News and Views - Imaging in Medicine (2013) Volume 5, Issue 1
New MRI tools shed light on Parkinson's disease progress Compact MRI system in development for improved joint imaging Diffusion-weighted MRI could guide development of new and improved tumor models, Cardiac CT reveals surprising racial differences in thoracic fat measurements, Analysis of multiple Alzheimers disease biomarkers might improve chance of predicting cognitive decline, Functional MRI could predict onset of bipolar disorder in at-risk individuals
Abstract
New MRI tools shed light on Parkinson’s disease progress
A team of scientists from Massachusetts Institute of Technology (MA, USA) have developed new imaging techniques that could have profound implications for the future diagnosis and management of Parkinson’s disease (PD).
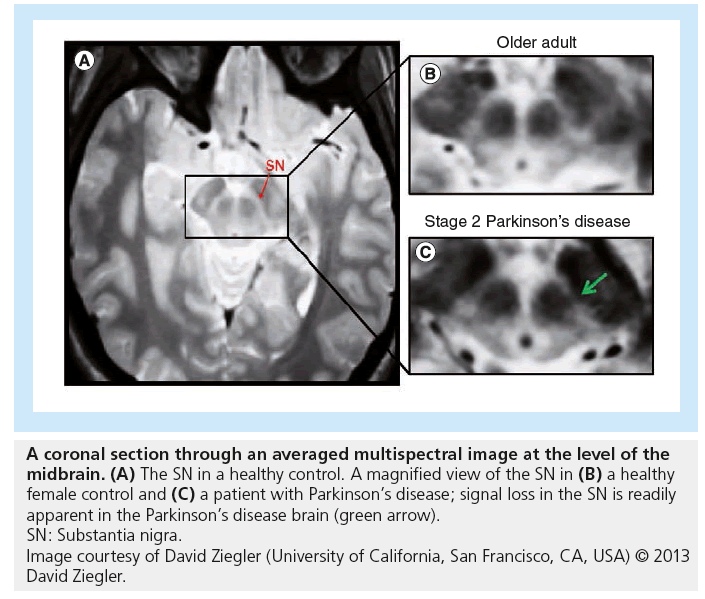
The research, published recently in Archives of Neurology, involved combining results from several different MRI scans in order to produce composite images. When the investigators applied these techniques to the study of patients with PD, the resultant composite images allowed degeneration within the brain to be viewed in unprecedented detail.
Using the composite imagery, the team were able to provide evidence that brain deterioration due to PD occurs within the substantia nigra pars compacta (SNc) prior to occuring within the cholinergic basal forebrain (BF). This is the first time that evidence from living patients has been gathered to support prominent anatomist Heiko Braak’s hypothesis that brain degeneration originates in deep brain structures before progressing to other parts of the brain.
To obtain their results, the scientists scanned the brains of 29 patients at various stages of PD (13 patients at Hoehn and Yahr [H&Y] stage 1 and 16 patients at H&Y stages 2–3) as well as 27 healthy control subjects. The team collected data for each patient by utilizing four different MRI techniques: multiecho proton density, multiecho T1-weighted, T2-weighted and T2-weighted fluid-attenuated inversion recovery. By combining the results of these scans, the researchers were able to construct images in which the SNc and the BF were clearly visible. They were then able to manually calculate the volume of both neural structures in each individual.
The scientists found that the SNc volume of the 13 patients diagnosed with H&Y stage 1 PD had been significantly decreased when compared with the healthy control subjects. On the other hand, relative to the control subjects, the 16 patients with H&Y stage 2 or 3 PD did not display significant signs of deterioration in this area of the brain, suggesting that the SNc is affected primarily in the earlier stages of PD.
The inverse was found when analyzing the relative volumes of the BF between the PD patients and the control group. When compared to the control subjects, patients with H&Y stage 2 or 3 PD displayed a statistically significant decrease in BF volume. However, the volume of the BF in patients with H&Y stage 1 PD was not significantly different to that of the healthy volunteers, suggesting that BF volume loss occurred later in the progression of the disease.
Researcher David Ziegler spoke to Imaging in Medicine to explain the significance of the results and to highlight the potential of the new techniques for the future of PD prediction and treatment. He noted that his team had established “the utility of these new MRI tools for measuring and tracking subtle disease-related changes in structures that are notoriously difficult to visualize with conventional MRI.”
As a consequence, he claimed the team had “provided much needed biomarkers for characterizing and tracking brain changes associated” with PD, which “can now be used to identify and track differences in disease progression” and “will enable clinicians to predict which patients are likely to develop particular symptoms and to tailor therapies to each patient’s needs.”
