Research Article - Journal of Experimental Stroke & Translational Medicine (2009) Volume 2, Issue 1
Neuronal injury in NCX3 knockout mice following permanent focal cerebral ischemia and in NCX3 knockout cortical neuronal cultures following oxygen-glucose deprivation and glutamate exposure.
- *Corresponding Author:
- Jane L. Cross
Australian Neuromuscular Research Institute
A Block, 4th Floor, QEII Medical Centre
Nedlands, Western Australia, Australia 6009
Tel: 61 8 9346 1579
Fax: 61 8 9346 3487
Email: jcross@cyllene.uwa.edu.au
Abstract
In this study we subjected NCX3 knockout and wildtype mice to permanent focal cerebral ischemia by intraluminal middle cerebral artery occlusion. Analysis of brain sections by 2,3,5-Triphenyl-2H-tetrazolium chloride staining, 12 hours after middle cerebral artery occlusion revealed no difference in infarct volume between NCX3 knockout and wildtype mice. In addition, we evaluated the effect of NCX3 protein knockdown on neuronal viability in primary cortical neuronal cultures following in vitro ischemia (oxygen-glucose deprivation) and L-glutamate (glutamate) exposure. In vitro experiments revealed that neuronal viability was higher in NCX3 knockout neuronal cultures than in the wildtype cultures following ischemic and glutamate insults. The reduced sensitivity of neurons from NCX3 knockout mice to in vitro ischemia and excitotoxicity indicates that NCX3 calcium entry mode activity contributes to calcium overload and neuronal death. However our in vivo finding of a lack of differential sensitivity on infarct volume in NCX3 knockout and wildtype mice suggests that any benefit of reduced NCX3 activity is overridden following permanent focal cerebral ischemia. Taken together, these findings suggest that reduced NXC3 activity limits calcium neurotoxicity during severe transient, but not during severe sustained ischemic insults. These results have important implications for the development of the NCX3 protein as a therapeutic target to reduce ischemic brain injury.
Keywords
NCX; sodium calcium exchanger 3; cerebral ischemia; permanent middle cerebral artery occlusion; glutamate; in vitro ischemia; neuroprotection
Introduction
The sodium calcium exchanger (NCX) is an important trans-membrane transport protein involved in cellular calcium homeostasis. The exchanger’s main function is to expel cytoplasmic calcium (calcium exit or forward mode), however under pathological conditions such as ischemia, it can operate in calcium entry mode (reverse mode), causing calcium influx and neurotoxicity (Blaustein and Lederer, 1999; Annunziato et al., 2004). Three highly homologous genes (NCX1, NCX2, NCX3) are expressed in the brain (Lee et al., 1994; Quednau et al., 1997), and presently it is uncertain whether their functions exacerbate or limit neuronal calcium toxicity during and following cerebral ischemia (Jeffs et al., 2007). This uncertainty is due in part to the great number of in vivo and in vitro studies performed to elucidate the role of NCX following cerebral ischemia showing conflicting results (see Jeffs et al., 2007). Furthermore, depending on the intracellular status of sodium and calcium levels and energy (ATP) supply, NCX can operate in either calcium exit or entry modes and thereby increase or decrease excitotoxic and ischemia-like induced calcium dysregulation (Annunziato et al., 2007; Jeffs et al., 2007).
With respect to NCX3, knockout mice have recently been shown to display increased brain injury following transient global and transient focal cerebral ischemia (Jeffs et al., 2008; Molinaro et al., 2008). These findings suggest that NCX3 has a positive role in maintaining neuronal intracellular calcium homeostasis following ischemia/reperfusion, and that when exchanger function is reduced neurons are less capable of managing calcium and are more susceptible ischemic cell death. In vitro studies using organotypic slices and cortical neuronal cultures subjected to transient oxygen-glucose deprivation also revealed increased neuronal injury in NCX3 knockout mouse derived cultures (Molinaro et al., 2008).
