Research Article - International Journal of Clinical Rheumatology (2022) Volume 17, Issue 1
New Quantitative Non-Radiologic Assessment of Osteoarthritis and Possible Infection Association
- *Corresponding Author:
- Rozin Alexander P
B. Shine Rheumatology Institute, Rambam Health Care Campus and Technion. Haifa. Israel
E-mail: rozin@rambam.health.gov.il
Received: 08-Oct-2021, Manuscript No. fmijcr-21-44249; Editor assigned: 12-Oct-2021, PreQC No. fmijcr-21-44249(PQ); Reviewed: 05-Jan-2022, QC No. fmijcr-21-44249; Revised: 10-Jan-2022, Manuscript No. fmijcr-21-44249(R); Published: 15-Jan-2022, DOI: 10.37532/1758-4272.2022.17(1).009-015
Abstract
Keywords
finger joints • osteoarthritis • non-radiologic assessment • deviation angle • visible palpable score • infection association
Introduction
Osteoarthritis (OA) remains one of the most common forms of musculoskeletal disease in the world. Some 75% of women aged 60-70 years had OA of their finger joints and, even by 40 years of age, 10-20% of subjects had evidence of severe radiographic disease of their hands or feet. The prevalence rate of knee OA increased from less than 1% for severe OA among people aged 25-34 to 30% in those aged 75 years and above. Eighty percent of people with OA have limitation of movement, and 25% cannot perform their major daily activities of living [1]. OA may have a rapidly progressive course, causing joint destruction within 3-7 years. Osteoarthritis pathology includes two main processes: osteophyte formation and joint narrowing due to assymmetric cartilage loss. Existing techniques for quantitative assessment of OA progression rely on the measurement of joint narrowing (joint width). The cartilage loss is not specific process observed also in rheumatoid arthritis and other inflammatory arthritides. However, osteophyte formation is specific for osteoarthritis. Today there is not quantitative non-X-ray assessment of osteophyte growth. Non-X-Ray quantitative assessment of fingers was previously reported for psoriatic dactylitis, but without taking into account individual finger joint characteristics [2]. Finger joint may be big because generic structure of the body. We decided to assess a relative input of the finger joint circle by measurement of distal periarticular, joint and proximal periarticular size and building curve with assessment of deviation curve angle. This instrument might be reflecting osteophyte enlargement and atrophy of soft periarticular tissues at independent manner of individual finger characteristic. That is why we decided to use a joint enlargement due to osteophyte formation as a corn stone object for measurement and assessment of OA progression. Above-mentioned data show that OA is mainly age related disease. Four processes become clinically relevant in most people facing age 40-60: hormonal involution, vascular disease, aging related decreased activity of immune system, and increased incidence of bacterial infections. The first three may be in a part implicated in increased frequency of the fourth one. We have, on one hand, growing prevalence of osteoarthritis in elderly population and, on the other hand, increased risk of bacterial infections, mainly urogenital, due to hormonal, vascular and immune impairments associated with aging. Therefore proposal, that there is a relationship between osteoarthritis and recurrent obvious or occult (subclinical) body infections. This proposal has become stronger after observation of 10 women with refractory osteoarthritis of knees and recurrent UTI when six of them responded favorably to cotrimoxazole prophylaxis against recurrent UTI with significant decrease of knee pain and improvement of knee function assessed with OARSI criteria [3]. We proposed every infection outbreak contributes to asymmetric cartilage loss and osteophyte formation.
Aims of the Study
1. To introduce a new method of quantitative assessment of knee and finger joints, affected with osteoarthritis.
2. To compare its results with OA-dependent factors and Visual Palpable Score (VPS) of joint enlargement.
3. To check proposed relationship between infectious events and osteoarthritis of finger and knee joints.
Patients and Methods
Two groups of patients investigated and compared were such as follow:
Group A
Ten patients, which are older 40 years of age with knee and finger joint pain and OA changes of 1 year duration at least, clinical evidence of knee OA: ACR1986 Criteria and EULAR 2009 criteria for knee OA [4,5] and ACR1990 Criteria and EULAR 2008 for OA of hand [6,7].
Knee OA criteria include persistent knee pain in patient of age 40 years or older, limited morning stiffness lasting 30 minutes or less, reduced function, crepitus on motion, restricted movement and bony enlargement.
