Review Article - Interventional Cardiology (2012) Volume 4, Issue 1
New and evolving indications for transcatheter aortic valve therapy
- Corresponding Author:
- Jonathan Byrne
Department of Cardiology Kings College Hospital, London, UK
E-mail: jonathan.byrne@nhs.net
Abstract
Keywords
access routes, transcatheter aortic valve therapy, transcatheter, valve-in-valve therapy
The development of transcatheter aortic valves has dramatically changed the landscape within cardiology and cardiothoracic surgery. Although surgical aortic valve replacement (AVR) remains the ‘gold standard’ for treatment of severe aortic stenosis (AS), high operative mortality (in certain populations [1]) has confined a significant proportion of patients to medical therapy. In 2002, the European Heart Survey found that 30% of patients were not offered AVR due to significant comorbidity; without definitive treatment, prognosis in this group of patients is dismal [2–4]. It is important to note that this survey only includes the patients referred for surgical assessment; a considerable number will not be referred because of the perceived risk of surgical AVR. Transcatheter aortic valve therapy (TAVI) was initially developed to address the unmet clinical need in this population of patients. There are currently two CE marked devices; the self-expanding CoreValve® system (Medtronic Inc., MN, USA) and the balloon expandable Edwards SAPIEN, now available as the smaller profile SAPIEN XT™ device (Edwards Lifesciences Inc., CA, USA). The evidence for their efficacy comes from a large number of registries across Europe and North America. The SAPIEN device also has strong supporting data from the randomized US trials, PARTNER A and B [5,6]. These studies demonstrate a prognostic benefit of TAVI in the inoperable cohort (PARTNER B), and equivalent outcomes when compared with conventional surgery in high-risk patients (PARTNER A).
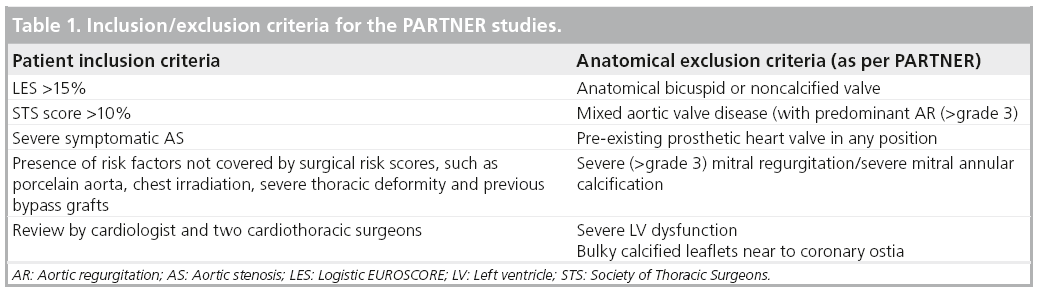
The decision to undertake TAVI is dependent on a number of factors, including selection of appropriate patients, choice of delivery route and prosthesis size and type. Initial studies mandated strict inclusion criteria for TAVI, with a number of contraindications. Some of these indications and contraindications are patientrelated, whereas others are more device specific and it is these conventional criteria that have formed the basis of international consensus documents regarding the use of this therapy [4]. As an example, Table 1 outlines the important inclusion and exclusion criteria for the PARTNER studies. As the use of TAVI has become more widespread and operator experience has grown, the indications and applications of the technology have broadened to include other patient groups. Indeed, it has become increasingly clear that many patients undergo TAVI in situations that fall outside the conventional criteria. This review will explore the indications and outcomes of the growing number of patients undergoing TAVI for reasons other than a logistic EUROSCORE (LES)of >15 and/or a Society of Thoracic Surgeons Score (STS) of >10 [7,8].
Patient selection, risk assessment & TAVI in apparently ‘lower risk’ cohorts
Appropriate patient selection remains the sine qua non of any successful TAVI service. A multidisciplinary approach is currently used, involving the expertise of cardiac surgeons, cardiologists, cardiac anesthetists, geriatricians and nurse specialists. In the very high-risk patient cohort, there is little controversy regarding the effectiveness of TAVI. The PARTNER B study examined outcomes in 358 patients who were deemed inoperable and were randomized to standard medical therapy (including balloon aortic valvuloplasty) or TAVI. TAVI conferred a prognostic benefit, with an absolute reduction in mortality of 20% at 1 year [5]. A second arm of the PARTNER study (Cohort A), included approximately 700 patients who were deemed operable, but had a high surgical risk score (30-day mortality >15%). Patients were randomized to receive either TAVI or conventional surgery. Periprocedural and 1-year outcomes were similar between the groups and there was a similar improvement in symptoms [6]. There was an important trend towards increased neurological events (including both stroke and transient ischemic attack) in the TAVI arm of the study, although the rate of major stroke was similar in both groups. The rate of vascular complications was higher in the transcatheter (transfemoral [TF]) group (18 vs 4.8% in the surgical cohort). The incidence of vascular complications mirror those observed in the SOURCE registry [9], although it must be emphasized that the transcatheter devices used in both studies were much larger than those being used presently in Europe (22–24 Fr for TF procedures, compared with 16–18 Fr currently). It is certainly reasonable to assume that the rate of vascular complications will fall as the device profile reduces, assuming that interventional cardiologist, follow the guidelines regarding vessel size and do not ‘push the envelope’.
