Case Series - Imaging in Medicine (2020) Volume 12, Issue 6
Neurological and neuromuscular manifestations in SARS-CoV-2: Review of Literature and Case Series
Robin Warner DO*Department of Neurology, Hospital for Special Surgery, New York
- Corresponding Author:
- Robin Warner DO
Department of Neurology
Hospital for Special Surgery, New York
E-mail: robin.petrizzo@gmail.com
Abstract
Introduction: The 2019 coronavirus, known as SARS-CoV-2 and COVID-19, was named a pandemic by the WHO in March 2020. It binds to the ACE-2 receptor and transmembrane serine protease 2 and is highly virulent. There are many sequelae of this virus, including neurological consequences. We have performed a literature review of the neurological sequelae of COVID-19 with relation to neuroimaging and then present a case series. Case Series: Seven cases were seen by neurology consultants at the Hospital for Special Surgery in New York City between February and May of 2020; 5 met criteria. Most of these consultations were called for encephalopathy. Some had neuroimaging of brain MRI or head CT, which all showed microvascular disease. One case had prior imaging without microvascular disease. Summary: It is known that vascular disease is a risk factor for severe COVID-19 infection. This case series demonstrates presence of microvascular disease in patients with encephalopathy. We know that microvascular disease can be a risk factor for toxic metabolic encephalopathy. It is unclear if the microvascular disease was present prior to infection, although at least one patient had prior imaging without microvascular disease. More research is needed to determine if COVID-19 infection can cause vascular disease.
Keywords
Neuroimaging ■ COVID-19 ■ SARS-CoV-2 ■ Neurological sequelae ■ Neuromuscular
Introduction
The 2019 coronavirus, known as SARS-CoV-2 and COVID-19 is a single stranded RNA beta corona virus enveloped in a 60-140nm lipid bilayer. It binds to the ACE-2 receptor and transmembrane serine protease 2 and was declared a pandemic in March 2020 [1]. This paper is a literature review of its neurological sequelae with focus on neuroimaging, then a case series of patients seen by neurology consultants at the Hospital for Special Surgery in New York City from February until May 2020.
Literature Review
SARS-CoV-2 arose in humans around December 2019 near Wuhan, China and likely originated in bats.2 It is unclear exactly how patient zero contracted the virus, but it is postulated that transmission occurred at a wildlife wet market [2]. Genetic analysis of this virus shows that it is of the genera beta corona virus, along with SARS and MERS. Infection occurs when the virus binds ACE-2 receptor or transmembrane serine protease 2 in lung epithelium. Direct infection of T-cells and macrophages occurs, activating them [3]. A cascade up-regulates pro-inflammatory cytokines, such as IL-1, IL- 6, IL-10 and TNF-a, and down-regulates antiinflammatory cytokines, such as IFN-g [2]. Typical symptoms include fever, cough, and dyspnea, but can develop into ARDS in about 1 week. Through molecular mimicry between virus and myelin basic protein, demyelination occurs [3].
In a 214 patient case series from Wuhan, 36.4% of patients had neurologic signs. They include headache (8%), confusion (9%), dizziness (16%), stroke (5.7%), ataxia, seizure (1 patient), anosmia (5.6%), ageusia (5.1%), visual disturbance, neuralgia, rhabdomyolysis and muscular weakness (17%) [4]. Animal models demonstrate respiratory failure due to brainstem involvement.3 Neurologic signs can be due to primary neurologic invasion or secondary injury, most commonly due to inflammation [4]. There are two possible mechanisms for nervous system spread: hematogenous spread or retrograde transport up axons [5]. Cytokine storm facilitates blood brain barrier breakdown and therefore hematogenous spread [6].
■ Central Nervous System Manifestations/Sequelae
Strokes are reported in patients with COVID-19, usually as a secondary neurological manifestation. Cardiotoxicity due to cytokine storm, hypercoagulability and direct myocardial invasion all serve as cardiac risk factors for stroke [4]. Direct invasion of vascular epithelium is possible. A case series from NEJM showed a cohort of five COVID-19 patients under 50 presented over a 2 week period with strokes. The average number of stroke patients under 50 in a given 2 week period at their institution is less than 1 [7].