Hand OA criteria (HOA) comprise hand pain, aching or stiffness along with hard tissue enlargement of two or more of 10 selected joints with distal interphalangeal predominance and deformity. Ten selected joints are the second and third distal interphalangeal joints, the second and third proximal interphalangeal joints and the first carpometacarpal joints (of both hands). According to EULAR recommendations should be taken into account risk factors for HOA, clinical hallmarks (Heberden and Bouchard nodes), functional impairment, increased risk generalized OA in patients with polyarticular HOA, special subsets of HOA such as erosive and thumb base, excluding common other arthritides (RA, gout, pseudo-gout, psoriatic) and analyzing blood tests indicating other diseases.
Group B
Ten healthy controls, which are older 40 years of age without knee and finger joint pain and OA changes. Because lack of clinical - X-Ray OA correlations we did not use X-Ray data for both groups. Patients of these two groups are to be assessed with study-specific questions (Table 1) including intensity (VAS) and duration of joint pain, and joint deformities, duration of history and relapse rate of uro-genital, respiratory, gastrointestinal tract and skin infections.
| History | OA group Controls |
|---|---|
| Age | 68.7+/-8.7 60.9+/-7.1 (p=0.043) |
| Sex | W/M 7/3 W/M 6/4 |
| Weight | 87.2+/-27.9 82.8+/-11.5 (p=0.6) |
| BMI | 31.2+/-8.0 31.7+/-5.3 (p=0.9) |
| Hand joint pain (I CMC, IP, PIP, DIP) (VAS)* | 6.2+/-1.3 0 |
| Knee pain (VAS) | 3.2+/-3.0 0 |
| Duration of joint pain | 12.1+/-8.6 0 |
| Duration of joint deformities | 6.9+/-7.2 0 |
| Family history of OA (7-one, 1-two ill parents) | 80% 0 |
| Urogenital tract infection (UTI) | |
| Prevalence | 8/10(80%) 1/10 (10%) |
| Time since the first UTI event (years) | 6.1 |
| Average number of UTI events per year | 1 0 |
| Respiratory tract infection (RTI) (tonsillitis, sinusitis, bronchitis, pneumonia, otitis, pharingitis) | |
| Prevalence | 6/10(60%) 1/10(10%) |
| Time since the first event of RTI (years) | 37.2 15 |
| Average number of RTI events per year | 2 0.3 |
| Gastro-intestinal tract infection (GITI) (gastritis, enteritis, colitis, pancreatitis, cholecystitis, cholangitis, hepatitis) | |
| Prevalence | 7/10 (70%) 0 |
| Time since the first event of GITI (years) | 21 0 |
| Average number of GITI events per year | 1.7 0 |
| Skin infection (erysipelas, cellulitis, folliculitis, furunculosis, impetigo, abscess, phlegmone) | |
| Prevalence | 3/10 (30%) 0 |
| Time since the first event of SI (years) | 15 0 |
| Average number of SI events per year | 1 0 |
* VAS – visual analogue scale: no pain 0 mm, maximal pain 100 mm. CMC – carpometacarpal, IP – interphalangeal, PIP – proximal interphalangeal, DIP – distal interphalangeal
Table 1. Questionnaire of patients' data and infection history
The examiners consisted of 3 consultant rheumatologists. Each examiner performed 2 assessments with each patient.
