Research Article - Neuropsychiatry (2017) Volume 7, Issue 3
Neurological presentations and therapeutic responses to cobalamin deficiency
- Corresponding Author:
- Min-Chien Tu
Department of Neurology, Taichung Tzu Chi Hospital
Buddhist Tzu Chi Medical Foundation
No. 88, Sec. 1, Fengxing Rd., Tanzi Dist., Taichung City 427, Taiwan
Tel: +886-4-3606-0666, ext: 4913
Fax: +886-4-3606-5928
E-email: tmctmc30@yahoo.com.tw
Abstract
The clinical phenotypes of cobalamin (Cbl) deficiency is often overlooked in clinical practice as their presentations vary according to the level of involvement between the hematologic and nervous systems. Although the negative impact of Cbl deficiency on cognition has been underpinned, the reported therapeutic responses after Cbl supplement therapy differ. Therefore, we aimed to describe the neurological presentations of patients with Cbl deficiency and investigate the potential biomarkers in predicting therapeutic responses with emphasis on cognitive aspects.
Keywords
Cobalamin deficiency, Therapeutic response, Vitamin B12, Cognitive functions, Magnetic resonance imaging
Introduction
The recognition and treatment of cobalamin (Cbl) deficiency is important, as it has been identified as a reversible cause of many hematopoietic and neurological diseases [1]. While a low Cbl status has been associated with pernicious anemia, leukopenia, thrombocytopenia, and even bone marrow dysfunction as the main hematopoietic presentations [1], subacute combined degeneration, polyneuropathies [1], dementia [2], depression [2], anxiety [2], and stroke [3] have also been documented to be related neurological diseases. Due to the fact that the clinical phenotypes of Cbl deficiency vary according to the level of involvement between the hematologic and nervous systems, it is often overlooked in clinical practice. Aside from sub-acute combined degeneration and polyneuropathies, the best known neurological presentations, the association between Cbl deficiency and cognitive impairment has been gaining attention recently. Although the negative impact of Cbl deficiency on cognition has been reported in several studies [4-8], the causality is unclear. One of the major issues is that the reported therapeutic responses after Cbl supplement therapy differ. Some cohort studies have shown promising results of Cbl supplement therapy in treating neuropsychiatric abnormalities related to Cbl deficiency [9,10], whereas other large-scale, prospective studies have failed to replicate these positive results [11,12]. Such inconsistencies in the response to Cbl supplement therapy might allude to the roles of other clinical and biochemical mediators. One observational study, in which patients with heterogeneous types of dementia were recruited, identified that patients with mild-to-moderate dementia responded better to Cbl supplement than those who were severely demented [10]. Stabler, et al. found that levels of methylmalonic acid and/or total homocysteine were elevated in 94% of Cbl-deficient patients with satisfactory hematological or neurological responses to replacement therapy [13]. In a randomized placebo-controlled study, a subgroup of patients with a level of methylmalonic acid between 0.60 and 2.00 μmol/l had neurological improvements after Cbl therapy [11]. In addition, limited studies have stressed the importance of evaluating both cognition and structural brain imaging in patients with a low Cbl status [8,14]. Taken together, changes in cognition related to replacement therapy may be affected by preexisting and relevant brain parenchymal damage. Therefore, in this study we aimed to (i) describe the various neuropsychiatric presentations before and after Cbl supplement treatment, and (ii) elucidate factors predicting therapeutic responses among patients with Cbl deficiency.
Materials and Methods
▪ Subjects and clinical registry
This study recruited 50 consecutive symptomatic patients with Cbl deficiency (≤ 250 pg/mL) [15], who visited the Department of Neurology of our hospital from March 2012 to March 2015. The initial neurological disease, clinical symptoms, nutritional status, serological evaluations, and other chronic diseases of all of the patients were recorded. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg in two separate blood pressure measurements [16], a self-reported diagnosis of hypertension, or taking antihypertensive medication. Diabetes mellitus was defined as a fasting blood sugar level ≥ 126 mg/dL, random postprandial blood sugar level ≥ 200 mg/dL, HbA1C ≥ 6.5% [17], a self-reported diagnosis of diabetes mellitus, or the use of insulin or oral hypoglycemics. Chronic kidney disease was defined as a glomerular filtration rate by Modifiable of Diet in Renal Disease Study equation [18] below 60 mL per minute per 1.73 m2 for 3 or more months with or without evidence of kidney damage [19]. Coronary artery disease was defined as an event and/or history related to stable angina pectoris, unstable angina pectoris, or myocardial infarction [20]. The predisposing factors related to Cbl deficiency including dietary habit, alcoholism, gastrointestinal surgery, infection [21], long-term use (> 16 weeks) of metformin [22], long-term use (> 6 years) of H2 blockers or pro-ton pump inhibitors [23], and pernicious anemia [24] were comprehensively reviewed. The inclusion criteria were: (1) a Cbl serum level ≤ 250 pg/mL [15]; and (2) complete clinical registry and serological evaluations. The exclusion criteria were: (1) delirium state; (2) severe psychiatric disorders; (3) stroke event within the previous 2 weeks; (4) serum folate level < 2 ng/mL [25]; (5) derangement in serology tests contributing to cognitive impairment, such as abnormal free T4, cortisol, or rapid plasma reagin; and (6) severe hearing or visual impairment. The study was approved by the Institutional Review Board of our hospital (REC 103-47).
▪ Serology tests
Antecubital venous blood after an 8-hour fast was collected for hemograms and levels of serum creatinine, homocysteine, folate, Cbl, free T4, thyroid stimulating hormones, cortisol (8 a.m.), and rapid plasma reagin. The samples were collected in evacuated tubes containing EDTA, centrifuged within 10 minutes and stored below -20°C until being analyzed.