The accurate assessment of patient risk remains a pressing priority for patients being considered for transcatheter therapy. Currently, two scoring systems are used (derived from large retrospective registries), the LES and the STS-predicted risk of mortality (STS-PROM). These scoring systems long predate the development of catheter valve therapy and there are major question marks over their applicability in the TAVI era. LES appears to be particularly inaccurate in high-risk populations, precisely those being assessed for potential transcatheter therapy [10–12]. The STS-PROM appears to be more accurate in high-risk patients and although more applicable to the TAVI cohort, remains far from perfect. This was perhaps best demonstrated in Cohort A of the PARTNER study, where the predicted STS score was far closer to the observed mortality than the LES [10]. Surgery still performed better than expected even with STS-PROM, but it remains unclear whether this was due to greater surgical expertise, or a failure of risk stratification. When considering an individual patient for a transcatheter procedure, a number of other variables should be considered; particularly frailty and comorbidities not included in standard risk scoring systems, such as chest deformity, previous radiation exposure or liver disease (although some of these are due to be included in the new STS-PROM algorithm). However, the vagaries of current risk scoring systems underline the importance of the multidisciplinary approach to patient assessment. The LES and STS assessment are only part of this process and the decision whether to undertake TAVI, rather than conventional surgery, is jointly made by the ‘heart team’. In the elderly patient, frailty remains a largely subjective assessment at present, but the development of a ‘frailty index’ is certainly an attractive concept. Although a number of parameters have been proposed, none have been prospectively validated for the TAVI population. Recently, slow gait speed has been examined in patients undergoing cardiac surgery [13]. In this study, 5-metre gait speed was shown to be an accurate and incremental predictor of mortality and morbidity in elderly patients undergoing cardiac surgery when used in addition to standard scoring systems and could potentially be extended for use in the TAVI cohort [13].
A further important and unanswered question, is whether TAVI should be performed in moderate- or low-risk cohorts. In light of the impressive surgical outcomes observed in the PARTNER A study, a widespread shift to transcatheter valve therapy in lower risk groups is unlikely at present. However, a more accurate individualized patient assessment, with a more sensitive scoring algorithm is likely to extend, rather than reduce, the numbers of eligible patients. It is also salient to point out that the current randomized data from the PARTNER A cohort includes early ‘learning curve’ experience with transcatheter therapy. An intriguing feature of the PARTNER trial was the dramatic difference between 30-day and 1-year mortality. This was noted in both the surgical (6.5 vs 26.8%, respectively) and the transcatheter cohorts (3.4 vs 24.2%, respectively) [6], findings that were mirrored in the SOURCE registry [9]. Importantly, only 25% of the deaths in the latter were classified as cardiac in origin and it is clear that patient comorbidity was the major driver of mortality at 1 year. Intriguingly, whilst the LES was a poor discriminator for procedural outcome, a lower LES did predict improved 1-year survival. Leontyev et al. reported a similar finding in octogenarian patients undergoing surgical AVR [14]. In this cohort of 282 patients, there was a linear relationship between the LES and medium term outcome. For lower-risk cohorts to be treated with TAVI, a far more refined method of risk assessment will be needed. It is likely that some of this information will come from analysis of the growing number of TAVI registries. Furthermore, technical advances in device technology will also lead to smaller delivery systems and refinement of percutaneous and surgical TAVI techniques, leading to an overall reduction in procedural risk. In time, it is likely that this, along with more refined patient selection, will extend the use of the device into lower-risk cohorts.
Cost–effectiveness is of key importance in the assessment of TAVI and will be a major determinant of its use, or lack of, in a lower-risk cohort. In those patients with no surgical option, the 20% reduction in mortality observed in the PARTNER B study was dramatic and was also associated with a 50% reduction in hospitalization rates [5]. These figures translate very readily into a costeffective treatment in this population. However, the PARTNER A study demonstrated equivalence in a population at high surgical risk [6]. With no difference in mortality when compared with the surgical arm, TAVI is unlikely to be a cost-effective treatment in this group of patients at present. However, in addition to the technical development of transcatheter devices, it is very likely that procedural costs will also diminish. This is certain to affect any future cost–effective analysis.