Finger and Knee Joints of the Both Groups Assessed with Quantitative Original Method
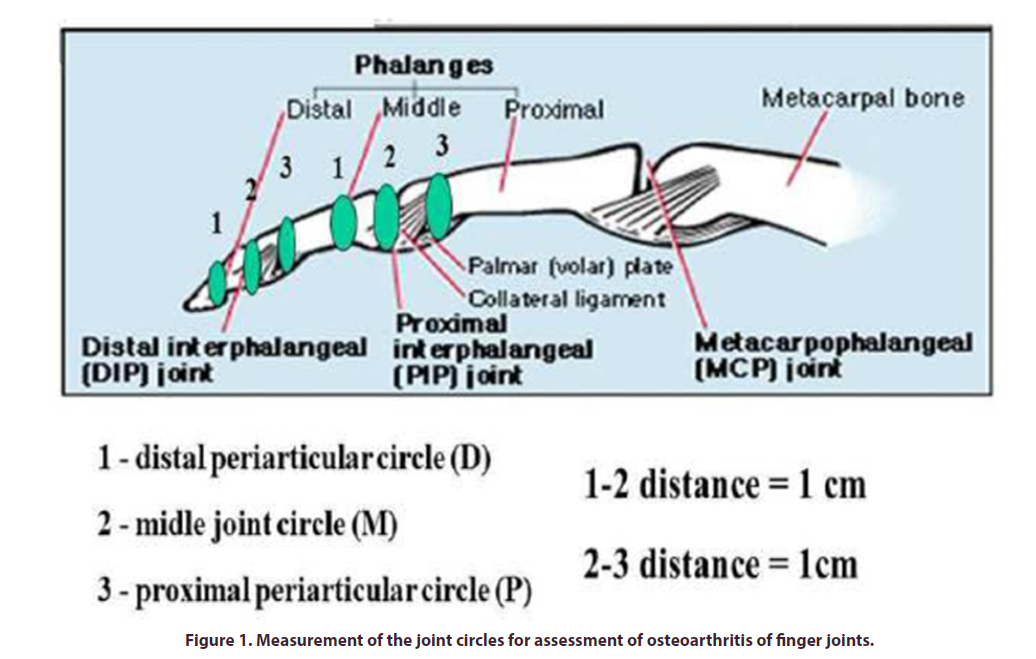
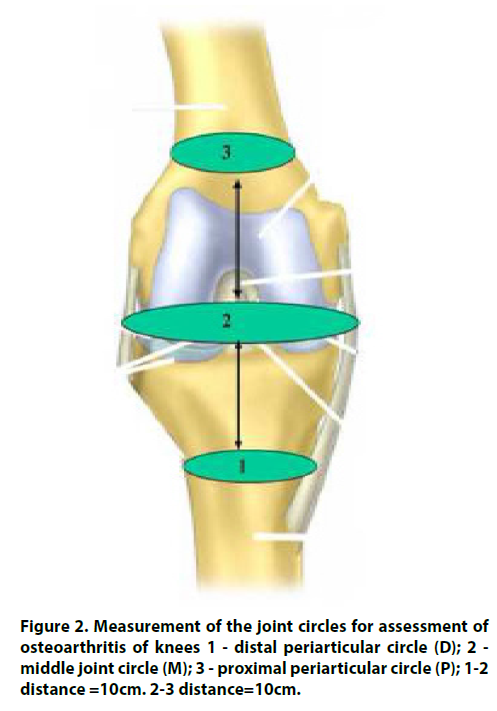
That we proposed for measurement of osteophyte formation for finger and knee joint. The method includes measurement of periarticular distal and proximal and articular circle of the joints (Figures 1 and 2) and calculation of a deviation angle α (Figure 3) as proposed indicator of osteophyte formation and joint enlargement. Non-X-Ray quantitative assessment of fingers was previously reported for psoriatic dactylitis, but without taking into account individual characteristics and with no assessment of the finger joints [2]. A finger joint may be big or small because generic structural factors. We decided to assess a relative input of finger joint circle by measurement of distal periarticular, joint and proximal periarticular circle size and building a curve with assessment of deviation curve angle. This approach might be reflecting osteophyte enlargement and atrophy of soft periarticular tissues at independent manner of individual finger characteristics. Rationale of the method is contribution of two bones proximal and distal to osteophyte formation. These differences of the circle size between enlargement of articular part of the bones and intact bone calculated for assessment of osteophyte growth. Proximal muscle atrophy as result of long standing disease is aggravated factor. In order to provide an instrument that is simple and easy to use, it was decided to use digital circumferometer [2]. Finger circumference was a frequent metric used in the 1980s to monitor the efficacy of antirheumatic drugs but was discontinued after poor inter-observer reliability was demonstrated [8].