▪ Cognitive evaluations
For patients with cognitive complaints, cognitive evaluations were routinely performed. Each assessment included Clinical Dementia Rating (CDR) [26], the Taiwanese version of the Mini- Mental Status Examination (MMSE) [27], and Cognitive Abilities Screening Instrument (CASI) [28]. The CDR is a semi-structured interview with the patients and reliable informants. It involves six domains of cognitive and functional performances, including memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. An overall score is reached according to a standardized algorithm. A CDR score of 0 indicates no cognitive impairment, and the remaining four scores represent various stages of severity (CDR-0.5: very mild; CDR- 1: mild; CDR-2: moderate; CDR-3: severe) [26]. The MMSE assesses the global cognition of subjects. The CASI provides quantitative assessments of nine cognitive domains including attention, orientation, short-term memory, long-term memory, language ability, drawing, verbal fluency, abstract thinking and mental manipulation [28]. Higher scores on the MMSE and CASI represent better cognitive performance. The total scores of the CDR, MMSE, and CASI, as well as sum-of-box of CDR and CASI index scores were recorded for analysis. The same cognitive evaluations were assessed again after normalization of serum Cbl. All of the subjects were free of psychotropic medications in the 2 weeks prior to the cognitive evaluations. Therapeutic responses were dichotomized according to MMSE changes (ΔMMSE=re-test MMSE - initial MMSE). Poor responders were defined as those with a decline in MMSE score (ΔMMSE < 0), whilst good responders were defined as those with an improved or no change in MMSE score (ΔMMSE ≥ 0). The rationale for using a dichotomized result was based on a previous study in which the average annual decline in MMSE score for a population of patients with dementia ranged from 1.8 to 6.7 points [29].
▪ White matter hyperintensity (WMH) assessments
For patients with cognitive complaints, brain magnetic resonance imaging (MRI) was performed on a 3.0T scanner (Discovery MR750, GE Medical System, Milwaukee, WI). WMHs were rated in accordance with Scheltens scale [30] from T2-fluid-attenuated inversion recovery (T2-FLAIR) sequences in an axial plane by a single rater (Min-Chien Tu). The parameters were as follows: repetition time 12000 msec, echo time 120 msec, inversion time 2200 msec, slices thickness 5 mm, field of view 24 cm, and matrix 256 x 256. Scheltens scale is a semi-quantitative visual rating method for WMHs with good intra- and inter-observer reliability [30]. For analysis of both the number and volume of WMHs, anchored 7-point severity ratings were applied in four regions, including periventricular (i.e., frontal horn, occipital horn, and lateral bands), deep (i.e., frontal, temporal, parietal and occipital lobes), basal ganglia (i.e., caudate nucleus, putamen, globus pallidus, internal capsule and thalamus), and infratentorial (i.e., mesencephalon, pons, medulla oblongata, and cerebellum) areas. We did not assess infratentorial regions due to the limited impact on cognition.
▪ Statistical analysis
Shapiro-Wilk test was used for confirming normality [31]. Independent-sample t and χ2 tests were used to detect group differences in demographic, morphometric and psychometric data where appropriate. Paired t-tests were used to compare cognitive performance of temporal pairs of observations (i.e., before and after treatment). Cohen’s d was used to determine the effect size on comparing two groups of similar size and standard deviation [32]. Hedges’ g was used alternatively under the condition that different standard deviation was identified by Levene test [33]. According to Cohen’s guidelines for interpreting measure of effect size, a Cohen’s d value of 0.10, 0.30, and 0.50 corresponds to the effect that could be described as small, median, and large, respectively [33]. For Hedges’ g, a value of 0.2 is considered to be small, 0.5 is a median effect, and 0.8 or greater is large [33]. Analysis of covariance (ANCOVA) was applied to determine certain variables that predict the outcome after elimination of some other confounds. All statistical tests were performed using IBM SPSS Stastistics version 19. A p value less than 0.05 was considered to be statistically significant.
Results
Table 1 shows the general clinical information of 50 participants with Cbl deficiency. The level of serum Cbl ranged from 104 to 250 pg/ml (mean = 194.7 pg/ml; standard deviation = 40.6 pg/dl), and was not significantly correlated with mean corpuscular volume (ρ = -0.085, p = 0.580), MMSE (ρ = -0.154, p = 0.349), or CASI (ρ = -0.150, p = 0.362). The level of both serum Cbl (ρ = -0.322, p = 0.035) and folate (ρ = -0.332, p = 0.030) had significant inverse correlation with homocysteine. With regards to predisposing factors of Cbl deficiency, 14 patients (28%) were vegetarians, 10 (20%) had used metformin for more than 16 weeks (long-term use), two were both vegetarian and long-term metformin users, two were alcoholics, and one had received a partial gastrectomy. None of the patients had a chronic infection or the long-term use (> 6 years) of H2 blockers or proton pump inhibitors. While four patients had macrocytosis (mean corpuscular volume >100 fL) [34], only two had macrocytic anemia, which had not yet been confirmed as pernicious anemia [24].
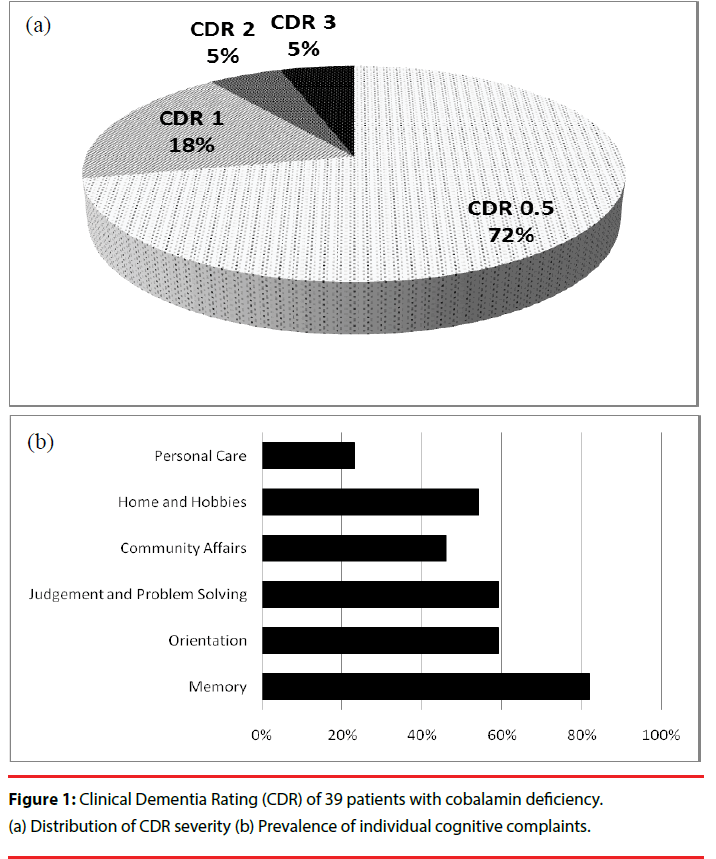
With regards to neuropsychiatric presentations, 39 patients (78%) had cognitive complaints, mostly in the form of memory or dysexecutive problems. Figure 1 illustrates the composition of the CDR score of the 39 patients with cognitive complaints. Their initial cognitive symptoms mainly manifested as memory complaints (32 patients; 82%), followed by judgment and problem solving (23 patients; 59%) and orientation (23 patients; 59%). During the initial assessment, 28 patients (72%) had cognitive complaints with very-mild severity (CDR = 0.5), and 11 patients (28%) had a CDR score ≥ 1.
