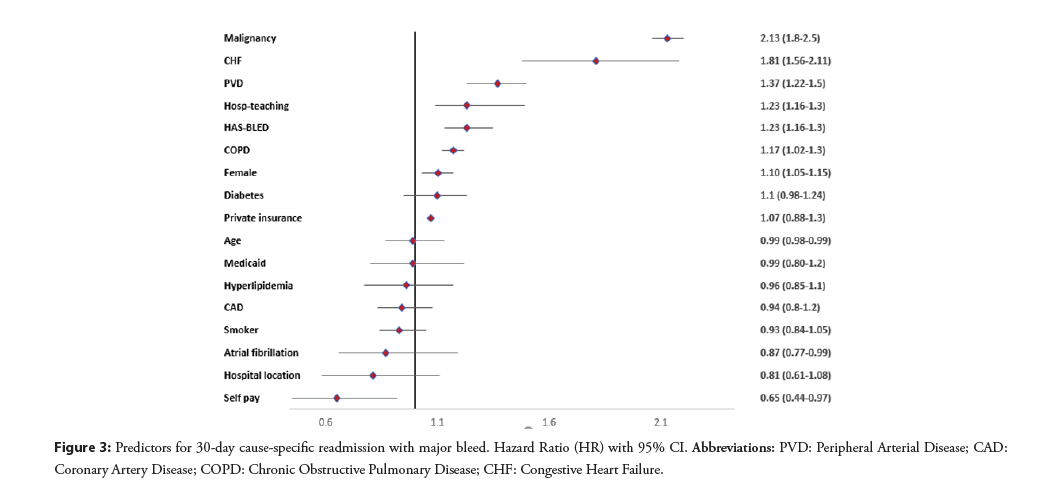
There were 5,829 (2.8%) readmissions during the first 30 days of which 4079 had recurrent major bleeding. Out of these readmissions, 1040 (2.7%) occurred in patients that had major bleeding as a cause of index hospitalization and 3039 (1.7%) occurred in patients that did not have major bleeding as a cause of index hospitalization. The predictors of 30-day cause specific readmission with major bleeding are given in Figure 3. We found a significant association of HAS-BLED with recurrent major bleeding (HR: 1.23; 95% CI: 1.16–1.30, p<0.001). The area under the curve for predictors without HAS-BLED to predict major bleeding was 0.626; 95% CI: 0.611–0.644 and it improved to (0.638; 95% CI: 0.622–0.654, p=0.0004) with HAS-BLED as shown in Figure 4.
Figure 3: Predictors for 30-day cause-specific readmission with major bleed. Hazard Ratio (HR) with 95% CI. Abbreviations: PVD: Peripheral Arterial Disease; CAD: Coronary Artery Disease; COPD: Chronic Obstructive Pulmonary Disease; CHF: Congestive Heart Failure.
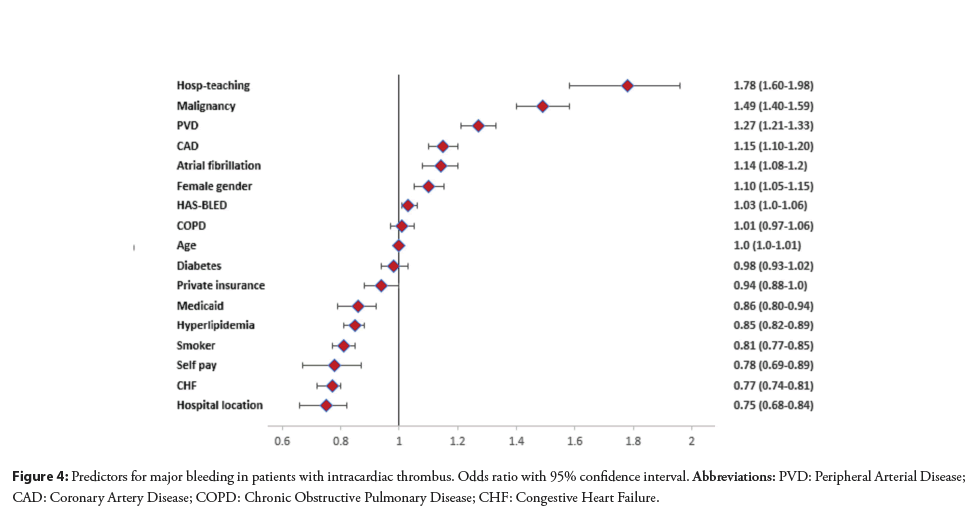
Figure 4: Predictors for major bleeding in patients with intracardiac thrombus. Odds ratio with 95% confidence interval. Abbreviations: PVD: Peripheral Arterial Disease; CAD: Coronary Artery Disease; COPD: Chronic Obstructive Pulmonary Disease; CHF: Congestive Heart Failure.