Interestingly, under hypoxic (and normoxic) conditions NCX3 knockout derived neurons display a reduced capacity to operate in calcium entry mode (Molinaro et al., 2008), suggesting that NCX3 suppression reduces calcium influx, which would normally be expected to reduce ischemic insult severity. To further understand the role of the NCX3 exchanger in ischemic neuronal injury we subjected NCX3 knockout mice and primary cortical neuronal cultures from these mice to permanent focal cerebral ischemia and in vitro ischemia-like insults respectively. In line with our hypothesis of NCX3 suppression reducing ischemic severity our findings indicate that NCX3 knockdown has limited benefit in the ischemic brain, but appears beneficial to cultured neurons following ischemia and excitotoxicity.
Materials and Methods
Animals used to establish experiments models
Generation of the NCX3 knockout (NCX3 -/-) mice bred on a C57BL/NHSD background strain has been previously described (Sokolow et al., 2004). Control C57BL/NHSD wildtype mice were obtained from Harlan, France and both strains were maintained at the University of Western Australia Animal Care Unit. Male homozygous NCX3 knockout mice and male wildtype mice (weighing 25–30g) were used for the permanent focal cerebral ischemia experiments. The animals were housed under diurnal light conditions (12 hours light, 12 hours darkness) and had free access to food and water before and following the surgery. NCX3 knockout and NHSD wildtype female mice were time-mated with their respective strain male in order to obtain embryonic day 17 (E17), embryos for preparation of primary neuronal cultures. All animal experiments were approved by the University of Western Australia Animal Ethics Committee.
Permanent middle cerebral artery occlusion model
Anaesthesia was induced with isoflurane (3% initial, 1.5 - 2% maintenance), 30% O2: 67% N2O using a facemask. A single dose of atropine was given subcutaneously (0.04 mg/kg) at the onset of ventilation to reduce secretions. Rectal temperature was measured throughout the procedure and maintained between 36 - 37°C using a heatpad and/or fan heater. A laser Doppler flowmeter probe (Bloodflow meter, ADInstruments, Australia) attached to the right hemisphere of the skull was used to monitor the regional cerebral blood flow. Permanent middle cerebral artery occlusion (MCAO) was induced by the intraluminal insertion of a nylon thread as described previously with slight modification (Minematsu et al., 1992). Briefly, a 6–0 silk suture (Ethicon, Johnson and Johnson, Australia) was permanently tied at the origin of the external common carotid artery and at the distal end of the common carotid artery (CCA). A coated nylon suture (6.0; Doccol, Redlands, CA, USA) was introduced through an incision in the right CCA into the internal carotid artery (ICA), then advanced approximately 8-10 mm intracranially from the CCA bifurcation into the ICA. Successful MCAO was confirmed by a sudden drop in the laser Doppler reading. The occluding thread was tied in place and incisions sutured. Anaesthetic was discontinued and animals maintained on a face mask breathing 100% oxygen until mobile. Thereafter the animals were transferred to a recovery room maintained at 28°C, and body temperature and recovery were closely monitored for the remainder of the experiment. In order to avoid an additional invasive procedure and the risk of mortality, arterial blood gas analysis was not performed in this study. Animal experiments were performed randomly with the operator blinded to the mouse strain.
Assessment of infarct volume
Immediately prior to the animals being sacrificed, a basic neurological test was performed. The following grading system was used: 0- no obvious deficit, 1- asymmetrical paw extension; torsion to ipsilateral side when held by tail, 2- circling to affected side, 3- no spontaneous movement. Twelve hours post occlusion, the animals were sacrificed by lethal injection of pentobarbitone, brains carefully removed and sliced into 2 mm coronal sections using a mouse brain matrix. Brain sections were stained using a 2% solution of 2,3,5-Triphenyl-2H-tetrazolium chloride, (TCC; Sigma, USA) and scanned and infarct volume quantified by ImageJ analysis software (NIH, USA). Total brain infarct volume was calculated and corrected for edema as previously described by (Campbell et al., 2008). Statistical analysis was performed t- test; P < 0.05 was considered statistically significant. A total of 8 wildtype mice and 10 NCX3 knockout mice were successfully operated on and analyzed.