When asked about the team’s plans for future research in this area, Ziegler informed Imaging in Medicine that they “are currently conducting additional analyses to correlate brain changes in the substantia nigra and BF with clinical and cognitive measures. In the future, we are hoping to conduct a longitudinal study in which we collect these imagining measures multiple times on patients as they progress.” The team will then “follow the patients to track whether the rate of disease progression or the development of specific cognitive impairments or dementia is associated with a particular pattern of brain changes.”
– Written by Michael Mansbridge
Source: Ziegler D, Wonderlick J, Ashourian P et al. Substantia nigra volume loss before basal forebrain degeneration in early Parkinson disease. Arch. Neurol. doi:10.1001/jamaneurol.2013.597 (2012) (Epub ahead of print).
Compact MRI system in development for improved joint imaging
Researchers at Imperial College London (London, UK) have embarked on a 3-year program to develop a compact, low-cost MRI system that could be used in hospitals to improve decision-making and patient care, particularly in knee replacement surgery. The system, which will be approximately the size of a coffee table, will use a new movable magnet to overcome the challenges faced when imaging areas with a high collagen content such as ligaments and tendons.
The first year of the program will focus on system design, including a new magnet; the second on manufacture of the main modules; and the third year will involve system integration, testing and trial imaging on volunteers. The researchers plan to then collaborate with industrial partners to produce a commercial product that they hope could be widely adopted by hospitals for improved MRI services.
“The MRI system that we are planning to build will be quite unique, because it will have a moveable magnet capable of rotating around the patient about two axes. This will enable us to exploit the ‘magic angle’ effect and to provide images of tissues such as tendons, ligaments and cartilage that would otherwise produce no recordable signal and appear black,” explained Mihailo Ristic when speaking to Imaging in Medicine. Ristic is from the Department of Mechanical Engineering at Imperial College London and his group will be developing the new MRI system. “The magic angle effect can be observed in certain tissue structures, such as those containing significant amounts of collagen, where the time constant of decay of the MR signal can vary by a very large amount with the angle of the tissue structures relative to the main magnetic field. This effect is conventionally a source of artefacts, but we intend to exploit it as the means of enhancing images,” he continued.
One of the key challenges of this project will be the magnet design itself, as Ristic explained: “In addition to being moveable, the magnet will adopt a unique, novel configuration needed for this purpose and still realize a highly uniform magnetic field in a volume of about 15-cm diameter that is necessary for MRI. Other aspects such as gradient coils and the radio frequency front end will also have to be developed for this magnet configuration.”
The new MRI system containing the novel magnet design is being designed specifically for imaging knee joints and will fit neatly around the knee. The Imperial College London researchers hope that their new system will lead to improved patient care: “Knee replacement is forecast to grow steadily for the foreseeable future with the vast majority of patients at present being offered total joint replacement,” explained Ristic. “The diagnostic precision in the decision to remove the entire knee is currently limited by poor imaging of the ligaments in particular, but also the articular surfaces. Over 90% of patients are currently only offered total knee replacement, in part because there is no reliable way of imaging the anterior cruciate ligament to confirm that it is still intact. This information would allow the surgeon the confidence to offer the more conservative, more effective and cheaper procedure of partial replacement, with obvious benefits to the patient who will suffer less trauma and also huge potential savings for healthcare purchasers.”
The compact MRI system will also be relatively cheap to manufacture (an estimated £200,000) in comparison to standard hospital MRI machines (which cost approximately £900,000) thanks to its small size and lower power requirements. In addition, there will be little special requirements for installation. “As such we envisage it being installed in patient surgeries and its widespread adoption would relieve the burden on general purpose whole-body MRI machines,” concluded Ristic.
– Written by Sarah Miller
Source: Imperial College London news release: www3.imperial.ac.uk/newsandeventspggrp/ imperialcollege/newssummary/ news_14-12-2012-12-2-41#fni-3
Diffusion-weighted MRI could guide development of new and improved tumor models
A recent study has demonstrated the ability of diffusion-weighted MRI (DW-MRI) to provide a quantitative measure of tumor development in preclinical triplenegative breast cancer models. The study, which was performed by researchers at Moffitt Cancer Center (FL, USA) and Arizona Cancer Center (AZ, USA), was published in the November 2012 issue of Experimental Biology and Medicine. The authors suggest the technique could be used to guide more successful development of new tumor models in the future.
New and improved preclinical tumor models are essential for ongoing cancer research; however, their production from patient-derived cancer cells can be laborious and success can be elusive. However, as Marty Pagel from Arizona Cancer Center, who was a coauthor of this recent study, explained, DW-MRI could shine a guiding light onto the process: “Noninvasive, quantitative imaging techniques have potential to accelerate and improve the success of establishing new tumor models for innovative cancer studies.”