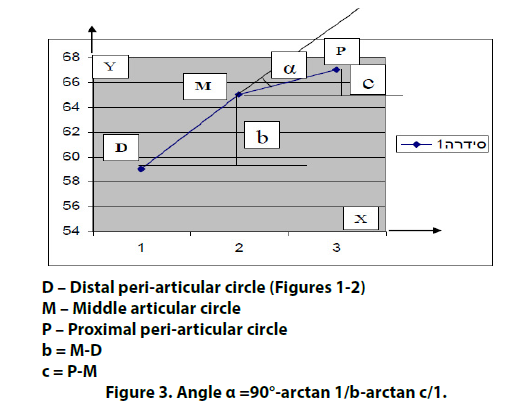
Trigonometric rapid tables may help for calculation of the deviation angle α according to formula (Figure 3). We did it simply by building of DMP curve (Figure 3) calculating deviation angle α by protractor. For example, distal peri-articular circle (D) is 40mm, middle articular circle (M) is 50mm and proximal peri-articular circle (P) is 45 mm. With interval 4 cm between points 1- 2 and 2-3 on X-axis, we mark D, M and P values on Y-axis. We connect points D, M, P, and calculate deviation angle α with protractor. The angle α is 23º.
Visual palpable assessment of osteophyte size procedure is original semi-quantitative joint bulging score as follows: 0 - no osteophyte, 1- osteophyte palpated, 2- osteophyte visible, 3- a big osteophyte or big osteophyte with joint deformity. This data was compared with deviation angle α and appropriate comparison of both groups for above-mentioned parameters performed.
Statistic assessment was performed using SPSS 12.0 soft-ware. Comparison between continuous variables of SSPR was done with the Mann-Whitney U non-parametric test. For group comparison of age variables a two-tailed Student'st-test was used. Inter-observer and intra-observer score was estimated. P-values of 0.05 or less were considered as statistically significant. The trial was approved by the hospital Helsinki board and written informed consent was got from investigated patients.
Results
Our patient and control groups were predominantly women 60-70% of age 60-69 years old and with overweight BMI 31+/-8 kg/m2 without statistical differences between groups (Table 1). OA patients had finger joint pain of grade 6.2+/1.3 and knee pain up to 6 points (VAS scale 0-10). Duration joint pain was average of 12 years duration and joint deformity of 7 years. OA group had 80% of family history of OA and none of controls. For the first time we measured and disclosed statistically higher level of the deviation angle (DA) for all finger joints in OA group compared with controls (Table 2). Visible palpable score (VPS) was also higher. For knees, we did not observed differences. IP, DIP II, PIP III-IV had biggest values of DA and DIP IV had minimal size. From our experience, really this joint is much more rarely involved in OA process.
| Joints | IP | DIP II | DIP III | DIP IV | DIP V | PIP II | PIP III | PIP IV | PIP V | Knee |
|---|---|---|---|---|---|---|---|---|---|---|
| OSTEOARTHRITIS | ||||||||||
| Deviation angle (DA) | 10.9+/-8.6 | 12.3+/-9.7 | 8.2+/-7.9 | 2.9+/6.3 | 7.7+/-7.4 | 8.7+/-7.4 | 12.7+/-9.2 | 12.7+/-8.3 | 8.6+/- 6.0 | 2.3+/-5.0 |
| Visible score (VS) | 1.4+/-0.8 | 1.8+/-1.1 | 1.4+/-1.2 | 0.6+/- 0.9 | 1.3+/-0.9 | 1.0+/-0.7 | 1.2+/-0.7 | 1.3+/-0.8 | 1.1+/-0.8 | 0.4+/-0.6 |
| HEALTHY CONTROLS | ||||||||||
| Deviation angle (DA) | 1.4+/-5.3 | -0.6+/- 4.2 | -1.7+/- 5.0 | -1.3+/- 4.9 | -0.3+/-4.2 | 2.9+/-6.7 | 4.5+/-4.8 | 4.9+/-4.9 | 3.8+/-5.7 | 2.8+/-4.4 |
| Visual Score (VPS) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| p-value | ||||||||||
| DA | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | 0.002 | 0.024 | 0.79 |
| VPS | <0.001 | <0.001 | <0.001 | 0.012 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.03 |
Abbreviations: IP – interphalangeal joint, DIP – distal interphalangeal joints, PIP – proximal interphalangeal joint, DA – deviation angle, VPS – visual palpable score (joint bulging score)
Table 2. Data of finger and knee joint measurment: deviation angle and visual palpable score.