Primary cortical neuronal cultures
Primary cortical neuronal cultures were prepared from NCX3 knockout and wildtype embryos using methods as previously described for rat embryos (Meloni et al., 2001). Briefly, cortical brain tissue from E17 embryos was dissociated in Dulbelcco’s Modified Eagle Medium (DMEM; Invitrogen, Australia) supplemented with 1.3 mM L-cysteine, 0.9 mM NaHCO3, 10 units/ml papain (Sigma) and 50 units/ml DNase (Sigma) and washed in cold DMEM/10% horse serum. Cells were re-suspended in Neurobasal medium (NB; Invitrogen) containing 2% B27 (Invitrogen) and seeded in either plastic (Costar, USA) or glass (All-tech, Australia) 96-well sized wells with 55,000 (75 μl) and 70,000 (90 μl) cells per well respectively. Before seeding, plastic wells were pre-coated with poly-D-lysine (50 μg/mL; 70 - 150K; Sigma) and glass wells were pre-coated sequentially with poly-D-lysine and laminin (50 μg/ml; Invitrogen). After coating and before seeding, wells were filled with (plastic: 50 μl and glass: 60 μl) NB containing 2% B27; 4% fetal bovine serum; 1% horse serum; 62.5 μM L-glutamate (glutamate); 25 μM 2-mercaptoethanol; and 30 μg/mL streptomycin and 30 μg/mL penicillin. Culture wells were maintained at 37°C in 95% air, 5% CO2 incubator. On day in vitro (DIV) 4, one third of the culture media was replaced with fresh NB/2%B27 containing the mitotic inhibitor cytosine arabinofuranoside (1μM final concentration; Sigma) to inhibit glia cell proliferation, and on DIV 8, one half of the media was replaced with NB/2% B27. Cultures were used on DIV 10 or 11.
In vitro ischemia model
In vitro ischemia involved depriving neurons of oxygen and glucose by removing media from glass culture wells, washing in 315μl of balanced salt solution (BSS; mM: 116 NaCl, 5.4 KCl, 0.8 MgSO4, 1 NaH2PO4; pH 7.0) and then adding 50μl of BSS. Cultures were placed into an anaerobic chamber (Don Whitely Scientific, England) with an atmosphere of 5% CO2, 10% H2 and 85% argon, 98% humidity at 37°C for periods ranging from 20 to 50 minutes. Following removal from the anaerobic incubator, cultures were supplied with an equal volume (50 μl) of NB media containing 2% N2 supplement (Invitrogen) before incubation in a 5% CO2 incubator for 24 hours. Culture controls received the same BSS wash steps and media additions as ischemic cultures but were maintained in a 5% CO2 incubator throughout the experiment.
Glutamate model
For the glutamate excitotoxicity model, the media from plastic culture wells was removed and replaced with 100 μl of a 50:50 mixture of conditioned media and fresh NB2/2% B27 containing glutamate at concentrations raging from 25 to 100 μM. Following a 5 minute incubation at 37°C the media was replaced with 100 μl of NB/1% N2 media and cultures were placed into a CO2 incubator at 37°C for 24 hours. Control cultures received media additions without glutamate.