“In the absence of imaging, such as we describe here, developers of tumor models are f lying blind,” commented Robert Gillies from the Moffitt Cancer Center, the researcher who lead this recent study. Gillies continued: “In this study, we show that the behavior of water in tissues can provide important information about tumor development. More specifically, the mobility of water is inhibited by biological barriers such as cell membranes, so that decreased mobility can be used as a measure of cell density and proliferation in tumor models.”
In this recent study, DW-MRI was used to evaluate the cellular proliferation of new xenograph tumor models of triple-negative breast cancer that were developed in severe combined immunodeficient mice from human breast cancer cell lines.
The apparent diffusion coefficient of tumors was measured using DW-MRI and found to correlate with expression of some markers associated with cellular proliferation, such as Ki-67, and molecules known to support processes key to proliferation, such as HIF-a1 and VEGFR2.
While the results also reflected the highly complex and difficult-to-interpret nature of the signaling pathways involved in tumor cell proliferation, overall, the authors concluded that “DW-MRI may be a more direct assessment of tumor growth and cancer cell proliferation.” The imaging technique therefore shows promise as a tool for guiding development of new tumor models for improved breast cancer therapies.
– Written by Sarah Miller
Sources: Stephen RM, Pagel MD, Brown K, Baker AF, Meuillet EJ, Gillies RJ. Monitoring the development of xenograft triple-negative breast cancer models using diffusionweighted magnetic resonance imaging. Exp. Biol. Med. (Maywood) 237(11), 1273–1280 (2012); Improving the development of new cancer models using an advanced biomedical imaging method: www.eurekalert.org/pub_ releases/2012-12/sfeb-itd121712.php
Cardiac CT reveals surprising racial differences in thoracic fat measurements
A recent cardiac CT study presented at the 2012 annual meeting of the Radiological Society of North America (RSNA) revealed unexpected findings relating to the amount of thoracic fat seen in African–Americans and Caucasian patients. Prior evidence has implicated fat around the heart as an independent marker of coronary artery disease (CAD) burden or a risk factor for future acute coronary events. However, in this study, African–American patients, who have a higher CAD prevalence compared with their Caucasian counterparts, were actually found to have a less fat around their hearts.
In 2010, the age-adjusted prevalence of CAD in the USA was 5.8% among Caucasians and compared with 6.5% among African–Americans. The pathophysiological explanation for the racial difference in CAD risk is not yet fully understood, but differences in thoracic adipose tissue between the races could be a contributing factor.
This recently presented study, which was performed in Charleston (SC, USA) in collaboration with coworkers from Germany, Italy and The Netherlands, evaluated cardiac dual-source CT images of 411 patients (204 self-identif ied African–Americans and 207 self-identified non-Hispanic Caucasians with a similar age and gender distribution) who had undergone coronary CT angiography for acute chest pain.
“Epicardial adipose tissue is visceral fat located between the myocardium and the pericardium, particularly around subepicardial coronary vessels. [Visualizing] epicardial adipose tissue on cardiac CT has been shown to be easy to perform and more reproducible than manual bidimensional measurements,” explained study author Paul Apfaltrer to Imaging in Medicine when discussing the imaging methods used in the recent study.
A single experience observer measured epicardial, mediastinal and pericoronary fat thickness in all 411 CT images, with some surprising results: “It was assumed that African–Americans, who have a higher prevalence of cardiovascular disease, CAD and higher disease-specific mortality rates, would present with higher rates of thoracic fat in an acute chest pain setting,” commented Apfaltrer. “However, contrary to what we hypothesized, the amount of epicardial fat was lower in African–Americans, while the prevalence of significant stenosis and of plaque was similar to Caucasian patients.”
“These demonstrated racial differences in the amount of fat surrounding the heart might be linked to differences in the prevalence of CAD and diseasespecif ic mortality rates,” explained Apfaltrer. “Understanding the mechanism behind these disparities may improve the prevention, risk stratification, and management of CAD. Population-based outcome studies are necessary to determine the prognostic value of these findings,” he concluded.
– Written by Sarah Miller
Source: Radiological Society of North America press release: www2.rsna.org/timssnet/Media/ pressreleases/pr_target.cfm?id=638
Analysis of multiple Alzheimer’s disease biomarkers might improve chance of predicting cognitive decline
A study published recently in Radiology involves the use of various biomarkers to track the progress of Alzheimer’s disease (AD). The team of researchers, based at the Duke University Medical Centre (NC, USA), believe that by using a combination of imaging and other diagnostic techniques it may be possible to more accurately predict the future cognitive decline of patients with mild cognitive impairment (MCI).
The researchers enrolled a cohort of 96 volunteers who had previously been diagnosed with MCI. The patients were selected from the Alzheimer’s Disease Neuroimaging Initiative, an ongoing US study following the cognitive decline of patients with the disease.