Figure 1: Clinical Dementia Rating (CDR) of 39 patients with cobalamin deficiency. (a) Distribution of CDR severity (b) Prevalence of individual cognitive complaints.
Other common presentations included dementia (38%), anxiety (38%), cerebrovascular disease (28%), and depression (20%) (Table 1). Of 14 patients with cerebrovascular disease, eight (57.1%) had recurrence and five (35.7%) had related major intracranial artery occlusion (two with internal carotid artery and three with middle cerebral artery occlusion) revealed on MR angiography. With regards to the type of cerebrovascular disease, one patient had an intracranial hemorrhage and another had frequent transient ischemic attacks. The remaining 12 patients (85.7%) had ischemic stroke, including eight with large-artery atherosclerosis and four with small vessel occlusion. No cardioembolisms were noted [35]. Polyneuropathy and cranial neuropathy were noted in only 12% and 4% of the patients, respectively. Of six patients with polyneuropathy, profound sensory complaints were the uniform clinical presentation. Of these six patients, four had sensorimotor demyelinating polyneuropathy, one had sensorimotor axonal polyneuropathy, and one had small-fiber polyneuropathy [36]. There was no overlapping clinical phenotype between polyneuropathy and cranial neuropathy.
| Demographic Data [mean (SD)] | |
| Age : 65.5 (15.6) Gender (M/F): 19/31 Education (years) : 6.7 (4.4) Cobalamin (pg/mL): 194.7 (40.6) Homocysteine (mg/L) : 3.1 (3.4) |
Hemoglobin (g/dL):13.0 (1.4) Folate (ng/mL): 12.3 (7.1) Mean Corpuscular Volume (fL/red cell): 94.2 (28.8) Creatinine (mg/dL): 1.0 (0.3) Cortisol (µg/dL) : 11.3 (3.0) |
| Chronic Diseases [number (%)] | |
| Coronary artery disease: 3 (6) Chronic kidney disease: 3 (6) |
Diabetes mellitus: 15 (30) Hypertension: 21 (42) |
| Predisposing Factors [number (%)] | |
| H2 blocker use> 6 years: 0 (0) Proton pump inhibitor use> 6 years: 0 (0) Gastrointestinal operation history: 1 (2) |
Metformin use> 16 weeks: 10 (20) Vegetarian diet: 14 (28) Alcoholism: 2 (4) |
| Neuropsychiatric Comorbidities [number (%)] | |
| Cerebrovascular disease: 14 (28) Polyneuropathy: 6 (12) Cranial neuropathy 2 (4) Myelopathy: 1 (2) Seizure: 1 (2) |
Cognitive Complaints: 39 (78) Dementia: 19 (38) Anxiety: 19 (38) Depression: 10 (20) Others:1(2) |
Table 1: Demographic data and neuropsychiatric presentations of 50 patients with cobalamin deficiency.
Thirty patients had complete cognitive evaluations before and after Cbl supplement therapy (mean treatment duration = 16.3 weeks, standard deviation = 7.9 weeks; mean corrected vitamin level = 705.0 pg/ml, standard deviation = 285.0 pg/dl). All patients had CDR ranging from 0.5 to 1. Sixteen and 14 patients were categorized as good and poor responders, respectively. Of the 16 good responders, 10 had an improvement in the MMSE ≥ 2, three had an improvement of 1, and three had the same MMSE score as at the initial assessment. The response rate was 53.3% (Table 2). Overall, there was only a trend in improvement in the total MMSE and CASI scores. However, there was a significant benefit on the cognitive domains of short-term memory and verbal fluency (both p < 0.05). Of the good responders, the benefit of treatment was most evident in total MMSE score (p < 0.01) and short-term memory domain (p < 0.05). There was a trend of general improvement in the rest of the cognitive parameters, except for long-term memory and drawing. For the poor responders, a significant decline of MMSE was noted (p < 0.05).
| Overall (n=30) |
Good Responders (n=16) |
Poor Responders (n=14) |
||||
|---|---|---|---|---|---|---|
| pre-Tx. | post-Tx. | pre-Tx. | post-Tx. | pre-Tx. | post-Tx. | |
| MMSE | 21.3 (6.9) | 22.1 (6.9) | 21.3 (7.0) | 24.4 (5.7)** | 21.3 (7.1) | 19.4 (7.4)* |
| CASI | 68.9 (22.8) | 71.2 (19.8) | 69.8 (23.4) | 75.0 (18.8) | 67.8 (22.8) | 66.6 (20.8) |
| LTM | 8.9 (2.2) | 8.9 (2.4) | 9.3 (1.7) | 9.2 (1.8) | 8.4 (2.6) | 8.5 (3.1) |
| STM | 6.3 (3.7) | 7.6 (3.4)* | 6.3 (4.0) | 8.3 (3.6)* | 6.5 (3.4) | 6.9 (3.1) |
| ATT | 6.6 (1.7) | 6.5 (1.1) | 6.6 (1.5) | 6.7 (0.9) | 6.5 (2.0) | 6.3 (1.3) |
| MENMA | 5.3 (3.8) | 5.7 (3.4) | 5.2 (4.0) | 6.1 (3.2) | 5.4 (3.8) | 5.2 (3.7) |
| ORIEN | 13.5 (5.5) | 13.6 (5.0) | 13.6 (5.6) | 15.0 (4.2) | 13.4 (5.6) | 11.9 (5.4) |
| AT | 7.0 (2,9) | 6.7 (2.8) | 6.8 (3.2) | 7.1 (2.7) | 7.3 (2.5) | 6.2 (3.0) |
| LAN | 8.3 (1.6) | 8.6 (1.7) | 8.4 (1.9) | 8.8 (1.5) | 8.2 (1.4) | 8.3 (1.9) |
| DRA | 7.4 (3.5) | 7.6 (2.8) | 8.0 (2.7) | 7.8 (2.2) | 6.7 (4.4) | 7.3 (3.6) |
| VF | 5.6 (2.9) | 6.3 (2.2)* | 5.7 (2.7) | 6.3 (2.3) | 5.5 (3.2) | 6.3 (2.2) |
*: p<0.05; **: p<0.01 on comparisons between pre-Tx. and post-Tx. within each group; data presented as [mean (SD)]; Tx.: Treatment; MMSE: Mini-mental state examination; CASI: Cognitive abilities screening instrument (total score); LTM: Long term memory; STM: Short term memory; ATT: Attention; MENMA: Mental manipulation; ORIEN: Orientation; AT: Abstract thinking; LAN: Language; DRA: Drawing; VF: Verbal fluency.