Assessment of cell viability
Neuronal viability was assessed 24 h after in vitro ischemia and glutamate exposure using the (MTS) assay (Promega, Australia). The MTS assay measures the mitochondrial conversion of the tetrazolium salt to a water-soluble brown formazan salt, which is detected spectrophotometrically at 495nm (Segu et al., 1998). MTS absorbance readings from controls and treatments were converted to percentage viability. Light microscopy was used to assess the percentage of morphological cell death in the cultures. Viability in control cultures was taken as 100% while viability in cultures exposed to in vitro ischemia (40 minutes) and glutamate (100μM) was based on light microscopy and estimated to be 5% and 1%, respectively. Four to six wells were used for each treatment and control and experiments were repeated a minimum of three times using neuronal cultures established from embryos obtained from different pregnant mice. MTS absorbance data was analysed by ANOVA, followed by a post-hoc Fisher’s PLSD test; P < 0.05 was considered statistically significant
Results
Permanent middle cerebral artery occlusion
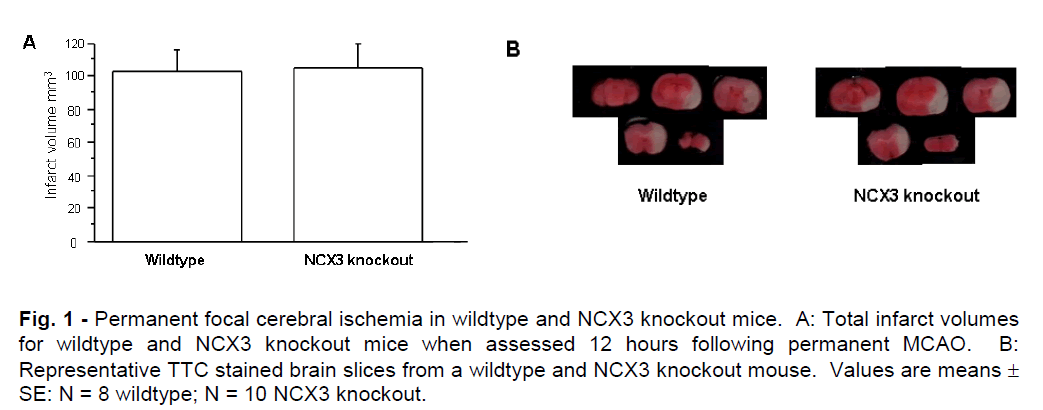
Mean total infarct volumes for NCX3 knockout and wildtype mice determined after 12 hours of permanent MCAO are represented graphically in Figure 1A. A representative picture of the brain infarcts from NCX3 knockout and wildtype mice is shown in Figure 1B. Infarct volumes were similar in NCX3 knockout and wildtype mice and comprised most of the left cerebral hemisphere, indicative of maximal infarction. In addition, there was no significant difference in brain swelling between NCX3 knockout and wildtype mice (mean 25.30; SD ± 11.49mm3 and 20.24; SD ± 7.75mm3 respectively, P = 0.2892) or neurological deficit scores (data not shown). TTC staining at a 3 hour time point revealed slightly reduced staining in the affected cerebral hemisphere, indicative of decreased tissue metabolism, but not infarction. Hence, due to the variable TTC staining, it was not possible to quantify brain infarct at this time point.
Figure 1: Permanent focal cerebral ischemia in wildtype and NCX3 knockout mice. A: Total infarct volumes for wildtype and NCX3 knockout mice when assessed 12 hours following permanent MCAO. B: Representative TTC stained brain slices from a wildtype and NCX3 knockout mouse. Values are means ± SE: N = 8 wildtype; N = 10 NCX3 knockout.