Maximal OA DA achieved 20º, average values for OA group were within 8-15º. DA less 7º observed in majority of controls and maximal DA rarely rised to 9 º. VPS of OA moved between 0.5 and 3 points. Controls had not OA changes. Urogenital tract infection history had 80% patients with OA and only 10% of control. Respiratory infections were in 60% of OA group and 10% of controls. Gastro-intestinal infectious problems had 70% of OA patients and none of controls. Skin infection history observed in 30% of OA and nothing in controls. Intra-observer and inter-observer score was good (>0.9).
Discussion
We presented new non-radiographic quantitative method for characterisation of finger joint and knee OA for comparision with semi-numeral array and infectious events. Several aims were as follow:
1. Diagnostic calculation assessment
2. Disease severity analysis
3. Dynamic progression or retardation of OA
4. Effect of therapy
5. Joint individual characteristic, like that of fingerprints.
We live in world of precise assessment of disease parameters. Such we can define early pathological change and make appropriate decision. OA therapy does not exist but may emerge. We delighted to propose a simple method of OA mesurement. For analysis of finger joint it looks useful by for knee joints it proved to be non-informative.
Relationship between OA and infection seems to be bizarry from only the first sight. Leading instrument of infection is lipopolysaccharide (LPS). That is bacterial endotoxin. LPS-induced cartilage damage is widely used as a model to investigate the effectiveness of cartilage protective agents in vitro. We proposed an analogical in vivo model of joint degenerative changes related to fatty liver. Might similar LPS-induced cartilage damage occur in vivo according to scenario such as follows? Overwhelming liver LPS of intestinal origin break vulnerable liver barriers of fatty liver, achieve blood stream in low but sustained accounts, and spread to joints.
This result in LPS-lipopolysaccharide-binding protein (LBP)-CD14 activation of inflammatory response associated with enhanced synthesis of interleukins (IL) IL-1, IL-6, tumor necrosis factor (TNF)-α, interferon (INF) INF-γ, nitric oxide (NO), TGF-β and development of degenerative joint changes. Spine osteophytes of patients with fatty liver had 50% bigger size compared with patients with normal liver [9].
Next line of suspicion for infectious origin of OA comes from natural remedies. Glucosamine sulfate (GS) and chondroitine sulfate (CS) were shown to delay X-Ray progression of OA [10,11]. Glucosamine chloride along with CS was shown to reduce symptoms of moderate-severe painful knee OA [12]. GS and CS supplements declined 5-year operative risk after its discontinuation [13]. The special once-a-day formulation, 1500mg of glucosamine sulfate as emphasized in the Glucosamine Unum In Die (once a day) Efficacy (GUIDE) trial recently reported, tended to be more efficacious than acetaminophen [14]. If GC and CS may help to OA should this agents have antibacterial properties? We decided to examine the antibacterial activity of GS, CS separately and both in one solution and as a trademark compound Megagluflex (MGF) on E. coli growth in vitro [15]. MGF inhibited E. coli growth significantly (p=0.001) in MIC of 1 mg/ml and higher. Close to expired time antibacterial activity declined and persisted at concentration of 100 mg/ml only. Solutions of vitamine C, a component of Megagluflex, and control media did not affect E. coli growth. Manganese sulfate solution in concentrations appropriate to the tested trademark Megagluflex, as well as solutions with appropriate pH and osmolality did not affect E.coli growth. Solutions of glucosamine sulfate and chondroitine sulfate separately and in one solution mildly inhibited E. coli growth in one of 5 experiments only in concentration of 50mg/ml, which was in accordance to GS and CS concentration in the MGF trademark. MGF, GS and CS testing was negative for possible bacterial contamination. The pH range of GC-containing solution was 5.0-5.3. The pH of control solution was 6.9. Further study showed antibacterial envirenmental activity of glucosamine sulfate [16,17]. We found high percentage of infection events in OA group (up to 80%) vs controls (10%). It does not look obvious. We would like to suggest two main reservoirs of pathogen collection. The upper one includes oral cavity, respiratory tract, nasopharyngeal and cervical lymph nodes. The lower collector comprises of gastro-colon, urinary tract, genital system and prostate. We suggest spread of PAMP’s (pathogen associated microbial particles) to nearest joints. Upper distribution is to hand-fingers joints and lower spread happens to knees, hip joints, pelvis joints. Spine might be damaged from portal and vena cava collectors. Skin may be origin for all joints. Fatty liver LPS-LBP-CD14 activation seems to be very expected mechanism. What conclusions follow such way of thinking? Should we start to treat OA with antibacterial medication and face complications of antibiotic resistance? Should we improve barrier function of liver? How will we be able to overcome the liver fat deposits? Should we assess risk-benefit ratio of profit and flaws of antibiotic therapy and use it any case in order to reduce bacterial mass and bacterial spread?