Anatomical considerations
The anatomy of the aortic valve and its relationship with the annulus and aortic root is closely examined when considering transcatheter valve implantation. Eccentric calcification, significant aortic regurgitation (AR) and the presence of a bicuspid aortic valve are often considered unfavorable for TAVI, but with careful selection, many of these patients can be successfully treated.
Bicuspid aortic valves
The presence of a bicuspid aortic valve is common, with a prevalence of 0.9–2% in the general population and accounts for a large number of patients presenting for conventional AVR. It has previously been considered a relative contraindication to transcatheter valve deployment, due to concerns about poor valve seating and paravalvular regurgitation, particularly with distortion of the native valve leaflets. However, TAVI is an option in some patients with bicuspid valves (particularly those that are functionally rather than anatomically biscupid), although case selection remains critical. In particular, the elliptical nature of the annulus often makes the choice of valve size more difficult. Wijesinghe et al. reported a case series of 11 patients with bicuspid valves, who underwent implantation with SAPIEN valves using a mixture of TF and transpical approaches [15]. Valve positioning was excellent in the majority of cases with no major AR. In general, the suitability of a bicuspid valve for TAVI will depend on the maximal size of the annulus and the degree of valvular calcification. A large, elliptical annulus with minimal or asymmetric calcification should still be treated with caution when considering TAVI. It must also be mentioned that the current available data relates only to the Edwards device; whether the self-expanding CoreValve can also be used safely and effectively in bicuspid aortic valves, remains unclear at present. It is important to note that if TAVI is adopted for the treatment of a younger population, the incidence of bicuspid valves will increase dramatically.
Severe AR
The presence of severe native valve AR (as the primary pathology) has been considered a contraindication to TAVI implantation. Both the Edwards and (to a lesser extent) CoreValve devices require a certain degree of calcification to anchor the valve. Furthermore, the annulus is usually enlarged to a greater degree in the presence of severe AR and a larger device is likely to be required. The presence of a large annulus with minimal or no calcification increases concern over valve migration during the procedure. In addition, AR is often associated with a dilated ascending aorta and treatment of the valve alone is unlikely to offer a definitive solution in many patients. However, since the CoreValve is less reliant on heavy calcification to ensure safe deployment, this device has been used to successfully treat severe AR in two cases where no surgical option was available [16,17]. The ability to retrieve a dislocated device with the CoreValve deployment system is certainly an advantage, but device migration remains a very real concern. It is highly likely that until new and novel devices are developed specifically for AR, TAVI will only ever be used in the surgically unfit patient.
Prosthetic valve failure: transcatheter ‘valve-in-valve’ therapy
Perhaps one of the most exciting ‘off-label’ uses of transcatheter technology is for the treatment of bioprosthetic valve failure. Repeat cardiac surgery may carry significant risks in this population, with a reported mortality of 6–15% [18,19], particularly in elderly patients and those with extensive comorbidities. In these groups a percutaneous (or limited surgical) ‘valve-in-valve’ approach is a very attractive concept. The first experimental reports confirmed the safety and feasibility of the technique, using a transapical approach for both mitral and aortic prostheses, with excellent hemodynamic function following valve deployment and minimal paravalvular regurgitation [20]. Subsequent case reports and series have confirmed the safety and efficacy of the technique in humans [21–24]. The largest series to date using the Edwards device examined the procedural outcomes in 24 patients undergoing valve-in-valve implantation in failed aortic, mitral, pulmonary and tricuspid positions [25]. Case selection is essential; failure of the prosthetic valve may well be associated with pannus formation, thrombus and mobile, degenerated leaflets, all of which will increase the complexity and potential risk of the procedure.
Aortic ‘valve-in-valve’
There is a growing body of evidence demonstrating short-term safety and medium-term efficacy of aortic valve-in-valve procedures. The procedure appears to be effective across a wide range of different prostheses and has been used to treat stented and stentless bioprostheses [21,25]. Although the etiology of valve failure will vary, transcatheter approaches are suitable for both severe stenosis and transvalvular regurgitation, although great care must be taken to exclude significant paraprosthetic AR.
The CoreValve system was the first device used to treat prosthetic aortic valve failure and has been reported in several small series [26–28]. Gotzman et al., reported a series of five patients treated for aortic bioprosthetic failure using a TF approach with favorable hemodynamic and short-term clinical results [28].
The largest series to date, included 10 patients treated with the Edwards device, and the majority using a transapical approach. The degree of postprocedural regurgitation was minimal in all cases [25]. Clearly, the size of the bioprosthesis is important to decision-making. Normal and sustained valve function will depend, at least partly, on full expansion of the device, which may be limited when using small bioprostheses. The technology is currently limited to a small number of valve sizes, although this is likely to change in the future. Follow-up is currently limited, but a reasonable medium-term outcome has been reported, although long-term durability remains unclear [29].