Table 2: Comparisons of cognitive performances before and after treatment.
Table 3 shows comparisons between the good and poor responders of Cbl supplement therapy. No differences in demographic data (i.e., age, education, gender, chronic diseases and neuropsychiatric comorbidities) and initial cognitive evaluations (i.e., MMSE, CASI, and CDR) were noted. However, the response rate in male and female patients was 33.3% and 66.6%, respectively. In serology tests, the good responders had a higher initial serum folic level than the poor responders (p < 0.05; Cohen’s d = 1.044). Comparisons in the level of initial/ corrected Cbl were not significant. There was no significant difference in the level of initial (male: 190.9 ± 38.6, female: 191.9 ± 31.6, p = 0.943) and corrected Cbl levels (male: 1089.0 ± 1000.5, female: 810.0 ± 504.8, p = 0.459) with regard to gender. There was a trend of higher serum homocysteine level among the good responders, yet not reaching statistical significance. With regards to WMH assessments, the poor responders had a greater total WMH load and deep white matter hyperintensities (DWMHs) 【both p < 0.05; Hedges’ g of total WMHs = 0.8429, Hedges’ g of DWMHs = 0.8890】. With regards to segregated regions, the poor responders had more DWMHs in frontal, parietal, occipital, and temporal regions (all p < 0.05). There was a significant effect of serum folate level on ΔMMSE after controlling for the effect of total WMHs and DWMHs【 F(24,1) = 2.26, p < 0.0001】
| Good Responders (n=16) | Poor Responders (n=14) | |
|---|---|---|
| Demographic Data | ||
| Age | 65.8 (13.2) | 67.1 (9.0) |
| Gender (M/F) | 4/12 | 8/6 |
| Education | 6.2 (4.0) | 6.6 (4.5) |
| Mini-Mental State Examination | 21.3 (7.0) | 21.3 (7.1) |
| Cognitive Abilities Screening Instrument | 69.8 (23.4) | 66.6 (22.4) |
| CDR Sum-of-box | 2.5 (2.1) | 2.8 (2.9) |
| CDR 0.5 (n) | 12 | 11 |
| CDR 1 (n) | 4 | 3 |
| Initial Cobalamin level (pg/mL) | 190.1 (33.7) | 202.0 (40.1) |
| Corrected Cobalamin level (pg/mL) | 744.9 (479.6) | 932.4 (921.5) |
| Hemoglobin (g/dL) | 12.9 (1.1) | 13.0 (1.2) |
| Mean Corpuscular Volume (fL/red cell) | 84.9 (8.0) | 103.1 (45.2) |
| Folate (ng/mL) | 16.8 (7.8) | 10.0 (4.9)* |
| Creatinine (mg/dL) | 0.9 (0.3) | 1.0 (0.3) |
| Homocysteine (mg/L) | 2.38 (1.43) | 1.76 (0.76) |
| Cortisol (µg/dL) | 11.9 (3.2) | 10.6 (2.8) |
| HbA1C (%) | 5.9 (0.6) | 5.9 (0.8) |
| Chronic Diseases (Y/N) | ||
| Coronary artery disease | 1/15 | 0/14 |
| Chronic kidney disease | 0/16 | 1/13 |
| Diabetes mellitus | 5/11 | 5/9 |
| Hypertension | 8/8 | 7/7 |
| Neuropsychiatric Comorbidities (Y/N) | ||
| Cerebrovascular disease | 6 | 4 |
| Dementia | 8 | 5 |
| Anxiety | 7 | 3 |
| Depression | 3 | 2 |
| Seizure | 0 | 1 |
| WMHs | ||
| PWMHs | 2.9 (1.3) | 3.5 (1.8) |
| Frontal | 1.1 (0.3) | 1.3 (0.6) |
| Occipital | 0.8 (0.8) | 1.2 (0.7) |
| Lateral ventricle | 0.9 (0.6) | 1.0 (0.7) |
| DWMHs | 5.5 (4.5) | 11.9 (9.3)* |
| Frontal | 1.9 (1.7) | 3.6 (2.5)* |
| Parietal | 1.5 (1.3) | 3.1 (2.5)* |
| Occipital | 1.6 (1.5) | 3.2 (2.4)* |
| Temporal | 0.4 (0.6) | 2.0 (2.5)* |
| Basal ganglia | 1.3 (2.0) | 4.6 (6.7) |
| Caudate nucleus | 0.2 (0.4) | 0.6 (0.9) |
| Putamen | 0.8 (0.9) | 1.4 (1.9) |
| Globus pallidus | 0.2 (0.5) | 0.9 (1.3) |
| Thalamus | 0.0 (0.0) | 0.9 (1.6) |
| Internal capsule | 0.2 (0.5) | 1.0 (1.5) |
| Total | 9.7 (6.5) | 20.0 (16.5)* |
Table 3: Comparisons of initial assessment variables between good and poor responders of cobalamin supplement.
Discussion
In this study, we described the clinical phenotypes of Cbl deficiency and investigated associations between therapeutic responses and clinical profiles. Our findings suggest dissociation between neurological and hematological involvement in patients with Cbl deficiency. Our preliminary results also support the benefit of replacement therapy in some patients with Cbl deficiency on the basis of standardized cognitive evaluations. Moreover, we identified that pretreatment serum folate levels and baseline WMH load could predict responses to subsequent Cbl replacement treatment. The current study also offers further insight into the pathogenesis of Cbl deficiency, and clarifies the role of biochemical and neuro-radiological factors.