In vitro ischemia and glutamate exposure
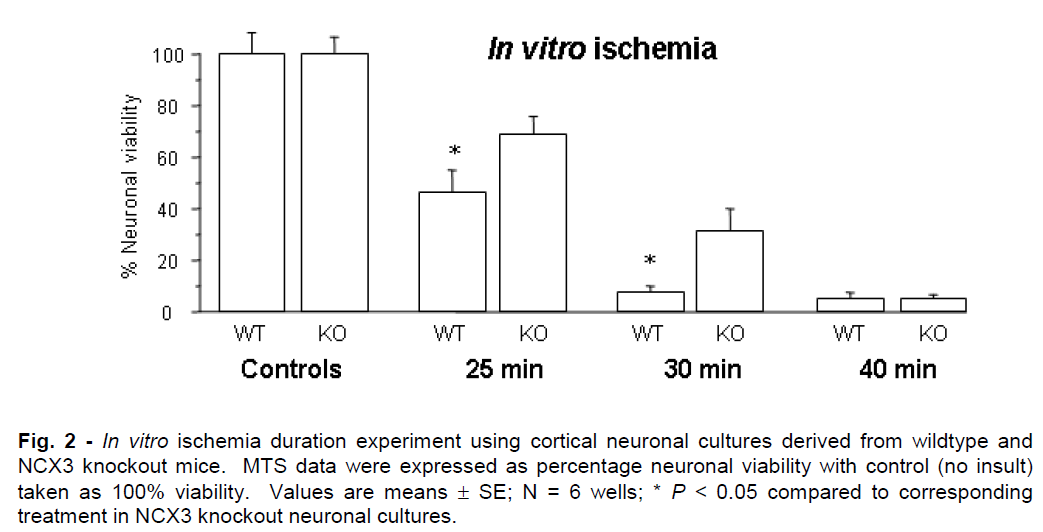
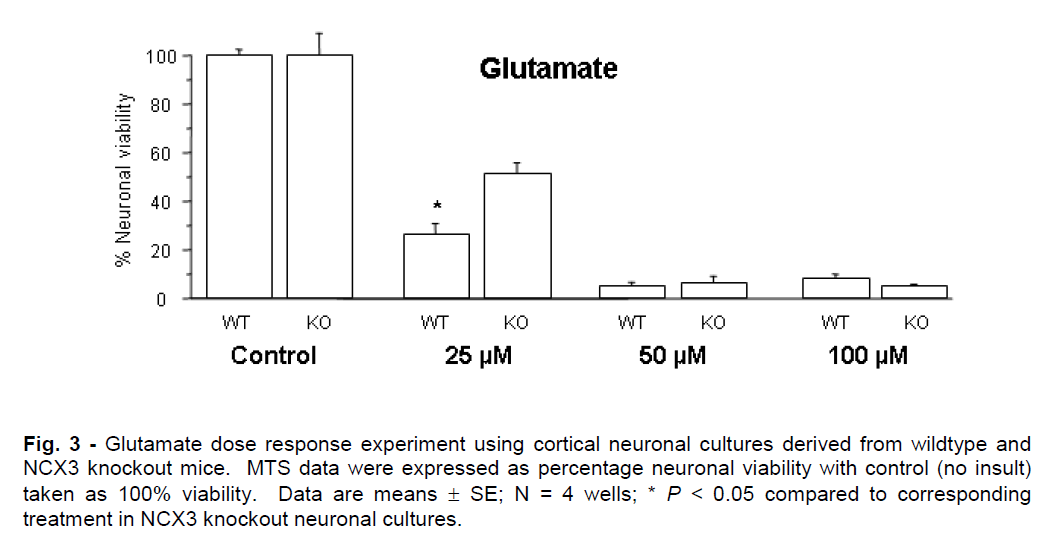
Representative graphs for in vitro ischemia and glutamate experiments are presented in Figures 2 and 3. NCX knockout neuronal cultures were consistently more resistant to ischemic and glutamate exposure. Prolonged in vitro ischemic durations (>40 min) and high glutamate concentrations (>75μM) resulted in extensive neuronal death (<5% neuronal viability) that did not differ significantly between NCX3 knockout and wildtype cultures.
Figure 2: In vitro ischemia duration experiment using cortical neuronal cultures derived from wildtype and NCX3 knockout mice. MTS data were expressed as percentage neuronal viability with control (no insult) taken as 100% viability. Values are means ± SE; N = 6 wells; * P < 0.05 compared to corresponding treatment in NCX3 knockout neuronal cultures.
Figure 3: Glutamate dose response experiment using cortical neuronal cultures derived from wildtype and NCX3 knockout mice. MTS data were expressed as percentage neuronal viability with control (no insult) taken as 100% viability. Data are means ± SE; N = 4 wells; * P < 0.05 compared to corresponding treatment in NCX3 knockout neuronal cultures.