Although a TF approach appears feasible, there are distinct advantages conferred by using a transapical approach for aortic valve-in-valve procedures. First, the valve can be positioned with relative ease in a coaxial fashion. Second, passage of the guidewire through the center of the valve leaflets is essential and the transapical approach allows the operator to avoid valve or stent struts.
Acute valve failure during transcatheter implantation can also necessitate a rather more urgent valve-in-valve procedure. Whilst suboptimal valve deployment may lead to occasional periprocedural complications (valve dislodgement or severe paravalvular AR), structural valve failure is far less common [30,31]. The failure of leaflets to open fully can lead to catastrophic valvular AR and hemodynamic collapse. In this situation the deployment of a further prosthesis is usually required [30,31].
Whilst the majority of aortic valve-in-valve procedures have been undertaken in failing ‘surgical’ prostheses, treatment of degenerate transcatheter valves in this manner may become more commonplace. First-generation devices are now over 5 years old and a recent report has described the successful use of a new device in a severely stenosed first-generation CoreValve prosthesis [32].
Valve-in-valve in ‘nonaortic’ positions
Valve-in-valve implantation has been reported in a number of nonaortic positions, using a number of different prosthetic devices.
▪ Mitral
Initial attempts to undertake mitral valve-invalve procedures were made using TF and direct transatrial approaches, but these were complicated by device embolization and an inability to position the valve coaxially [25]. The majority of procedures have subsequently been undertaken using a transapical approach and this mode of access offers similar advantages to the aortic procedure, primarily the ability to position the valve coaxially. Cheung et al., reported a series of 11 patients treated over a 3-year period [33]. The majority had severe transvalvular regurgitation. Procedural success was achieved in all cases with one in-hospital death and acceptable short-term results with clinical improvement were observed in all but one patient. In addition to this larger cohort, a number of successful individual cases and smaller case series has been reported [33–35]. Case selection remains vital; the presence of significant paravalvular pathology remains a contraindication to the procedure and the long-term efficacy of these newer devices in the mitral position remains uncertain. The use of the transapical approach has distinct advantages over the TF venous approach for mitral valve-invalve procedures, with coaxial device positioning and manipulation being far less predictable using the retrograde approach. Recently, the successful transapical use of a SAPIEN XT valve was reported in a patient without a bioprosthesis, who had undergone previous mitral valve repair and placement of an annuloplasty ring [36]. There are a number of devices on the horizon, specifically designed to treat failing native and prosthetic mitral valves, ranging from several direct ‘valvein- valve’ technologies (using both trans-septal and transapical approaches), to percutaneous repair of chordae and papillary muscle pathology.
▪ Pulmonary
A percutaneous approach to the failing right ventricular conduit was first developed by Bonhoeffer et al. in the late 1990s, and therefore predates the clinical application of aortic transcatheter therapy by several years [37,38]. Pulmonary conduits are tubular structures and are well suited to transcatheter valve implantation. Bonhoeffer et al. have reported medium-term outcomes on a series of 155 patients [38]. As well as excellent procedural outcomes, they also noted a relatively low rate of surgical reintervention and valve dysfunction [39]. Surgical or percutaneous reintervention was required for 24 patients over a 7-year period, although the incidence of valve failure was extremely low. A small number of devices were subject to stent fracture and required a further valve-in-valve procedure [39]. The approach in almost all cases was transvenous (primarily femoral), although a modified surgical subxiphoid approach has been used [40]. The use of an Edwards device in the pulmonary position has also been described; Boone et al. reported an excellent medium-term outcome in seven patients with pulmonary homografts, with retained valve function at 3.5 years of follow-up [41].
▪ Tricuspid
The first report of a tricuspid valve-in-valve procedure used an intercostal surgical approach to access the right atrium and enable direct coaxial positioning across the failing prosthesis [42]. A true percutaneous approach has also recently been described, with several reports outlining a similar approach from the right internal jugular vein (using a Sapien Edwards device and a Melody® pulmonary valve prosthesis [Medtronic Inc., CA, USA]) to treat failing tricuspid bioprostheses [42–45]. All three cases were successful, with no periprocedural complications. Whether rapid pacing is required during right sided valve deployment remains open to interpretation; right heart pressures are low, and valve movement during deployment is likely to be a less important issue when compared with intervention in the left heart. Again, the long-term durability of these devices within existing bioprostheses remains unknown.