COVID-19 RNA has been detected in CSF [2]. Autopsies of COVID-19 patients showed hyperemic, edematous brain tissue with degeneration of neurons [4]. Dysregulation of ACE-2 receptors implicated in animal models of autoimmune encephalomyelitis [4]. MRI brain shows thalamic and brainstem hyperintensities [5]. COVID-19 encephalitis may manifest with seizures, as it did in this case report of a 6-week old [8]. Patient’s EEG showed interictal abnormalities of excessive temporal sharp waves and intermittent delta vertex slowing. One patient in a study of 214 COVID-positive subjects had seizure [4].
Encephalitis is another serious manifestation of COVID-19. Detection of virus in CSF has occurred [2]. Post-mortem studies of COVID-19 showed hyperemic, edematous brain tissue with degeneration of neurons [4]. SARS-CoV viral RNA was seen in brain tissue in 2003 [3]. Dysregulation of ACE-2 receptors have been implicated in animal models of autoimmune encephalomyelitis [5]. MRI brain shows hyperintensities invariably in bilateral thalami. A case report of a patient presenting with confusion and meningeal signs was diagnosed with encephalitis [9-13]. Encephalitis may manifest with seizures. A case report of a COVID-positive 6-week-old with a 3-minute event of tonic stiffening and upgaze showed interictal abnormalities on EEG of excessive temporal sharp waves, intermittent delta vertex slowing [8]. A 24-year-old presented in convulsive status epilepticus, found to have COVID-19. CSF OP was 32 and the rest of the CSF analysis was normal. Brain MRI showed diffusion restriction along the wall of the right temporal horn, and FLAIR revealed hyperintensity of the right mesial structures common in herpes encephalitis [14]. Several case reports of a patients in their 70’s with no seizure history, who presented in convulsive and non-convulsive status epilepticus [12,15].
In a report of 99 patients from Wuhan, 9% had confusion, which is thought to be a function of disease severity [16]. A case series of 58 patients in France with encephalopathy presented with confusion (65%), agitation (69%), corticospinal tract signs (67%), or dysexecutive syndrome (36%) [17]. Imaging of confused patients in our case series revealed non-specific findings, such as microvascular disease. Although microvascular disease, hypoxia, critical illness, multiorgan failure and old age can be a risk factor for encephalopathy, it is unclear if microvascular disease always precedes encephalopathy in COVID-19.
A case report demonstrated hemorrhagic necrotizing encephalopathy as another serious neurologic complication of COVID-19. MRI shows rim enhancing hemorrhagic lesions in bilateral thalami, medial temporal lobes, and sub insula. CT shows hypoattenuation in symmetric regions that include the thalami [6].
■ Peripheral Nervous System Manifestations/Sequelae
COVID-19 has been linked to cases of Guillain- Barre syndrome (GBS) and other inflammatory neuropathies, which sometimes precede or is concurrent with the respiratory illness. Molecular mimicry between viral RNA and myelin basic protein is the proposed mechanism of demyelination of both the central and peripheral nervous systems [5]. A case series from Italy of 5 patients [9] and a single case report from Iran [10] with GBS have been reported. The syndrome began with typical symptoms with neurological symptoms beginning 5-10 days later. Evolution of GBS symptoms occurred over about 3 days. Most cases were typical GBS, but two were Miller Fischer variant [9-11]. MRI lumbar spine with gadolinium showed cauda equina enhancement [9]. Unfortunately, treatment with IVIG or PLEX did not result in favorable outcomes in most of these cases. Three patients required another treatment and one recovered one-month post treatment. Two remained tetraplegics.
Myopathy or myositis can occur as a late manifestation of COVID-19 and is associated with multi-organ damage. Direct damage is implicated by the presence of ACE-2 receptors in skeletal muscle [4]. Significantly elevated CK (and other muscle markers), CRP, and D-dimer can be seen [5]. Critical illness myopathy is associated with multi-organ damage. A case report from Wuhan describes a patient with leg weakness and pain 9 days into his COVID-19 illness, which was treated with aggressive hydration, PLEX, then IVIG with rapid improvement [12].
A less serious sequela of COVID-19 is anosmia or ageusia. Post-viral anosmia occurs in 40% of a sample of 99 patients in Wuhan and can be permanent [1]. Coronaviruses can invade CN1 via the cribriform plate. Invasion of the olfactory bulb can lead to decreased volume on brain MRI [16]. The remainder of anosmia is caused by nasal turbinate obstruction of CNI observed on imaging, after invasion vis ACE-2 receptors on microvillar Bowman’s gland cells [1,3]. This differs from post-infectious anosmia in that there is no ageusia and it is temporary.