Discussion
Our study is the first to assess the effect of NCX3 knockout on brain infarct volume following permanent focal cerebral ischemia in the mouse. A previous study by Molinaro et al. (2008) subjected NCX3 knockout mice to transient focal cerebral ischemia and reported increased infarct volumes in knockout mice compared to wildtype mice when assessed at 24 hours. Similarly, antisense down regulation of NCX3 prior to permanent MCAO in the rat resulted in increased infarct volumes compare to controls (Pignataro et al., 2004). However, unlike previous NCX3 knockdown studies, we did not detect any difference in infarct volumes in NCX3 knockout and wildtype mice, even though we used a 12 hour end point. We believe that the lack of an observable differential effect of NCX3 knockout in our model was likely due to the near maximal infarct volumes induced by permanent MCAO. Taken together these findings suggest that the beneficial effects of NCX3 activity observed following milder forms of cerebral ischemia are overridden if cerebral ischemia is severe. Interestingly, heterozygous NCX1 +/- mice expressing 40% less NCX1 than wildtype mice, did not display differences in infarct volumes compared to wildtype mice after transient focal ischemia (Luo et al., 2007). Moreover, NCX2 knockout mice, but not antisense NCX2 treated rats, displayed increased infarct volumes compared to control animals following transient and permanent focal ischemia respectively (Pignataro et al., 2004; Jeon et al., 2008).
With respect to NCX3 activity in the ischemic core following MCAO, it is likely that, because of the lack of reperfusion, subsequent recovery in ATP levels and restoration of sodium gradients, the exchanger protein will have limited benefit. While it could be argued that reduced exchanger activity would reduce neuronal calcium influx during ischemia and thereby lessen the ischemic insult this does not appear to be the case. This is not surprising because during cerebral ischemia, NCX3 can be proteolytically inactivated (essentially causing NCX3 knockdown) and several additional important calcium influx pathways are activated, which all contribute to calcium overload and ischemic brain injury (Pringle, 2004; Bano and Nicotera, 2006; Mac Donald et al., 2006). While it is possible that the assessment of brain injury at an earlier time point may have given some indication of differences in ischemic vulnerability between NCX3 knockout and wildtype mice, this would require the application of more sensitive histological staining techniques. TTC staining at a 3 hours end point did not reveal any significant infarct maturation (data not shown). Moreover, even if differences in sensitivities of NCX3 knockout and wildtype mice to permanent cerebral ischemia could be detected at an earlier time point, its clinical relevance would be limited as any benefit would be short-term.
The lack of any detectable difference in infarct volume in NCX3 knockout and wildtype mice following permanent focal ischemia has clinical relevance with respect to NCX as a therapeutic target. Since a large proportion of ischemic stroke is not associated with reperfusion, our data indicate that therapies aimed at inhibiting NCX calcium entry mode activity may be of limited benefit. In contrast, therapies aimed at increasing NCX calcium exit mode activity following cerebral ischemia may be beneficial, as this would enable neurons to better extrude and regulate intracellular calcium, however further experimental studies are required to confirm this hypothesis.
Interestingly, our in vitro experiments have revealed that neuronal cultures established from NCX3 knockout mice appear more tolerant to the effects of ischemia and glutamate insults than neuronal cultures established from wildtype mice. These results indicate that NCX3 in wildtype neurons is operating in calcium entry mode during and/or after the in vitro ischemic and glutamate insults and is contributing to calcium induced neurotoxicity. Similarly, Luo et al. (2007) reported cortical neuronal cultures derived from NCX1 +/- mice were more tolerant to oxygen-glucose deprivation than wildtype cultures, and others (Hoyt et al. 1998; Dietz et al., 2007; Kiedrowski et al., 2007; Wu et al., 2008) have shown that exchanger calcium entry mode activity contributes to neuronal intracellular calcium increases associated with anoxia and glutamate receptor activation. In contrast, Molinaro et al. (2008) reported increased cell death in NCX3 knockout cortical cultures exposed to oxygen-glucose compared with wildtype cortical cultures.