TAVI in the presence of existing mitral prostheses
There are a number of technical concerns regarding TAVI in the presence of an existing mitral valve prosthesis. During deployment, the rigid prosthetic mitral struts may reduce stability and increase the risk of device embolization, during or following deployment. Furthermore, close interaction between the valve struts, over time, has the potential to reduce valve durability. Rodés-Cabau et al. first described the use of a transapical approach to place an Edwards prosthesis, although a TF balloon valvuloplasty was used initially to define the interaction with the mitral prosthesis [46]. Bruschi et al. described a series of four TF cases using the CoreValve device with similar acute procedural success and no compromise to either aortic or mitral prosthetic valve function [47]. Positioning of the transcatheter prosthesis is more challenging and there is a tendency for the device to move forwards into the left ventricular outflow tract during deployment. The degree of movement will also vary according to the type of mitral bioprosthesis in place; Soon et al. reported a series of ten patients with both mechanical and bioprosthetic mitral prostheses, all of whom underwent transapical Edwards valve implantation. Mechanical valves had less material protruding into the left ventricular outflow tract and balloon and device displacement was less commonly seen when compared with the bioprosthetic valves [48]. Positioning of the transcatheter valve may initially be more ‘ventricular’ when compared with standard TAVI deployment, to avoid excessive movement and potential embolization of the device into the outflow tract [48]. The behavior of the vavluloplasty balloon prior to valve deployment will also give a useful insight into the interaction between the existing prosthesis and the transcatheter device during implantation, in order to guide accurate valve positioning.
Alternative access routes for TAVI implantation
The two currently available devices are licensed for use via the TF or transapical (TA) approaches in the case of the Edwards device, and the TF route for the CoreValve system. However, there are a number of patients who are not suitable for conventional forms of transcatheter access. In some, the presence of diffuse and generalised peripheral vascular disease prevents the use of the TF route. In patients with severe left ventricular dysfunction, or extensive apical scarring, use of the transapical approach is associated with increased procedural risk and may contribute to an increase in postprocedural morbidity.
Transaortic access
A direct, transaortic (TAo) approach was initially described in a patient with severe kyphoscoliosis who was unsuitable for a femoral or apical approach [49]. A mini-sternotomy allowed direct ascending aortic puncture, passage of a transapical delivery system and valve deployment. In a subsequent series, the TAo approach was used in a cohort of 17 patients, the majority of whom had severe lung disease or marked chest wall deformities [50]. The authors suggest that this group may benefit most from the TAo approach, with the avoidance of thoracic wall trauma seen with the TA approach (leading to increased pain and the potential to impair respiration postoperatively). Overall, both the risk profile and procedural outcomes were similar to the TA cohort [50]. Although largely confined to the Edwards device, the TAo approach has also been used to deliver the CoreValve in both native and bioprosthetic valves in small numbers of patients [51,52].
Subclavian/axillary access
A direct transaxillary/subclavian (TAx) approach for transcatheter valve deployment has also been described. The axillary artery is large and usually free from atherosclerosis, although it may often be rather tortuous. Access is obtained by direct surgical cut down. The use of TAx access has been widely described with the CoreValve system as an alternative in patients with unsuitable femoral access, and was initially described in the Siegburg First in Man study and subsequently, in a number of case reports [53–55]. Petronio et al. described 54 patients (just over 10% of the entire TAVI cohort) who underwent a subclavian TAVI, with low procedural complications and equivalent outcomes to the standard TF approach in their institution approaches at 6 months [56], results that were replicated in two other, smaller series [57,58]. Despite the tortuosity of the axillary artery, the shorter distance between device and aortic valve allow for greater control and manipulation of the delivery system during valve deployment. The use of the Edwards device via the TAx approach is less common, initially because of the larger size of the delivery system (previously 22–24 Fr) compared with the 18 Fr CoreValve system. However, there are some reports of successful Edwards Sapien devices deployed via the TAx approach in both native AS and following bioprosthetic valve failure [58,59].
Considerable debate exists regarding the best choice of access for the patient undergoing transcatheter valve implantation. The choice will generally depend upon anatomical/patient related factors and the expertise of the center undertaking the procedure. Whether more widespread use of non-TF/TA access sites becomes commonplace is unclear, but these approaches are certainly attractive for specific cohorts of patients being considered for TAVI.
Conclusion
TAVI remains a relatively new technology; it is less than 10 years ago that Alain Cribier implanted the first device in a patient. In that time, the procedure has moved rapidly from the experimental arena to mainstream therapy, as a treatment for a selected group of patients with severe AS. It is probable that the indications and inclusion criteria for TAVI will further expand over the next decade. It is tempting to speculate that lower-risk groups will benefit from TAVI, but clearly this will need assessment in carefully conducted, randomized trials and registries. Further refinements are also required in patient selection for TAVI, not only to extend the patient groups that may benefit, but also to define those who will not. The development of a ‘TAVI risk score’ will take considerable time but will ultimately inform and guide clinical decision making.
It seems highly likely that the indications for TAVI will expand over the next decade, and that many of the procedures detailed in this review are also likely to become routine. This seems particularly relevant with respect to the growing number of ‘valve-in-valve’ procedures outlined above, which have the potential to become the definitive therapy for failing bioprosthestic valves.