In summary, there are several neurological sequelae of this coronavirus, and other coronaviruses. It is important for clinicians to be aware of these issues.
■ Case Series
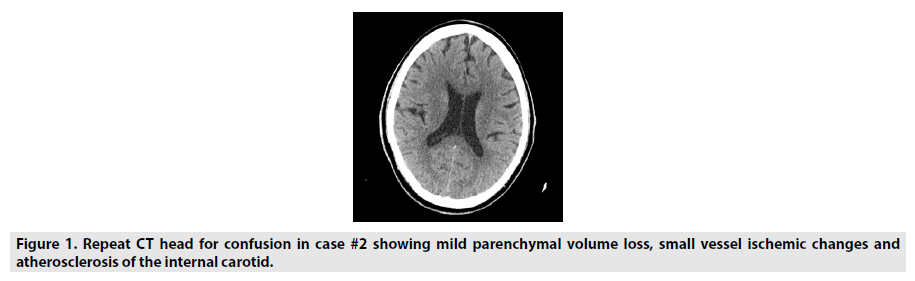
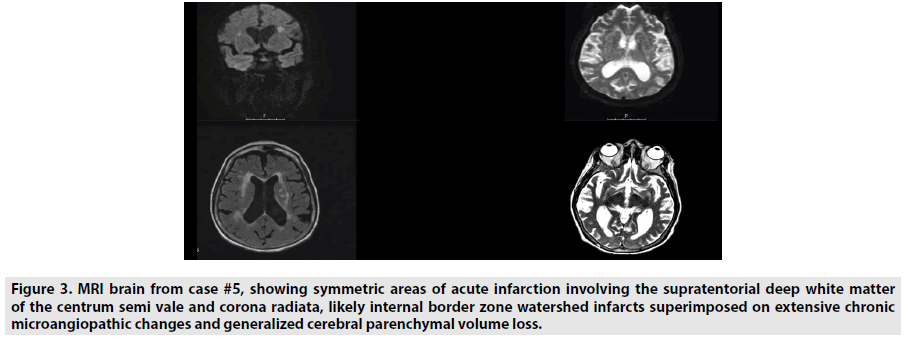
Seven cases were seen by neurology consultants at the Hospital for Special Surgery in New York City between February and May of 2020. Two were excluded for lack of neuroimaging. Most of these consultations were called for encephalopathy after extubating. They had typical symptom onset initially with subsequent confusion, described mainly as inattentiveness and poor short-term memory. Five had neuroimaging of brain MRI or head CT, which all showed microvascular disease (FIGURES 1 AND 2). There were two cases of watershed infarctions (FIGURE 3). One case had prior imaging without microvascular disease. Most had a past medical history that can lead to microvascular disease, but one had no prior history. Please refer to the table for more details (TABLE 1).
Figure 3: MRI brain from case #5, showing symmetric areas of acute infarction involving the supratentorial deep white matter of the centrum semi vale and corona radiata, likely internal border zone watershed infarcts superimposed on extensive chronic microangiopathic changes and generalized cerebral parenchymal volume loss.
| Age/Sex | Case | Medical History | Presentation | Exam | Notes | Imaging | Diagnosis |
|---|---|---|---|---|---|---|---|
| 59M | 1 | Hyperlipidemia | Dysgeusia, abnormal behavior, dizziness. Fever, anorexia, diarrhea | Inattentive, 4/5 bilateral proximal leg weakness |
EEG- diffuse background slowing CSF- elevated protein (98) |
MRI brain with gadolinium- mild microvascular disease | Encephalopathy |
| 61M | 2 | Hypertension, diabetes mellitus type 2, coronary artery disease, hyperlipidemia and chronic kidney disease | Confusion. Fever, cough, hypoxia. | Few words, not following commands. 2 weeks later, was inattentive, poor short term memory. 4 weeks later, normal. |
Normal initial CT head. CT head after confusion- chronic microvascular disease (Figure 1) |
Encephalopathy | |
| 57M | 3 | None | Fever, cough, dyspnea, AKI, atrial fibrillation. | Generalized weakness. Mental status was intact. | Treated with hydroxychloroquine, antibiotics and toculizumab. CK >7,000 | MRI brain- mild chronic subinsular white matter disease without stroke. | Rhabdomyolysis |
| 84M | 4 | Diabetes mellitus type 2 and prostate cancer metastatic to the lung | Cough, diarrhea, dyspnea, septic shock, AKI, hypernatremia and atrial flutter. | Aroused to voice, followed commands, and was diffusely weak | Treated with hydroxychloroquine. | MRI brain- subacute watershed infarcts, microangiopathic changes, and generalized cerebral parenchymal volume loss. (Figure 2) | Watershed infarcts |
| 81F | 5 | Hyperlipidemia | Found down. Fever, hypoxia. | Non-verbal, does not follow commands, does not withdraw to noxious stimuli in the legs. | Treated with high dose steroids and hydroxychloroquine | CT head-moderate parenchymal volume loss, severe microvascular changes, and old lacunes in the right thalamus and caudate head. MRI brain- acute bilateral watershed area infarcts superimposed on microangiopathic changes.(Figure 3) |
Watershed infarcts |
Table 1. Characteristics of patients seen with COVID-19.