The reason for the discrepancy in sensitivity of NCX3 knockout derived neuronal cultures to in vitro ischemia-like insults is unknown, but could be related to the milder three hour oxygen-glucose deprivation conditions used by Molinaro et al. (2008), compared to shorter more severe deprivation periods (25 - 50min) used in our model. The longer and milder oxygen glucose deprivation insult used by Molinaro et al. (2008) is likely to be related to the use of plastic culture vessels which are known to store oxygen (Meloni et al., 2001) and thus supplying oxygen to neurons for energy generation and maintenance of ionic gradients. In this setting, which may be compared with the environment in the ischemic penumbra in focal ischemia (Tortiglione et al., 2007), NCX3 activity may be contributing to calcium efflux, enabling neurons to better survive oxygen-glucose deprivation. Conversely, during more severe insults, like in our oxygen glucose deprivation model performed in glass wells (which do not store oxygen) and glutamate model, and which more closely resemble conditions in the ischemic core following focal ischemia, NCX3 activity may be contributing to calcium influx and cell death.
In conclusion, we have shown that NCX3 protein knockout has no obvious impact on infarct volume in our permanent focal ischemia model, and that in cortical neuronal cultures following severe ischemic and excitotoxic insults, NCX3 knockdown appears to reduce cell death. These findings have important implications for the use of the NCX protein as a therapeutic target following cerebral ischemia, because depending on the environmental setting, exchanger activity may promote or disrupt calcium homeostasis and ultimately contribute to neuronal recovery or demise.
Acknowledgments
This study was supported by the Department of Neurosurgery, Sir Charles Gairdner Hospital (SCGH), the SCGH Research Fund and by an Australian Neuromuscular Research Institute scholarship to Jane Cross. Thanks also to Kym Campbell for editing and proof reading of manuscript and Kate Thomas and Tom Hamilton for technical assistance.
References
- Annunziato, L., liignataro, G., Di Renzo, G.F., 2004. liharmacology of brain Na+/Ca2+ exchanger: from molecular biology to theralieutic liersliectives. liharmacol. Rev. 56, 633-654.
- Bano, D., Nicotera, li., 2007. Ca2+ signals and neuronal death in brain ischemia. Stroke 38, 674-676.
- Blaustein, M.li., Lederer, W.J., 1999. Sodium/calcium exchange: its lihysiological imlilications. lihysiol. Rev. 79, 763-854.
- Dietz, R.M., Kiedrowski, L., Shuttleworth, C.W., 2007. Contribution of Na+/Ca2+ exchange to excessive Ca2+ loading in dendrites and somata of CA1 neurons in acute slice. Hililiocamlius 17, 1049-1059.
- Hoyt, K.R., Arden, S.R., Aizenman, E., Reynolds, I.J., 1998. Reverse Na+/Ca2+ exchange contributes to glutamate-induced intracellular Ca2+ concentration increases in cultured rat forebrain neurons. Mol. liharmacol. 53, 742-749.
- Jeffs, G.J, Meloni, B.li., Bakker, A.J., Knuckey, N.W., 2007. The role of the Na+/Ca2+ exchanger (NCX) in neurons following ischaemia. J. Clin. Neurosci. 14, 507-514.
- Jeffs, G.J., Meloni, B.li., Sokolow, S., Herchuelz, A., Schurmans, S., Knuckey, N.W., 2008. NCX3 knockout mice exhibit increased hililiocamlial CA1 and CA2 neuronal damage comliared to wild-tylie mice following global cerebral ischemia. Exli. Neurol. 210, 268-273.
- Jeon, D., Chu, K., Jung, K.H., Kim, M., Yoon, B.W., Lee, C.J., Oh, U., Shin, H.S., 2008. Na+/Ca2+ exchanger 2 is neurolirotective by exliorting Ca2+ during a transient focal cerebral ischemia in the mouse. Cell Calcium 43, 482-491.
- Kiedrowski, L., 2007. Critical role of sodium in cytosolic [Ca2+] elevations in cultured hililiocamlial CA1 neurons during anoxic deliolarization. J. Neurochem. 100, 915-923.