The analysis of baseline biochemical factors showed an interaction between Cbl and folate. In the context of Cbl deficiency, the patients with a lower, while still normal, serum folate level (10.0 ± 4.9 ng/mL) tended to have a worse response to Cbl supplement therapy than those with a higher level (16.8± 7.8 ng/mL). The impact of serum folate level on cognition changes remained to be significant even after controlling the effect of WMHs. This seems reasonable, as a low serum folate level has also been correlated with the severity of cortical atrophy [37] and worse cognitive performances [38]. From a biochemical perspective, both Cbl and folate are thought to be essential in maintaining homeostasis of the nervous system, as they share some common pathways and act as critical enzymatic cofactors that reduce levels of the pro-oxidant homocysteine in the S-adenosylmethionine cycle [39]. However, some epidemiological studies have suggested that the impact of these two substances on cognition may be modified by various biochemical conditions. A large crosssectional survey reported that patients with a low serum Cbl level (< 65.3 ng/mL) and high serum folate concentration (> 26.0 ng/mL) were at greater risk of cognitive impairment, compared to those with a low serum Cbl level and normal serum folate concentration [40]. Conversely, in the same cohort, a high folate concentration was found to be a protective factor in elderly patients with a normal serum Cbl level, and a high serum folate concentration was associated with less cognitive impairment compared to those with a normal serum folate concentration [40]. Another prospective study also reported that a faster rate of cognitive decline was associated with high folate intake, regardless of the source of intake (e.g., food and/or folate vitamin supplementation). In addition, those with a high folate intake (median, 742 μg/d) had a significantly faster rate of cognitive decline than those with a low intake (median, 186 μg/d) [41]. These unexpected findings raise the concern of folate fortification, as this may mask the neurological complications related to Cbl deficiency [41] and possibly impair the activity of the two Cbl-dependent enzymes, methionine synthase and MMA-coenzyme A mutase [40]. Our cohort received no folate supplementation, and their serum folate concentrations were still below the previous warning level (26.0 ng/ mL). We therefore suggest that a synergistic benefit of folate and Cbl on cognition still exists when the serum folate level is controlled within an acceptable range (16.8~24.6 ng/mL). Besides, a trend of higher serum homocysteine level among the good responders also corroborated the previous study [13], in which hyperhomocysteinemia was identified among the majority of Cbl-responsive patients. Taken together, we regarded serum homocysteine level to be a biological indicator for Cbl-deficient state and a potential prognostic factor.
From our neuro-radiological observations, total WMH load and DWMHs had a large power in predicting therapeutic responses among patients with Cbl deficiency. WMHs have been associated with cognitive decline [42], dementia [43], and a higher dementia conversion rate [44]. However, such associations appear to be modulated by load and spatial distribution of the lesions [45,46]. Current WMH studies often classify lesions into DWMHs and periventricular white matter hyperintensities (PWMHs), given that their appearances have overlapping but distinct pathogeneses and clinical significance [47,48]. Histopathologically, DWMHs but not PWMHs correlate with the severity of demyelination of the corresponding area, and their volumes correlate with the presence of cortical infarcts and cerebral hemorrhage [49]. PWMHs, on the other hand, correlate with the severity of atherosclerosis [49]. Clinically, although age has been shown to be significantly associated with the severity of both PWMHs and DWMHs, hypertension and diabetes mellitus have been more strongly associated with severe DWMHs [48]. Increasing evidence has also shown the different impact of DWMHs and PWMHs in patients with dementia or mild cognitive impairment. Some researchers have emphasized the detrimental role of frontal PVMHs on related cognitive function and/or decline [45,46], whilst others have reported negative but dissociative domainspecific impacts resulting from both DWMHs and PWMHs [50]. We therefore assume that the total WMH load and DWMHs represented more powerful biomarkers than PWMHs, as they reflected a reduced brain reserve. Moreover, the existence of WMHs may represent damage far beyond cerebral vascularity. WMHs have frequently been identified with vessels affected by small vessel disease [51], in which chronic hypoperfusion of the white matter and disruption of the blood-brain barrier is regarded as the main pathogenesis. Pathological studies have further identified pallor myelin, tissue rarefaction associated with the loss of myelin and axons, and mild gliosis in the regions of WMHs [52,53]. The accrual of WMHs seems to be associated with neurobiological properties in patients with low Cbl and folate levels. Both folate and Cbl deficiencies have been associated with impaired myelin integrity and nerve cell homeostasis, potentially through mechanisms of homocysteine-induced increased neurotoxicity, vasotoxicity, and inefficient S-adenosylmethionine methylation [54,55]. Therefore, the existence of WMHs represents a surrogate marker of damage to nerve cells, myelin, and microvasculature.
Although we were unable to identify a potential cognitive marker to predict the therapeutic response, Cbl supplement therapy was shown to exert domain-specific benefits. Overall, the performance of short-term memory and verbal fluency improved. Among the good responders, profound improvements in short-term memory and global cognitive score serve as robust evidence for the prompt correction of Cbl deficiency. Our results provide an interesting parallel to the report of Kalita, et al. in which a profound improvement in hospital anxiety depression scores and a concomitant benefit in executive function were identified after Cbl supplement therapy [14]. Both studies reported benefits in verbal fluency, suggesting that frontalsubcortical circuit dysfunction is one of the main pathogenesis related to Cbl deficiency [14]. This hypothesis is also supported by a functional imaging study, in which hypoperfusion within frontal regions and basal ganglia were associated with dysexecutive syndrome in patients with Cbl deficiency [7]. Additionally, we also found therapeutic benefits in short-term memory, in which both frontal and temporal lobes are regarded as pivotal neural substrates. The cognitive profile in our cohort is compatible with previous literatures, as dysfunction of fronto-temporal regions has been implied in both electrophysiological [14] and functional imaging studies [7]. Together with our study, the importance of prompt recognition and treatment of Cbl deficiency should be highlighted, in the hope of reversing cognitive impairment before brain volume shrinkage.
It may be argued that the results can be explained by co-existing neurodegenerative diseases such as Alzheimer’s disease (AD), since a subset of patients had a non-preferable therapeutic response. However, the changes in cognitive profiles among the patients in the current study are far different from therapeutic responses identified in patients with AD, where benefits from cholinesterase inhibitors mainly occur in attention, executive, language and visuospatial functions, in the presence of inevitable declines in global cognitive score [56,57]. As none of our patients received cholinesterase inhibitors during Cbl supplement therapy, the reversibility and unique domain-specific benefits corroborate that the cognitive deficits in our cohort were primarily due to Cbl deficiency.
The descriptions of the predisposing factors and clinical phenotypes related to Cbl deficiency are also of clinical value. Common causes of Cbl deficiency in the elderly include food-cobalamin malabsorption, pernicious anemia, and dietary deficiency [21]. Around a quarter and one fifth of the patients with Cbl deficiency were vegetarians (dietary deficiency) and long-term metformin users (food-cobalamin malabsorption), respectively. Still, a considerable subset of the patients had no predisposing factors according to their medical history. As most of our patients received therapy with oral supplements, atrophic gastritis may explain a poor response to Cbl supplement therapy [21].