In multivariable logistic regression model, we also evaluated association of several risk factors with the major bleeding as given in Figure 4. HAS-BLED proved to be a weak predictor of major bleeding.
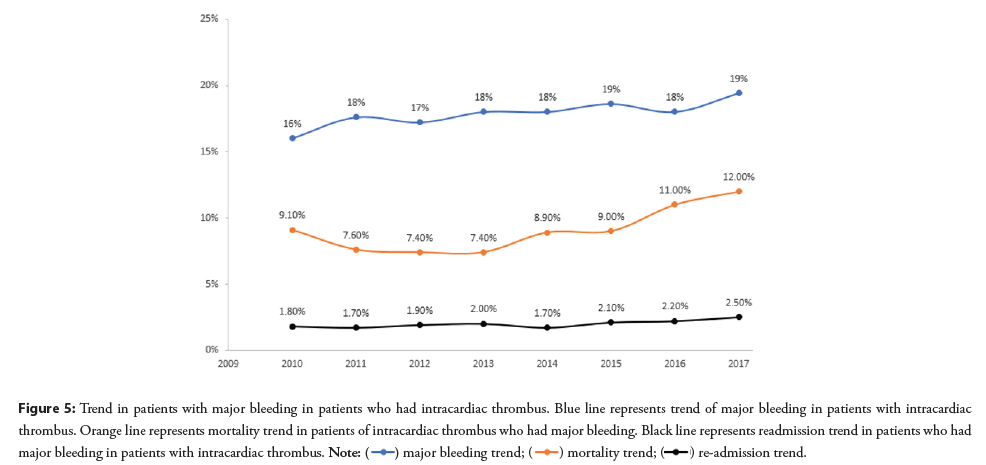
From 2010–2017, there was increase in mortality observed in intracardiac thrombus patients that had major bleeding (linear trend p=0.03). The trend for hospitalizations for major bleeding or readmissions remained increased from 2010 to 2017 (linear trend p=0.004 and trend p=0.01 respectively) (Figure 5).
Figure 5: Trend in patients with major bleeding in patients who had intracardiac thrombus. Blue line represents trend of major bleeding in patients with intracardiac
thrombus. Orange line represents mortality trend in patients of intracardiac thrombus who had major bleeding. Black line represents readmission trend in patients who had
major bleeding in patients with intracardiac thrombus.
Discussion
To our knowledge, this is the first study to evaluate major bleeding in intracardiac thrombus patients. In our study, we observed 18% of patients with intracardiac thrombus are hospitalized with major bleeding. We also observed a significant association of major bleeding with mortality in this group of patients. There was also increased risk of 30-day readmission with these patients. The risk score HASBLED marginally improved predictability of 30-day readmission with major bleeding over and above the conventional risk factors. Malignancy had a predominant association among the predictors with major bleeding and readmissions secondary to major bleeding. Interestingly, atrial fibrillation was associated with major bleeding in patients with intracardiac thrombus. Teaching hospital was seen associated with major bleeding and readmission secondary to major bleeding. Mortality was observed in 1/10th of major bleeding patients with an increasing over the study years.
HAS-BLED score has been validated by multiple studies as a predictive score to assess the risk of major bleeding in patients for assessment for anticoagulation [6,11-13]. It has been validated for assessment of vitamin K anticoagulants and non-vitamin K anticoagulants for venous thromboembolism or atrial fibrillation [6,11-13]. A prospective study of 1,370 patients with atrial fibrillation revealed a high predictive value of HAS-BLED score for major bleeding in patients with atrial fibrillation. A score of ≥ 3 has been reported to indicate a high risk of bleeding [8]. However, these studies did not evaluate the HAS-BLED score in intracardiac thrombus patients. Our study revealed an association of HAS-BLED score with major bleeding readmissions, however, the sensitivity was low but in line with the prior study reference [14]. This finding would suggest more research for bleeding risk scoring scales in patients with intracardiac thrombus.