The majority of TAVI procedures are currently performed via the TF or TA routes; however, alternatives are required for specific patients, particularly those with extensive peripheral vascular disease or significant respiratory disease. The development of TAo and TAx routes considerably expands the options available in these particular patients.
These remain exciting times for transcatheter therapy, with a growing list of novel indications for the procedure and innovative methods of valve delivery.
Future perspective
The field of TAo and transcatheter valve therapy will continue to evolve over the next 5–10 years. A move into the moderate-risk surgical cohort appears inevitable, particularly as the procedural cost reduces and the size of the delivery system further ameliorates the vascular complication rates seen with the current generation. There is the very real possibility that transcatheter therapy will become the dominant treatment for aortic valve disease over the coming decade. Furthermore, treatment of bioprosthetic valve failure with transcatheter valves may become the predominant method of treatment for this particular pathology. Treatment of native mitral valve disease with transcatheter technology will be the next major hurdle to overcome and although an effective solution is probable, this is likely to be a far more challenging prospect.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Executive summary
Refinements in risk stratification & treatment of lower-risk cohorts
▪ The current surgical risk stratification algorithms are inadequate for the transcatheter aortic valve therapy (TAVI) population and more
sensitive scoring systems will be required in future.
▪ These are likely to take into account a number of patient-related factors, including an objective assessment of frailty.
▪ TAVI remains an excellent treatment option for patients with high surgical risk. For those at moderate and low operative risk,
conventional surgery remains an excellent treatment option.
Anatomical considerations when undertaking TAVI
▪ Anatomical considerations, such as bicuspid aortic valves and primary aortic regurgitation, have previously excluded some patients from
TAVI, but careful patient selection may allow many more to be treated.
Valve-in-valve therapy
▪ Transcatheter valves are increasingly being used successfully to treat bioprosthetic failure.
▪ Although the numbers remain relatively small, the current generation of devices have been used to treat bioprosthetic failure in all four
cardiac valves.
▪ With prosthetic valves in the mitral and aortic positions, there appear to be clear advantages in using a transapical rather than a
transfemoral approach.
Alternative access routes
▪ A number of novel access routes have been successfully used during transcatheter procedures.
▪ A direct transaortic approach is an attractive alternative in patients with severe peripheral vascular disease and poor respiratory function.
▪ The subclavian approach is also an option with the CoreValve device, with encouraging acute and medium-term results.
Conclusion
▪ An increasingly large number of patients are being treated with transcatheter valve therapy and many of the current ‘off-label’
indications are likely to become routine in coming years.
▪ As device design evolves and operator experience increases, use of the technology is likely to expand further.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Bose AK, Aitcheson JD, Dark JH. Aortic valve replacement in octogenarians. J. Cardiothorac. Surg. 2, 33 (2007).
- Lung B, Butchart EG, Delahaye F et al. A prospective study of patients with valvular heart disease in Europe: the Euro Heart survey in valvular heart disease. Eur. Heart J. 24, 1231–1234 (2003).
- Braunwald E. On the natural history of severe aortic stenosis. J. Am. Coll. Cardiol. 5, 1018–1020 (1990).
- Vahanian A, Alfieri OR, Alfieri OR. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. J. Cardiothorac. Surg. 34, 1–8 (2008).
- Leon MB, Smith CR, Mack M et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 363, 1597–1607 (2010).
- Smith CR, Leon MB, Mack MJ et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 364(23), 2187–2198 (2011).
- Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur. J. Cardiothorac. Surg. 16, 9–13 (1999).
- Hattler BG, Madia C, Johnson C et al. Risk stratification using the Society of Thoracic Surgeons Program. Ann. Thorac. Surg. 58, 1348–1352 (1994).
- Thomas M, Schymik G, Walther T et al. One-year outcomes of cohort 1 in the Edwards SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry: the European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 124, 425–433 (2011).
- Brown ML, Schaff HV, Sarano ME et al. Is the European system for cardiac operative risk evaluation model valid for estimating the operative risk of patients considered for percutaneous aortic valve replacement? J. Thorac. Cardiovasc. Surg. 136(3), 566–571 (2008).
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J. Thorac. Cardiovasc. Surg. 135, 180–187 (2008).
- Wendt D, Osswald B, Thielmann M et al. The EuroSCORE: still helpful in patients undergoing isolated aortic valve replacement? Interact. Cardiovasc. Thorac. Surg. 10, 239–244 (2010).
- Afilalo J, Eisenberg MJ, Morin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J. Am. Coll. Cardiol. 56(20), 1668–1676 (2010).
- Leontyev S, Walther T, Borger MA et al. Aortic valve replacement in octogenarians: utility of risk stratification with EuroSCORE. Ann. Thorac. Surg. 87, 1440–1445 (2009).