Except for the strong association with cognitive deficits, the prevalence of recurrent stroke and major cerebral vascular diseases in our cohort appeared to be higher than that expected in stroke patients. Previous studies have reported an annual recurrence rate of 4-14% in large-scale ischemic stroke registries [58-61], and 14.18% and 5.17% of stroke patients were diagnosed with middle cerebral artery and intracranial internal carotid artery occlusion, respectively, under MRI screening in the Chinese Intracranial Atherosclerosis Study [62]. Our observations support the hypothesis that the downstream metabolite changes related to Cbl deficiency, and mostly homocysteine, may trigger vasculopathy either by hastening atherosclerosis [63] or by creating thrombophilic conditions [64].
The dissociation between neurological and hematological systems involvement also raises an interesting point. Hematological impairment has been thought to start early and precede neuropsychiatric manifestations, typically in the context of subacute combined degeneration, in patients with Cbl deficiency [65]. However, an increasing number of patients have been reported to have neuropsychiatric presentations in the absence of abnormal hemograms [9,66]. The exact mechanisms determining whether the clinical phenotype will be predominantly neuropsychiatric rather than hematological, or vice versa, remain to be elucidated. Several animal studies have reported that severe neurological deficits related to Cbl deficiency may be induced in the absence of abnormalities in the blood or bone marrow [67,68]. The dissociative presentations may be partly explained by the distinct biochemical properties of two active forms of Cbl, methylcobalamin and adenosylcobalamin. Methylcobalamin and folate undergo interlocking reactions accounting for deoxyribonucleic acid synthesis in cells where chromosomal replication and division occur, most notably in hematological systems [69]. Whereas, adenosylcobalamin acts as a coenzyme for methylmalonyl coenzyme A mutase, a process which is unrelated to folate in the synthesis of fatty acids in the myelin sheaths that surround nerve cells [70]. The aim of the current study was to present clinical and neuroimaging information, so that identifying the determining biochemical factors related to the clinical phenotypes is beyond the scope of this study.
A trend of female predominance among good responders would be another interesting point (the response rate in male/female patients = 33.3/66.6%). The gender effect toward Cbl status is complex and remains to be elucidated. On the basis that women have a higher Cbl level than men across age and race categories [71- 74], female gender might be associated with neuroprotection and better clinical prognosis. An increment in serum estradiol caused by shortterm multi-vitamin supplementation including Cbl was reported from a study recruiting 20 postmenopausal women [75]. However, the biological interactions may vary according to exogenous or endogenous properties of sex hormone. Hormonal contraception has been reported to cause serum Cbl to decrease [76]. Although several studies suggested redistribution of Cbl to be the biological process responsible for clinical observations [77,78], other studies argued such hypothesis and proposed a deficiency in the level of serum Cbl binders resulting in a false low Cbl to be the reason for subclinical biochemical changes [79,80]. To sum up, our study results reflected the possibility that the bioavailability and/or concentrations of Cbl might be modulated by sex hormonal status.
There are several limitations to this study. First, similar to most other studies investigating the relationships between Cbl deficiency and cognition, excluding comorbid neurodegenerative diseases, and especially AD, is of great clinical challenge. However, the likelihood of such diseases being present in our cohort is relatively low, as initial brain MRI of our patients showed no obvious volume reduction within medial temporal lobes, and the changes in cognitive profiles after therapy were very different from those observed in the treatment responses of patients with AD. Second, it remains to be clarified to what extent the levels of Cbl, folate, and cognitive impairment are associated. Although deficiency of Cbl and/or folate is a reasonable cause for cognitive decline, a low nutritional intake could also be the consequence of impaired cognition. Third, as a certain number of our patients had lacunar strokes, comparisons of cognitive profiles/therapeutic responses between groups with similar WMH loads but different serum Cbl levels would also have been of value to confirm the causality between Cbl and cognition. However, as a considerable portion of responders showed robust reversal or maintenance of cognition, we believe that our observations are primarily relevant to a low Cbl status. Fourth, although generalization of the results of current study would warrant future researches of larger sample size, we suggest that serum folate level and WMH load in patients with Cbl deficiency could serve as pertinent markers in predicting therapeutic responses due to larger effect size as evident from statistical analysis.
Conclusion
In conclusion, among the variable clinical phenotypes related to Cbl deficiency, a high prevalence of cognitive deficits, recurrent stroke, and major intracranial artery occlusion relevant to the index events warrant further research. We found dissociation between involvement of the nervous and hematological systems. Except for vegetarians (dietary deficiency) and long-term metformin users (food-cobalamin malabsorption), a considerable subset of patients had no identifiable predisposing factors according to their medical history, indicating the need of more extensive evaluations among patients refractory to supplement therapy. Although Cbl supplement therapy led to positive and possibly domain-specific benefits on cognition, the therapeutic responses were modulated by serum folate level and initial WMH load. A serum folate level between 16.8 and 24.6 ng/mL led to a synergistic benefit on cognition during Cbl supplement therapy. Greater total WMHs and DWMHs were associated with unfavorable therapeutic response. Comprehensive assessments of serum folate level, WMHs, and exclusion of atrophic gastritis are advised for patients with a poor response to Cbl supplement therapy.
Acknowledgements
The authors thank the patients and their caregivers for their time and commitment to this research.
References
- Stabler SP. Clinical practice. Vitamin B12 deficiency.New. Engl. J. Med368(2), 149-160 (2013).
- Lachner C,Steinle NI,Regenold WT. The neuropsychiatry of vitamin B12 deficiency in elderly patients.J. Neuropsychiatry. Clin.Neurosci24(1),05-15 (2012).
- Penix LP. Ischemic strokes secondary to vitamin B12 deficiency-induced hyperhomocystinemia.Neurology51(2), 622-624 (1998).
- Tucker KL,Qiao N, Scott T,et al.High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study.Am. J. Clin.Nutr82(3), 627-635 (2005).
- Tangney CC, Tang Y, Evans DA,et al.Biochemical indicators of vitamin B12 and folate insufficiency and cognitive decline.Neurology72(4), 361-367 (2009).
- Vogiatzoglou A,Refsum H, Johnston C,et al.Vitamin B12 status and rate of brain volume loss in community-dwelling elderly.Neurology71(11), 826-832 (2008).
- Tu MC, Lo CP, Chen CY,et al.Correlation of Tc-99 m ethyl cysteinate dimer single-photon emission computed tomography and clinical presentations in patients with low cobalamin status.BMC.Neurology 15, 251 (2015).
- Hsu YH, Huang CF, Lo CP, et al.Vitamin B12 deficiency: Characterization of psychometrics and MRI morphometrics.Nutr.Neurosci19(2),47-54 (2016).
- Lindenbaum J,Healton EB, Savage DG,et al.Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis.New. Engl. J. Med318(26), 1720-1728 (1988).