A prospective study of 780 patients evaluated bleeding risks in patients who were treated with anticoagulants. The study reported higher risks of bleeding in elderly females and malignancy [15,16]. Our study revealed a prominent predictive ability of malignancy on major bleed readmission and as a major bleeding risk in patients with intracardiac thrombus. Malignancy predisposes for thromboembolism, and anticoagulation in malignancy increases the risks for bleeding. The bleeding risk is directly related to the severity of cancer [2,17].
Atrial fibrillation was associated with bleeding risks in patients with intracardiac thrombus. Patients with cardiovascular risk factors are at higher risks of atrial fibrillation [18]. Since atrial fibrillation, systemic thromboembolism, and bleeding have common risk factors (including advanced age, uncontrolled hypertension, ischemic cardiomyopathy, prior stroke, or previous bleeding events) which can explain the association of atrial fibrillation with major bleeding [19]. Besides, patients with atrial fibrillation are on anticoagulation which adds to risks of major bleeding. We observed positive association of the peripheral arterial disease with major bleeding. This could be secondary to increased use of antiplatelet and anticoagulant combination in this group of patients.
In our study, we observed 18% of patients with intracardiac thrombus had major bleeding. We noticed a flat trend in bleeding risks in patients with intracardiac thrombus over the study years, 2010-2017. Over the last decade, direct oral anticoagulants have been used with improved rates of bleeding in patients with venous thromboembolism [20]. Direct oral anticoagulants are not yet approved for treatment for intracardiac thrombus. However, if we assume that there is increase in use of direct oral anticoagulants, we did not observe a drop in the major bleeding in these patients.
We observed higher mortality rates in patients with major bleeding compared to patients without major bleeding. Mortality trends were up trending in major bleeding patients over the study years 2010-2017. The higher mortality rates can be a reason for low 30-day readmission for major bleeding group as survival for such patients is low compared to no-bleeding patients. The worsening trend in mortality suggests concerns and further research into the field. A systematic review of 69 prospective and randomized trials on case fatality from anticoagulation in patients with Venous Thromboembolism (VTE) revealed 0.2% rates of fatal major bleeding and 11.3% case fatality rate in patients with VTE on anticoagulation [21].
Our study has several implications. Firstly, there was a significant high risk of major bleeding in patients that initially present with intracardiac thrombus. This risk is also present in patients that did not have major bleeding during the first hospitalization. Surveilling these patients during the first few months of discharge and close follow up is very important since we observed a high risk of mortality associated with major bleeding. Secondly, HASBLED score was able to predict increased risk of mortality however the sensitivity was mild which means the other risk factors such as malignancy, peripheral vascular disease, coronary artery disease, and atrial fibrillation need to be considered while deciding the risk of bleeding in these patients. Third, significant mortality rates secondary to major bleeding were observed in our study. Fourth, we observed a worsening trend in mortality among major bleeding patients over the study years. This suggests more research into the field to improve scoring scales for improvement in mortality rates.
Our study does have several limitations mainly secondary to the retrospective nature of the study and the fact that it is based on an administrative database. Since administrative data are often created for financial and administrative purposes, it lacks certain pertinent clinical information such as laboratory data, medications use, and physical examination. We could not access the type of anticoagulation used. The data regarding anticoagulation is not available. However, since patients with intracardiac thrombus are usually on anticoagulation, we presume the bleeding risk should be while the patients were on anticoagulation. Further randomized studies will be needed to evaluate the bleeding risks with particular types of anticoagulation used in patients with intracardiac clot. Despite these limitations, the NRD still provides an important insight with a large statistical power into real-world data to study the prevalence and management of this subgroup of patients.
Conclusion
In this large nationwide study, we observed significant rates of major bleeding in patients with intracardiac thrombus. HASBLED score was observed to have an association with intracardiac thrombus who had major bleeding, however, the sensitivity of the score was mild. The mortality in intracardiac thrombus patients with major bleeding is significant with a worsening trend. This would suggest more research to develop a better scale for bleeding risk assessment for improvement in major bleeding and mortality rates.