Discussion
It is known that vascular disease is a risk factor for severe COVID-19 infection. This case series demonstrates presence of microvascular disease in patients with encephalopathy. We know that microvascular disease can be a risk factor for toxic metabolic encephalopathy. It is unclear if the microvascular disease was present prior to infection, although at least one patient had prior imaging without microvascular disease [18].
Conclusion
COVID-19 infection has been shown to invade vascular endothelium in-vitro and on autopsy. Inflammatory cells, viral inclusion bodies, endotheliitis, and apoptotic bodies are observed on histopathology. More research is needed to determine if direct viral invasion of the epithelium or interruption of clotting cascades by COVID-19 can cause cerebral microvascular disease, thereby making patients more susceptible to encephalopathy and stroke sequelae compared with other viruses.
Acknowledgements
I would like to acknowledge Dr. Robert Bard of Bard Cancer Diagnostics (Bard@cancerscan. com) for encouraging me to write this report. I would also like to acknowledge Dr. Jeffery Schacter for seeing and examining the patients.
Coinvestigators
Dr. Robert Bard Encouraged writing of the review portion of this report.
Dr. Jeffery Schacter Saw and examined patients.
Funding
None
Declaration of interests
None.
References
- Galougahi MK, Ghorbani J, Bakhshayeshkaram M et al. Olfactory Bulb Magnetic Resonance Imaging in SARS-CoV-2-Induced Anosmia: The First Report. Acad. Radiol. 27, 892-3, (2020).
- Nath A. Neurologic complications of coronavirus infections. Neurology. 94, 809-10, (2020).
- Pérez CA. Looking ahead: The risk of neurologic complications due to COVID-19. Neurol. Clin. Pract. 10, 371-4, (2020).
- Mao L, Jin H, Wang M et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. J. Am. Med. Ass. Neurol. (2020).
- Pesce A, Palmieri M, Armocida D et al. Letter: Neurosurgery and Coronavirus (COVID-19) Epidemic: Doing our Part. Neurosurgery. 87, E48-E49, (2020).
- Poyiadji N, Shahin G, Noujaim D et al. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology. 296, E119-E120, (2020).
- Oxley TJ, Mocco J, Majidi S et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N. Engl. J. Med. e60, 382-20, (2020).
- Dugue R, Cay-Martínez KC, Thakur K et al. Neurologic manifestations in an infant with COVID-19. Neurology. 68, 952-4, (2020),
- Toscano G, Palmerini F, Ravaglia S et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 382, 2574-6, (2020).
- Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: A case report. J. Clin. Neurosci. 76, 233-35, (2020).
- Gutiérrez-Ortiz A, Méndez S, Rodrigo-Rey et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 95, e601-e605, (2020).
- Filatov A, Sharma P, Hindi F et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 12, (2020).
- Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain. Behav. Immun. (2020).
- Moriguchi T, Harii N, Goto J et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int. J. Infect. Dis. 94, 55-58, (2020).
- Sohal S, Mossammat M. COVID-19 Presenting with Seizures. IDCases. (2020).
- Eliezer M, Hautefort C, Hamel A et al. Sudden and Complete Olfactory Loss Function as a Possible Symptom of COVID-19. Otolaryngol. Head. Neck. Surg. 277, 2625-30, (2020).
- Helms J, Kremer S, Merdji H et al. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 382, 2268-70, (2020).
- Varga Z, Flammer AJ, Moch H et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 395, 1417 – 18, (2020).