- Nilsson K,Warkentin S,Hultberg B, et al.Treatment of cobalamin deficiency in dementia, evaluated clinically and with cerebral blood flow measurements.Aging (Milano)12(3), 199-207 (2000).
- Hvas AM,Ellegaard J,Nexo E. Vitamin B12 treatment normalizes metabolic markers but has limited clinical effect: a randomized placebo-controlled study.Clin.Chem47(8), 1396-1404 (2001).
- Hoey L, Strain JJ, McNulty H. Studies of biomarker responses to intervention with vitamin B-12: a systematic review of randomized controlled trials.Am. J. Clin.Nutr89(6), 1981s-1996s (2009).
- Stabler SP, Allen RH, Savage DG, et al.Clinical spectrum and diagnosis of cobalamin deficiency.Blood76(5), 871-881 (1990).
- Kalita J, Agarwal R, Chandra S,et al.A study of neurobehavioral, clinical psychometric, and P3 changes in vitamin B12 deficiency neurological syndrome.Nutr.Neurosci16(1), 39-46 (2013).
- Stott DJ,MacIntosh G, Lowe GD,et al.Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease.Am. J. Clin.Nutr82(6), 1320-1326 (2015).
- Chobanian AV,Bakris GL, Black HR,et al.The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report.JAMA289(19), 2560-2572 (2003).
- Garber AJ, Abrahamson MJ,Barzilay JI,et al.Consensus Statement By The American Association Of Clinical Endocrinologists And American College Of Endocrinology On The Comprehensive Type 2 Diabetes Management Algorithm - 2015 Executive Summary.Endocr.Pract 21(12), 1403-1414 (2015).
- Levey AS, Bosch JP, Lewis JB,et al.A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med130(6),461-470 (1999).
- Snyder S,Pendergraph B. Detection and evaluation of chronic kidney disease.Am. Fam. Physician72(9), 1723-1732 (2005).
- Cannon CP,Brindis RG,Chaitman BR, et al.2013 ACCF/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes and coronary artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Acute Coronary Syndromes and Coronary Artery Disease Clinical Data Standards).Circulation127(9), 1052-1089 (2013).
- AndresE,LoukiliNH, Noel E,et al.Vitamin B12 (cobalamin) deficiency in elderly patients.CMAJ171(3), 251-259 (2004).
- Wulffele MG,Kooy A,Lehert P,et al.Effects of short-term treatment with metformin on serum concentrations of homocysteine, folate and vitamin B12 in type 2 diabetes mellitus: a randomized, placebo-controlled trial.J. Intern. Med254(5), 455-463 (2003).
- Dharmarajan TS,Kanagala MR,Murakonda P, et al.Do acid-lowering agents affect vitamin B12status in older adults?J. Am. Med. Dir.Assoc9(3), 162-167 (2008).
- Annibale B,Lahner E,Fave GD. Diagnosis and management of pernicious anemia.Curr.Gastroenterol. Rep 13(6), 518-524 (2011).
- Fischbach F, Dunning MB. Manual of Laboratory and Diagnostic Tests. 8th ed. Lippincott Williams & Wilkins, Philadelphia (2008)
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules.Neurology43(11), 2412-2414 (1993).
- Shyu YI, Yip PK. Factor structure and explanatory variables of the Mini-Mental State Examination (MMSE) for elderly persons in Taiwan. J.Formos. Med. Assoc100(10), 676-683 (2001).
- Lin KN, Wang PN, Liu HC,et al.Cognitive Abilities Screening Instrument, Chinese Version 2.0 (CASI C-2.0): administration and clinical application.Acta. Neurol. Taiwan21(4), 180-189 (2012).
- Clark CM, Sheppard L,Fillenbaum GG,et al.Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer's disease.Arch.Neurol56(7), 857-862 (1999).
- Scheltens P,Barkhof F, Leys D,et al.A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging.J. Neurol.Sci114(1), 7-12 (1993).
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika52, 591–611 (1965).
- Jacob C. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates (1988)
- Levene H. Robust tests for equality of variances. In: Ingram Olkin, Harold Hotelling, editors. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling. Stanford University Press, 278-292 (1960).
- Kaferle J,Strzoda CE. Evaluation of macrocytosis.Am. Fam. Physician79(3), 203-208 (2009).
- Goldstein LB, Jones MR,Matchar DB,et al.Improving the reliability of stroke subgroup classification using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria.Stroke 32(5), 1091-1098(2001).
- Donofrio PD, Albers JW. AAEM minimonograph#34: polyneuropathy: classification by nerve conduction studies and electromyography.Muscle.Nerve13(10), 889-903 (1990).
- Snowdon DA, Tully CL, Smith CD,et al.Serum folate and the severity of atrophy of the neocortex in Alzheimer disease: findings from the Nun study.Am. J. Clin.Nutr71(4), 993-998 (2000).
- Hassing L,Wahlin A,Winblad B,et al.Further evidence on the effects of vitamin B12 and folate levels on episodic memory functioning: a population-based study of healthy very old adults.Biol. Psychiatry45(11), 1472-1480 (1999).
- Coppen A,Bolander-Gouaille C. Treatment of depression: time to consider folic acid and vitamin B12.J.Psychopharmacol19(1), 59-65 (2005).
- Morris MS, Jacques PF, Rosenberg IH,et al.Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification.Am. J. Clin.Nutr85(1), 193-200 (2007).
- Morris MC Evans DA,Bienias JL, Tangney CC,et al.Dietary folate and vitamin B12 intake and cognitive decline among community-dwelling older persons. Arch.Neurol62(4), 641-645 (2005).
- Kuller LH, Lopez OL, Newman A, et al.Risk factors for dementia in the cardiovascular health cognition study.Neuroepidemiology22(1), 13-22 (2003).
- Debette S,Beiser A,DeCarli C, et al.Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study.Stroke41(4), 600-606.
- Jokinen H,Kalska H,Ylikoski R, et al.Longitudinal cognitive decline in subcortical ischemic vascular disease--the LADIS Study.Cerebrovasc. Dis27(4), 384-391 (2009).
- Tu MC, Huang CW, Chen NC, et al.Hyperhomocysteinemia in Alzheimer dementia patients and cognitive decline after 6 months follow-up period.Acta. Neurol. Taiwan19(3), 168-177 (2010).
- SeoSW, Lee JM,Im K, et al.Cortical thinning related to periventricular and deep white matter hyperintensities.Neurobiol. Aging33(7), 1156-1167 (2012).