Acknowledgments
TM and WTQ did formal analysis, wrote the manuscript, made figures. TH, WA, JS helped in manuscript writing, formatting figures and review of manuscript. JI and MS helped in manuscript review and final critical review of the manuscript. All authors have contributed and reviewed the manuscript. No authors have any conflicts of interest to disclose. No funding was required for the project. The project did not require ethical clearance as it is done from a public database with deidentified patient data. TM will be corresponding author and guarantor of content of manuscript.
References
- Selak V, Kerr A, Poppe K, et al. Annual risk of major bleeding among persons without cardiovascular disease not receiving antiplatelet therapy. JAMA. 319(24): 2507-2520 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 100(10): 3484-3488 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Bourezg A, Bochaton T, Mewton N, et al. Atrial fibrillation, intra-ventricular thrombus, and other anticoagulant indications relationship with adverse outcomes in acute anterior myocardial infarction patients. J Cardiol. 72(4): 277-283 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Noubiap JJ, Bigna JJ, Agbor VN, et al. Meta-analysis of atrial fibrillation in patients with various cardiomyopathies. Am J Cardiol. 124(2): 262-269 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Di Minno MN, Ambrosino P, Russo AD, et al. Prevalence of left atrial thrombus in patients with non-valvular atrial fibrillation. A systematic review and meta-analysis of the literature. Thromb Haemost. 115(3): 663-677 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest. 138(5): 1093-1100 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in non-warfarin anticoagulated atrial fibrillation patients. J Am Coll Cardiol. 61(3): 386-387 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Kooiman J, van Hagen N, Iglesias Del Sol AI, et al. The HAS-BLED score identifies patients with acute venous thromboembolism at high risk of major bleeding complications during the first six months of anticoagulant treatment. PLoS One. 10(4): e0122520 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Roldán V, Marín F, Manzano-Fernández S, et al. The HAS-BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2-VASc scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 62(23): 2199-2204 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Austin SR, Wong Y-N, Uzzo RG, et al. Why summary comorbidity measures such as the Charlson comorbidity index and Elixhauser score work. Medical Care. 53(9): e65 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Poli D, Antonucci E, Testa S, et al. The predictive ability of bleeding risk stratification models in very old patients on vitamin K antagonist treatment for venous thromboembolism: Results of the prospective collaborative EPICA study. J Thromb Haemost. 11(6): 1053-1058 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Lip GY, Lin H-J, Hsu H-C, et al. Comparative assessment of the HAS-BLED score with other published bleeding risk scoring schemes, for intracranial haemorrhage risk in a non-atrial fibrillation population: The Chin-Shan Community Cohort Study. Int J Cardiol. 168(3): 1832-1836 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Apostolakis S, Lane DA, Guo Y, et al. Performance of the HEMORR2HAGES, ATRIA, and HAS-BLED bleeding risk–prediction scores in patients with atrial fibrillation undergoing anticoagulation: The AMADEUS (Evaluating the Use of SR34006 compared to warfarin or Acenocoumarol in patients with atrial fibrillation) Study. J Am Coll Cardiol. 60(9): 861-867 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Chang G, Xie Q, Ma L, et al. Accuracy of HAS‐BLED and other bleeding risk assessment tools in predicting major bleeding events in atrial fibrillation: A network meta‐analysis. J Thromb Haemost. 18(4): 791-801 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Kuijer PM, Hutten BA, Prins MH, et al. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 159(5): 457-460 (1999).
[CrossRef] [Google Scholar] [PubMed]
- Vedovati MC, Mancuso A, Pierpaoli L, et al. Prediction of major bleeding in patients receiving DOACs for venous thromboembolism: A prospective cohort study. Int J Cardiol. 301: 167-172 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Hutten BA, Prins MH, Gent M, et al. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: A retrospective analysis. J Clin Oncol. 18(17): 3078-3083 (2000).
[CrossRef] [Google Scholar] [PubMed]
- Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 96(7): 2455-2461 (1997).
[CrossRef] [Google Scholar] [PubMed]
- Hughes M, Lip G. Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: A systematic review. QJM. 100(10): 599-607 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Xu Y, Schulman S, Dowlatshahi D, et al. Direct oral anticoagulant-or warfarin-related major bleeding: characteristics, reversal strategies, and outcomes from a multicenter observational study. Chest. 152(1): 81-91 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Carrier M, Le Gal G, Wells PS, et al. Systematic review: Case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 152(9): 578-589 (2010).
[CrossRef] [Google Scholar] [PubMed]