- Wijesinghe N, Ye J, Rodés-Cabau J et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. JACC Cardiovasc. Interv. 3, 1122–1125 (2010).
- Ducrocq G, Himbert D, Hvass U et al. Compassionate aortic valve implantation for severe aortic regurgitation. J. Thorac. Cardiovasc. Surg. 140, 930–932 (2010).
- Krumsdorf U, Haass M, Pirot M et al. Technical challenge of transfemoral aortic valve implantation in a patient with severe aortic regurgitation. Circulation 4, 210–211 (2011).
- Eitz T, Fritzsche D, Kleikamp G et al. Reoperation of the aortic valve in octogenarians. Ann. Thorac. Surg. 82, 1385–1391 (2006).
- Jamieson WRE, Burr LH, Miyagishima RT et al. Reoperation for bioprosthetic aortic structural failure – risk assessment. Eur. J. Cardiothorac. Surg. 24, 873 (2003).
- Walther T, Falk V, Dewey T et al. Valve-in-a-valve concept for transcatheter minimally invasive repeat xenograft implantation. J. Am. Coll. Cardiol. 50(1), 56–60 (2007).
- Walther T, Kempfert J, Borger MA et al. Human minimally invasive off-pump valve-in-a-valve implantation. Ann. Thorac. Surg. 85, 1072–1073 (2008).
- Cheung A, Webb JG, Wong DR et al. Transapicaltranscatheter mitral valve-in-valve implantation in a human. Ann. Thorac. Surg. 87, e18–e20 (2009).
- Ruiz CE, Laborde JC, Condado JF et al. First percutaneous transcatheter aortic valve-in-valve implant with three-year follow-up. Catheter. Cardiovasc. Interv. 72, 143–148 (2008).
- Ye J, Webb JG, Cheung A et al. Transcatheter valve-in-valve implantation. 16 month follow-up. Ann. Thorac. Surg. 88, 1322–1324 (2009).
- Webb JG, Wood DA, Ye J et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation 121, 1848–1857 (2010).
- Wenaweser P, Buellesfeld L, Gerckens U et al. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve-in-valve procedure using the CoreValve Revalving system. Catheter. Cardiovasc. Interv. 70, 760–764 (2007).
- Bruschi G, DeMarco F, Oreglia J et al. Transcatheter aortic valve-in-valve implantation of a CoreValve in a degenerated aortic bioprosthesis. J. Cardiovasc. Med. 11, 182–185 (2010).
- Gotzmann M, Mügge A, Bojara W. Transcatheter aortic valve implantation for treatment of patients with degenerated aortic bioprostheses-valve-in-valve technique. Catheter. Cardiovasc. Interv. 76, 1000–1006 (2010).
- Ye J, Webb JG, Cheung A et al. Transcatheter valve-in-valve aortic valve implantation: 16-month follow-up. Ann. Thorac. Surg. 88, 1322–1324 (2009).
- Pasupati S, Puri A, Devlin G, Fisher R. Transcatheter aortic valve implantation complicated by acute structural valve failure requiring immediate valve-in-valve implantation. Heart Lung Circ. 19, 611–614 (2010).
- Miranda-Balbuena N, Araji OA. Management of aortic valve dysfunction after transapical approach using the technique ‘valve after valve.’ Ann. Thorac. Surg. 92, 1102–1104 (2011).
- Hammerstingl C, Nickenig G, Grube E. Treatment of a degenerative stenosed CoreValve aortic bioprosthesis by transcatheter valve-in-valve insertion. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.23228 (2011) (Epub ahead of print).
- Cheung AW, Gurvitch R, Ye J et al. Transcatheter transapical mitral valve-in-valve implantations for a failed bioprosthesis: a case series. J. Thorac. Cardiovasc. Surg. 141, 711–715 (2011).
- de Weger A, Tavilla G, Ng AC et al. Successful transapical transcatheter valve implantation within a dysfunctional mitral bioprosthesis. JACC Cardiovasc. Imaging 3, 222–230 (2010).
- Seiffert M, Franzen O, Conradi L et al. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter. Cardiovasc. Interv. 76, 608–615 (2010).
- de Weger A, Ewe SH, Delgado V, Bax JJ. First-in-man implantation of a transcatheter aortic valve in a mitral annuloplasty ring: novel treatment modality for failed mitral valve repair. Eur. J. Cardiothorac. Surg. 39, 1054–1056 (2011).
- Bonhoeffer P, Boudjemline Y, Saliba Z et al. Transcatheter implantation of a bovine valve in pulmonary position: a lamb study. Circulation 102, 813–816 (2000).
- Bonhoeffer P, Boudjemline Y, Saliba Z et al. Percutaneous replacement of a pulmonary valve in a right ventricle to pulmonary artery conduit. Lancet 356, 1403–1405 (2000).