- Simpson JE,Ince PG,Higham CE, et al. Microglial activation in white matter lesions and nonlesional white matter of ageing brains.Neuropathol. Appl.Neurobiol33(6), 670-683 (2007).
- Lazarus R,Prettyman R, Cherryman G.White matter lesions on magnetic resonance imaging and their relationship with vascular risk factors in memory clinic attenders. Int. J.Geriatr. Psychiatry20(3), 274-279(2005).
- Shim YS, Yang DW, Roe CM,et al.Pathological correlates of white matter hyperintensities on magnetic resonance imaging.Dement.Geriatr.Cogn.Disord39(1-2), 92-104 (2015).
- Delano-Wood L, Bondi MW, Sacco J, et al.Heterogeneity in mild cognitive impairment: differences in neuropsychological profile and associated white matter lesion pathology.J. Int.Neuropsychol.Soc15(6), 906-914 (2009).
- vanSwieten JC, van den Hout JH, van Ketel BA,et al.Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain 114(Pt2), 761-774 (1991).
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review.Stroke28(3): 652-659 (1997).
- Fazekas F,Kleinert R,Offenbacher H, et al.Pathologic correlates of incidental MRI white matter signal hyperintensities.Neurology43(9), 1683-1689 (1993).
- Kim HJ, Cho HK, Kwon YH.Synergistic induction of ER stress by homocysteine and beta-amyloid in SH-SY5Y cells.J.Nutr.Biochem19(11), 754-761 (2008).
- Pizzolo F,Blom HJ, Choi SW, et al.Folic acid effects on s-adenosylmethionine, s-adenosylhomocysteine, and DNA methylation in patients with intermediate hyperhomocysteinemia.J. Am. Coll.Nutr30(1), 11-18 (2011).
- Behl P,Lanctot KL,Streiner DL,et al.Cholinesterase inhibitors slow decline in executive functions, rather than memory, in Alzheimer's disease: a 1-year observational study in the Sunnybrook dementia cohort. Curr. Alzheimer. Res3(2), 147-156 (2006).
- Bracco L,Bessi V,Padiglioni S,et al.Do cholinesterase inhibitors act primarily on attention deficit? A naturalistic study in Alzheimer's disease patients. J.Alzheimers. Dis40(3), 737-742 (2014).
- Sacco RL, Wolf PA,Kannel WB,et al.Survival and recurrence following stroke. The Framingham study.Stroke13(3): 290-295 (1982).
- Sage JI, Van Uitert RL. Risk of recurrent stroke in patients with atrial fibrillation and non-valvular heart disease.Stroke14(4), 537-540 (1983).
- Viitanen M, Eriksson S,Asplund K. Risk of recurrent stroke, myocardial infarction and epilepsy during long-term follow-up after stroke.Eur.Neurol28(4), 227-231 (1988).
- Hier DB,Foulkes MA,Swiontoniowski M, et al.Stroke recurrence within 2 years after ischemic infarction.Stroke22(2), 155-161 (1991).
- Wang Y, Zhao X, Liu L, et al.Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study.Stroke45(3), 663-669 (2014).
- Refsum H,Ueland PM, Nygard O,et al.Homocysteine and cardiovascular disease.Annu. Rev. Med49(1), 31-62 (1998).
- O'Donnell J, Perry DJ. Pharmacotherapy of hyperhomocysteinaemia in patients with thrombophilia.Expert.Opin.Pharmacother3(11), 1591-1598(2002).
- Woltmann HW. The nervous symptoms in pernicious anemia: an analysis of one hundred and fifty cases. Am. J. Med.Sci157(1), 400-409 (1919).
- Kim HI,Hyung WJ, Song KJ,et al.Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann. Surg.Oncol18(13), 3711-3717 (2011).
- Agamanolis DP, Chester EM Victor M,Kark JA,et al.Neuropathology of experimental vitamin B12 deficiency in monkeys. Neurology26(10), 905-914 (1976).
- Metz J, van der Westhuyzen J. The fruit bat as an experimental model of the neuropathy of cobalamin deficiency.Comp.Biochem. Physiol. A Comp.Physiol88(2), 171-177 (1987).
- Cosar A,Ipcioglu OM,Ozcan O,et al.Folate and homocysteine metabolisms and their roles in the biochemical basis of neuropsychiatry.Turk. J. Med.Sci44(1), 1-9 (2014).
- Scalabrino G. Subacute combined degeneration one century later. The neurotrophic action of cobalamin(vitamin B12) revisited.J.Neuropathol. Exp.Neurol60(2), 109-120 (2001).
- González-Gross M, Benser J, Breidenassel C, et al.Gender and age influence blood folate, vitamin B12, vitamin B6, and homocysteine levels in Europeanadolescents: the Helena Study. Nutr. Res 32(11), 817-826 (2012).
- Akanji AO, Thalib L, Al-Isa AN. Folate, vitamin B₁₂ and total homocysteine levels in Arab adolescent subjects: reference ranges and potential determinants.Nutr.Metab.Cardiovasc. Dis 22(10), 900-6 (2012).
- Lim HS, Heo YR. Plasma total homocysteine, folate, and vitamin B12 status in Korean adults.J.Nutr. Sci.Vitaminol (Tokyo) 48(4), 290-297 (2002).
- Hinds HE, Johnson AA, Webb MC, et al.Iron, folate, and vitamin B12 status in the elderly by gender and ethnicity. J. Natl. Med.Assoc103(9-10), 870-877 (2011).
- Palmas W. Effects of short-term supplementation with ascorbate, folate, and vitamins B6 and B12 on inflammatory factors and estrogen levels in obese postmenopausal women. Int. J.Vitam.Nutr. Res 76(1), 34-38 (2006).
- Berenson AB, Rahman M.Effect of hormonal contraceptives on vitamin B12 level and the association of the latter with bone mineral density.Contraception86(5), 481-487 (2012).
- Wertalik LF, Metz EN, LoBuglio AF, et al.Decreased serum B12 levels with oral contraceptive use. JAMA221(12), 1371-1374 (1972).
- Costanzi JJ, Young BK, Carmel R. Serum vitamin B12 and B12-binding protein levels associated with oral contraceptives. Tex. Rep. Biol. Med 36(1), 69-77 (1978).
- Lussana F, Zighetti ML, Bucciarelli P, et al.Blood levels of homocysteine, folate, vitamin B6 and B12 in women using oral contraceptives compared to non-users.Thromb. Res 112(1-2), 37-41(2003).
- Gardyn J, Mittelman M, Zlotnik J, et al.Oral contraceptives can cause falsely low vitamin B(12) levels. Acta.Haematol104(1), 22-24(2000).