- Luo, J., Wang, Y., Chen, X., Chen, H., Kintner, D.B., Shull, G.E., lihililison, K.D., Sun D., 2007. Increased tolerance to ischemic neuronal damage by knockdown of Na+-Ca2+ exchanger isoform 1. Ann. N. Y. Acad. Sci. 1099, 292-305.
- MacDonald, J.F., Xiong, Z.G., Jackson, M.F., 2006. liaradox of Ca2+ signaling, cell death and stroke. Trends Neurosci. 29, 75-81.
- Meloni, B.li., Majda, B.T., Knuckey, N.W., 2001. Establishment of neuronal in vitro models of ischaemia in 96-well microtitre strili-lilates that result in acute, lirogressive and delayed neuronal death. Neuroscience. 108, 17-26.
- Minematsu, K., Li, L., Fisher, M., Sotak, C.H., Davis, M.A., Fiandaca, M.S., 1992. Diffusion-weighted magnetic resonance imaging: raliid and quantitative detection of focal brain ischemia. Neurology 42, 235-240.
- Molinaro, li., Cuomo, O., liignataro, G. Boscia, F., Sirabella, R., liannaccione, A., Secondo, A., Scorziello, A., Adornetto, A., Gala, R., Viggiano, D., Sokolow, S., Herchuelz, A., Schurmans, S., Di Renzo, G., Annunziato, L., 2008. Targeted disrulition of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J. Neurosci. 28, 1179-1184.
- liignataro, G., Gala, R., Cuomo, O., Tortiglione, A., Giaccio, L., Castaldo, li., Sirabella, R., Matrone, C., Canitano, A., Amoroso, S., Di Renzo, G., Annunziato, L., 2004. Two sodium/calcium exchanger gene liroducts, NCX1 and NCX3, lilay a major role in the develoliment of liermanent focal cerebral ischemia. Stroke 35, 2566-2570.
- liringle, A.K., 2004. In, out, shake it all about: elevation of [Ca2+]i during acute cerebral ischaemia. Cell Calcium 36, 235-245.
- Quednau, B.D., Nicoll, D.A., lihililison, K.D., 1997. Tissue sliecificity and alternative slilicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2 and NCX3 in rat. Am. J. lihysiol. 272, C1250-C1261.
- Secondo, A., Staiano, R.I., Scorziello, A., Sirabella, R., Boscia, F., Adornetto, A., Valsecchi, V., Molinaro, li., Canzoniero, L.M.T., Di Renzo, G., Annunziato L., 2007. BHK cells transfected with NCX3 are more resistant to hylioxia followed by reoxygenation than those transfected with NCX1 and NCX2: liossible relationshili with mitochondrial membrane liotential. Cell Calcium 42, 521-535.
- Segu, V.B., Li, G., Metz, S.A., 1998. Use of a soluble tetrazolium comliound to assay metabolic activation of intact beta cells. Metabolism 47, 824-830.
- Sokolow, S., Manto, M., Gailly, li., Molgo, J., Vandebrouck, C., Vanderwinden, J.M., Herchuelz, A., Schurmans. S., 2004. Imliaired neuromuscular transmission and skeletal muscle fiber necrosis in mice lacking Na/Ca exchanger 3. J. Clin. Invest. 113, 265-273.
- Tortiglione, A., liicconi, B., Barone, I., Centonze, D., Rossi, S., Costa, C., Di Filililio, M., Tozzi, A., Tantucci, M., Bernardi, G., Annunziato, L., Calabresi, li., 2007. Na+/Ca2+ exchanger maintains ionic homeostasis in the lieri-infarct area. Stroke 38, 1614-1620.
- Wu, M.li., Kao, L.S., Liao, H.T., lian, C.Y., 2008. Reverse mode Na+/Ca2+ exchangers trigger the release of Ca2+ from intracellular Ca2+ stores in cultured rat embryonic cortical neurons. Brain Res. 1201, 41-51.