- Lurz P, Coats L, Khambadkone S et al., Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation 117, 1964–1972 (2008).
- Ferrari E, Sulzer C, Rizzo E, von Segesser LK. Transcatheter stent-valve implantation in a stenotic pulmonary conduit via a subxyphoidian access. Eur. J. Cardiothorac. Surg. 36, 595–541 (2009).
- Boone RH, Webb JG, Horlick E et al. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheter. Cardiovasc. Interv. 75, 286–294 (2010).
- Hon JK, Cheung A, Ye J et al. Transatrial transcatheter tricuspid valve-in-valve implantation of balloon expandable bioprosthesis. Ann. Thorac. Surg. 90, 1696–1697 (2010).
- Van Garsse LA, Ter Bekke RM, van Ommen VG. Percutaneous transcatheter valve-in-valve implantation in stenosed tricuspid valve bioprosthesis. Circulation 123, e219–e221 (2011).
- Roberts P, Spina R, Vallely M et al. Percutaneous tricuspid valve replacement for a stenosed bioprosthesis. Circ. Cardiovasc. Interv. 3, e14–e15 (2010).
- Cerillo AG, Chiaramonti F, Murzi M et al. Transcatheter valve-in-valve implantation for failed mitral and tricuspid bioprosthesis. Catheter. Cardiovasc. Interv. 121(16), 1848–1857 (2011).
- Rodés-Cabau J, Dumont E, Miró S et al. Apical aortic valve implantation in a patient with a mechanical valve prosthesis in mitral position. Circ. Cardiovasc. Interv. 1(3), 233 (2008).
- Bruschi G, De Marco F, Oreglia J et al. Percutaneous implantation of CoreValve aortic prostheses in patients with a mechanical mitral valve. Ann. Thorac. Surg. 88, e50–e52 (2009).
- Soon JL, Ye J, Lichtenstein SV et al. Transapical transcatheter aortic valve implantation in the presence of a mitral prosthesis. J. Am. Coll. Cardiol. 58, 715–721 (2011).
- Bapat V, Thomas M, Hancock J, Wilson K. First successful transcatheter aortic valve implantation through ascending aorta using Edwards SAPIEN THV system. Eur. J. Cardiothorac. Surg. 38, 811–813 (2010).
- Bapat V, Khawaja MZ, Attia R et al. Transaortic transcatheter aortic valve implantation using Edwards Sapien valve: a novel approach. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.23276 (2011) (Epub ahead of print).
- Latsios G, Gerckens U, Grube E. Transaortic transcatheter aortic valve implantation: a novel approach for the truly ‘no-access option’ patients. Catheter. Cardiovasc. Interv. 75, 1129–1136 (2010).
- Cockburn J, Trivedi U, Hildick-Smith D. Transaortic transcatheter aortic valve implantation within a previous bioprosthetic aortic valve replacement. Catheter. Cardiovasc. Interv. 78, 479–484 (2011).
- Grube E, Laborde J C, Gerckens U et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg First-In-Man study. Circulation 114, 1616–1624 (2006).
- Latsios G, Gerckens U, Grube E et al. First successful aortic valve implantation with the CoreValve ReValving System via right subclavian artery access: a case report. Heart Surg. Forum 11, E323–E324 (2008).
- Asgar AW, Mullen MJ, Delahunty N et al. Transcatheter aortic valve intervention through the axillary artery for the treatment of severe aortic stenosis. J. Thorac. Cardiovasc. Surg. 137, 773–775 (2009).
- Petronio AS, de Carlo M, Bedogni F et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ. Cardiovasc. Interv. 3, 359–366 (2010).
- Modine T, Obadia JF, Choukroun E et al. Aortic valve implantation using the axillary/ subclavian access: feasibility and early clinical outcomes. J. Thorac. Cardiovasc. Surg. 141, 487–491 (2011).
- Taramasso M, Maisano F, Cioni M et al. Transapical and transaxillary percutaneous aortic valve implantation as alternatives to the femoral route: short- and middle-term results. Eur. J. Cardiothorac. Surg. 40, 49–55 (2011).
- Sharp AS, Michev I, Colombo A. First transaxillary implantation of Edwards Sapien valve to treat an incompetent aortic bioprosthesis. Catheter. Cardiovasc. Interv. 75, 507–510 (2010).
▪▪ Major study comparing medical therapy with transcatheter aortic valve therapy (TAVI) in patients unsuitable for conventional surgery, showing unequivocal benefit of transcatheter therapy in this group.
▪▪ Randomized trial demonstrating equivalence between TAVI and conventional aortic valve replacement in a ‘high-risk’ surgical population.
▪ Large registry examining outcomes in patients treated with TAVI.
▪▪ Important study examining outcome in series of patients treated with ‘valve-in-valve’ therapy.
▪ Novel transaortic approach to transcatheter valve implantation.