The team measured cerebrospinal fluid proteins and conducted MRI and 18F-fluorodeoxyglucose-PET scans for each patient, using independent components analysis to extract meaningful data from the images. The resultant data were then entered into logistic regression models, correlating the data with the subsequent level of cognitive decline recorded for each patient after 2 and 3 years into the study. The models also controlled for factors such as the participants’ age and level of education.
By combining the imaging techniques and cerebrospinal fluid data, the researchers found that accuracy of predicting AD in patients previously diagnosed with MCI was significantly improved when compared with clinical testing alone. Out of the tests, 18F-fluorodeoxyglucose-PET was found to be the most effective tool in predicting the onset of AD.
Furthermore, the process of combining these additional techniques with existing clinical neuropsychological and genetic blood tests significantly reduced the likelihood of misclassifying AD in patients with MCI. Whereas the risk of misclassification was previously 41.3%, it was lowered to 28.4% when imaging and cerebrospinal fluid data were included.
In future, the team “would like to add PET amyloid imaging to our markers and do a cost analysis to determine how much additional information each biomarker” provides for the money spent, researcher Jeffrey Petrella explained. He believes this “may help physicians make more costeffective decisions,” although he accepts that their “results need to be validated in a larger sample first.”
– Written by Michael Mansbridge
Source: Shaffer JL, Petrella JR, Sheldon FC et al. Predicting cognitive decline in subjects at risk for Alzheimer disease by using combined cerebrospinal fluid, MR imaging, and PET biomarkers. Radiology doi:10.1148/ radiol.12120010 (2012) (Epub ahead of print).
Functional MRI could predict onset of bipolar disorder in at-risk individuals
A recent functional MRI (fMRI) study from Australian researchers has revealed that individuals with an increased genetic risk of bipolar disorder actually have clear and quantifiable differences in brain activity compared with controls before clinical signs of the condition are detectable.
Dysfunctional neural mechanisms involved in cognitive control of emotion have been previously associated with genetic risk of bipolar disorder but the triggers are not yet understood. Bipolar disorder has the highest suicide rate of all psychiatric disorders.
“This study is important for two reasons: first, any differences (such as those we identified in our recent study) may indicate endophenotypes (trait abnormalities) that are indicative of a vulnerability to bipolar disorder and not a consequence of developing this condition,” explained study leader Philip Mitchell from the Black Dog Institute (Randwick, Sydney, Australia) and University of New South Wales (Sydney, Australia), two institutions involved in this recent study, when speaking to Imaging in Medicine. “Second, demonstration of biological differences in those at increased risk will be important in the development of prevention and early intervention programs for those at heightened risk to this condition.”
In this recent study, fMRI was used to monitor functional brain activity during a facial-emotion go/no-go task, in which participants were shown images of happy, fearful or calm (neutral) human faces. There was a total of 47 study participants aged between 18 and 30 years old with a high genetic risk of developing bipolar disorder and a total of 47 control subjects of the same age without a family history of bipolar or other severe mental illness.
Whole-brain corrected fMRI analyses revealed signif icantly reduced brain activity in response to the facial emotions in individuals with a high risk of bipolar compared with the control group. Researchers observed an impaired inhibitory function of the inferior frontal cortex in response to fearful faces in at-risk individuals. This brain area is known to regulate emotional responses and the study authors suggest that this may be a trait marker of bipolar vulnerability.
“A number of our subjects (at-risk or controls) were either currently depressed or on psychotropic medications, both of which could potentially confound our findings,” explained Mitchell when discussing the challenges faced in this recent study. “After we removed those subjects from the analyses, the results persisted; giving us increased confidence in the validity of the findings.”
In addition to providing evidence that those at a high risk of bipolar could have pre-existing functional disturbances, a recent study also suggests that fMRI could be used in the future as a tool for improving the lives of young people at risk of the condition in the future by allowing early detection, leading to improved outcomes, and even prevention of onset.
The researchers are also analyzing other imaging data on this same clinical population and are following participants up annually to determine predictors (neuroimaging, genetic, neuropsychological and clinical) of which subjects eventually go on to develop bipolar disorder.
Mitchell explained to Imaging in Medicine that his group has a longstanding interest in identifying biological and psychological mechanisms in bipolar disorder: “We commenced the current study over 3 years ago, and will be commencing our fourth year of follow-up of this clinical sample later this year. We have found the families very interested in this study, as they are all concerned with the risk of their children developing the illness, and wish to assist research that aims to eventually reduce this.”
– Written by Sarah Miller
Sources: Roberts G, Green MJ, Breakspear M et al. Reduced inferior frontal gyrus activation during response inhibition to emotional stimuli in youth at high risk of bipolar disorder. Biol. Psychiatry doi:10.1016/j.biopsych.2012.11.004 (2012) (Epub ahead of print); University of New South Wales news release: http:// newsroom.unsw.edu.au /news /heal th / brain-imaging-identifies-bipolar-risk