May be there is a sense to turn to natural antibacterial substances (honey) and plants, bacterial lowering diet, colon cleaning procedures, oral cavity sanitation, effective control of chronic infections? How we can estimate efficacy of our means? You are right! We can do it by dynamic measuring of the deviation angle and visible palpable joint score.
Conclusion
In conclusion we proposed new non radiological quantitative method for assessment of finger joint OA. That was not informative for knee OA. We observed high percentage of infection history in OA group. This trial is only initial step. Larger cohort studies are needed to confirm or deny validity of quantitative assessment of finger and knee osteoarthritis and its relation to infectious factors.
References
- Brooks PM. Impact of osteoarthritis on individuals and society: How much disability? Social consequences and health economic implications. Curr. Opin. Rheumatol. 14, 573-577 (2002).
- Helliwell PS, Firth J, Ibrahim GM et al. Development of an Assessment Tool for Dactylitis in Patients with Psoriatic Arthritis. J. Rheumatology. 32, 1745-1750 (2005).
- Rozin AP, Militianu D, Edoute Y. OARSI response criteria in assessment of co-trimoxazole influence on refractory knee osteoarthritis during prophylaxis of recurrent UTI. EULARBerlin 2004, Ann. Rheum. Dis. 63, 358 (2004).
- Altman R, Asch E, Bloch D et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the knee. Arthritis. Rheum. 29, 1039-1049 (1986).
- Zang W, Doherty M, Peat G et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann. Rheum. Dis. 69, 483-9 (2010).
- Altman R, Alarcon G, Appelrouth D et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis. Rheum. 33, 1601-1610 (1990).
- Zang W, Doherty M, Leeb BF et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis. Report of task force of ESCISIT. Ann. Rheum. Dis. 68, 8-17 (2009).
- Anderson JJ, Felson DT, Meenan RF et al. Which traditional measures should be used in rheumatoid arthritis clinical trials? Arthritis. Rheum. 32, 1093-9 (1989).
- Rozin AP, Gaitini D, Toledano K et al. Is spinal osteophytosis associated with fatty liver? Rheumatology Reports. 4, e:4: 9-12 (2012).
- Reginster JY, Deroisy R, Rovati LC. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomized, placebo-controlled clinical trial. Lancet. 357, 251-256 (2001).
- Reginster JY, Kahan A, Vignon E. A two-year prospective, randomized, double-blind, controlled study assessing the effect of chondroitine4&6 sulfate on the structural progression of knee osteoarthritis: STOPP (Study on Osteoarthritis Progression Prevention). ACR. L42 (2006).
- Clegg DO, Reda DJ, Harris CL et al. Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis. NEJM. 354, 795-808 (2006).
- Altman RD, Abadie E, Avouac B et al. Total joint replacement of hip or knee as an outcome measure for structure modifying trials in osteoarthritis. Osteoarthritis. Cartilage. 13, 13-19 (2005).
- Herrero-Beaumont G, Roman Ivorra JA, del Carmen-Trabado M et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: a randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis. Rheum. 56, 555-567 (2007).
- Rozin AP, Goldstein M, Sprecher H. Antibacterial activity of glucosamine sulfate and chondroitine sulfate? Clin. Exp. Rheum. 26, 509-510 (2008).
- Rozin AP. Glucosamine sulfate – environmental antibacterial activity. Clin. Rheumatol. 1221-1223 (2009).
- Rozin AP. Antibiotic therapy for osteoarthritis? Medicina. Interna (Rom). 7, 61-66 (2010).
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref