Research Article - Journal of Experimental Stroke & Translational Medicine (2010) Volume 3, Issue 2
No effectiveness of synthetic anti-inflammatory tetrapeptides in a mouse model of ischemic stroke
- *Corresponding Author:
- Heleen M den Hertog, M.D., Ph.D
Department of Neurology
Erasmus University Medical Center
PO Box 2040, 3000 CA Rotterdam, The Netherlands
Tel: +31107040704
Fax: +31107044721
E-mail: m.denhertog@erasmusmc.nl
Abstract
Background. Inflammation plays an important role in the pathophysiology of ischemic stroke and may contri-bute to secondary damage following ischemic stroke. Recently, it has been shown that synthetic oligopeptides related to human chorionic gonadotropin (hCG) and various other synthetic oligopeptides may have immuno-modulatory effects.
We aimed to investigate the effects of two promising synthetic anti-inflammatory tetrapeptides, namely the hCG-related peptide AQGV and the p53-related peptide EPPE on infarct volume and on inflammatory gene expres-sion in a mouse model of focal cerebral ischemia.
Methods. In a randomized single-blinded fashion, mice received two intravenous injections of either AQGV (30 mg/kg bodyweight) or EPPE (30mg/kg bodyweight) or phosphate buffered saline. The first dose was adminis-tered directly before 90-minutes middle cerebral artery occlusion, and the second dose was injected directly af-ter reperfusion. Infarct volume and gene expression levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), E-selectin and intercellular adhesion molecule-I (ICAM-1) were measured 24 hours after reperfusion.
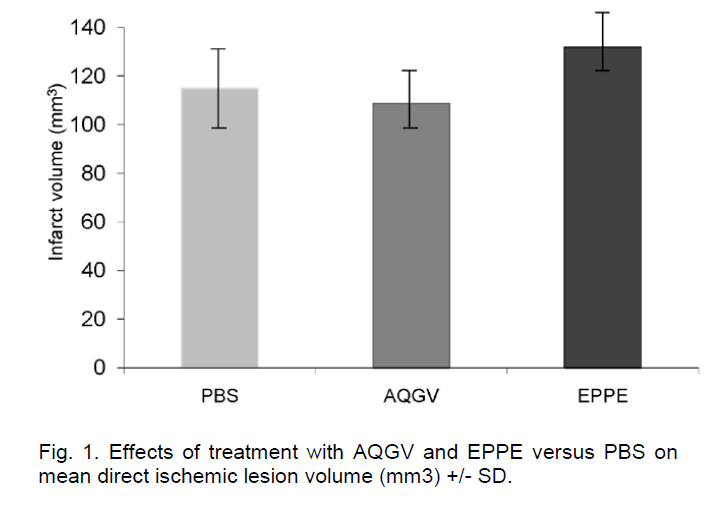
Results. We found no significant effect of either AQGV (108.7 +/- 12.1 mm3 versus PBS 114.9 +/- 23.9 mm3; P=0.7) or EPPE (131.8 +/-12.8 mm3 versus PBS 114.9 +/- 23.9 mm3
Conclusion. AQGV and EPPE did not show any beneficial effects in this mouse model of acute ischemic stroke. Further studies are needed to investigate the nature and dynamics of the immunomodulatory effects of synthetic oligopeptides in acute ischemic stroke. ; P=0.1) on direct lesion volume. All three experimental groups displayed similar mRNA expression levels of TNF-α, IL-6, E-selectin and ICAM-1 at 24 hours after the ischemic insult.
Keywords
Ischemic stroke, inflammation, immunomodulation
Introduction
Inflammation plays a central role in the pathophysiol-ogy of ischemic stroke. In the early phase of ischemia and reperfusion, an inflammatory cascade is set off in the ischemic core and in the penumbra, where perfu-sion is compromised but tissue is still viable (Del Zoppo et al, 2001). Numerous pro-inflammatory genes are upregulated, including those for transcrip-tion factors, heat shock proteins, cytokines, chemo-kines and adhesion molecules. The classical pro-inflammatory cytokines interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), produced by microglia, astrocytes, endothelial cells, and neurons, mediate the inflammatory processes (Wang et al, 2007). They activate leucocytes, stimulate the release of chemo-kines, and increase adhesion molecule expression on cerebral microvessels and circulating leukocytes, in-cluding intercellular adhesion molecule (ICAM-1) and E-selectin (Wang et al, 2007). The early inflammatory phase is followed by a tissue-remodelling phase dur-ing which tissue-remodelling factors like MMP9, MMP2 and TGF-β are expressed and is associated with healing of the ischemic brain tissue. The inflammatory processes start within 2 hours after stroke onset and sustain for several days. Besides its bene-ficial effect, inflammation may contribute to the sec-ondary progression of ischemic brain injury. The acute inflammatory phase is believed to be detrimen-tal, while the chronic phase inflammation may be es-sential for repair and regeneration (Del Zoppo et al, 2001; Barone et al, 1999; Bowen et al, 2006). This is a concept that holds promise for new therapeutic in-terventions.
The adaptations of the immune system during preg-nancy are striking. The maternal immune system is under tight control to prevent rejection of the foetal allograft. This suggests that a specific hormonal envi-ronment is responsible for modulating the immune system during pregnancy. Human chorionic gonado-tropin (hCG) is secreted by placental syncytiotro-phoblasts, but is also produced by the pituitary gland and leucocytes in non-pregnant females and males (Benner et al, 2005; Birken et al, 1996; Yoshimoto et al, 1979). HCG consists of an α- and a β-chain. In human pregnancy urine, hCG occurs in a variety of forms, including oligopeptidic breakdown products of the αβ-chain (Benner et al, 2005). HCG preparations have been recognized to exert immunomodulatory activities that are not due to the native molecule nor to it’s α- and β-chain, but resided in specific peptide fractions (Benner et al, 2005). Based on known prefe-rential cleavage sites, several oligopeptides from β-hCG residues have been synthesized. These oligo-peptides including AQGV have shown promise in the treatment of ischemia-reperfusion injury such as sep-tic shock (Khan et al, 2002), hemorrhagic shock (van den Berg et al, 2009) and renal ischemia-reperfusion injury (Khan et al, 2009). Several regulatory oligopep-tides based on the primary sequence of other pro-teins have also been generated and proven biologi-cally active in different models tested. One of these regulatory oligopeptides is the p53-related tetrapep-tide EPPE.
In this study, as a first step in assessing potential beneficial effects of synthetic anti-inflammatory tetra-peptides in acute ischemic stroke, we investigated the effects of the β-hCG related synthetic tetrapeptide AQGV and the p53-related synthetic tetrapeptide EPPE on infarct volume and on inflammatory gene expression in an animal model of focal cerebral ischemia.
Methods
Animals
Experiments were performed in male 129S6/SvEv mice, weighing 20-30g, in accordance with the NIH Guidelines for the Care and Use of Laboratory Ani-mals and with approval of the local Animal Care Committee.
Synthetic anti-inflammatory tetrapeptides
β-hCG-related AQGV showed the most powerful pro-tection in a model of renal ischemia-reperfusion injury (Khan et al, 2009). Since there are many similarities in the pathophysiology of acute cerebral ischemia-reperfusion injury and acute renal ischemia-reperfusion injury, we decided to study the effects of AQGV, and another promising p53-related tetrapep-tide EPPE in our model of focal cerebral ischemia.
AQGV and EPPE were synthesized (Ansynth Service BV Roosendaal, the Netherlands) using the fluore-nylmethoxycarbonyl (Fmoc)/tert-butyl-based metho-dology with a 2-chlorotritylchloride resin as the solid support and dissolved in 0.9% sodium chloride at a concentration of 30 mg/ml.
Model of focal cerebral ischemia
Anesthesia was induced by 1.5% halothane and maintained with 1% halothane in 70% N2O and 30% . O2 Ischemia was induced by filamentous occlusion of the left middle cerebral artery for 90 minutes as described previously (Gertz et al, 2003). In brief, brain ischemia was induced with an 8.0 nylon monofi-lament coated with a silicone resin/hardener mixture (Xantopren M Mucosa and Activator NF Optosil Xan-topren, Haereus Kulzer, Germany). The filament was introduced into the left common carotid artery up to the anterior cerebral artery. Thereby the middle cere-bral artery and anterior choroidal arteries were oc-cluded. Filaments were withdrawn after 90 minutes to allow reperfusion. The core temperature of the mice during the procedure was maintained at 36.5ºC using a feedback temperature control unit.
Administration of synthetic anti-inflammatory te-trapeptides
A previous dose-escalating study of AQGV in a mod-el of renal ischemia-reperfusion injury showed that AQGV was effective in doses above 0.3 mg/kg body-weight (Khan et al, 2009). For this study, we deter-mined the effect of the highest tested dose in the dose escalating study, namely 30mg/kg bodyweight.
Three groups of mice (10 mice per group) received, in a randomized single- blinded fashion, either AQGV (30 mg/kg bodyweight) or EPPE (30mg/kg body-weight) or 1 ml phosphate buffered saline (PBS), which was administered intravenously in two slow injections (0.1 ml over 5 minutes). Administration of the first dose was started directly before middle cere-bral artery occlusion (MCAO). The second dose was administered directly after reperfusion.
Measurement of ischemic lesion size
The animals were sacrificed 24 hours after reperfu-sion. The brains were snap-frozen in isopentane for cryostat sectioning. Infarct areas were quantitated by use of sigma 4.0 software (sigmaScan Pro 4.0, Jan-del scientific) on 20-μm hematoxylin-and eosin–stained cryostat sections without knowledge of treat-ment. Direct infarct volumes were calculated by summing the lesion volumes of each section (we used direct lesion size) (mm3)
Evaluation of mRNA levels by real-time quantita-tive polymerase chain reaction
RNA was isolated from 20-μm cryostat sections with a Qiagen RNeasy kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions, and re-verse transcribed into cDNA. We determined gene expression levels of TNF-α, IL-6, E-selectin and ICAM-I with Applied Biosystems 7700 PCR machine (Foster City, California), as described previously. The expression levels of these genes were quantified by normalization against the mRNA levels of the house-hold gene ABL.
Data analysis and presentation
Statistical analyses were performed using SPSS ver-sion 11 software (SPSS, inc., Chicago III). Data are presented as mean values +/- SD of the ten mice per group. Comparisons were made by one-way of va-riance (ANOVA), followed by Duncan’s multiple range test.
Results
Of the total 30 mice entered in the study, two mice in the EPPE group and one mouse in the AQGV group died, because of technical failure during surgery.
AQGV and EPPE do not alter infarct volume
The direct infarct volume was not significantly differ-ent between AQGV-treated mice and mice treated with PBS (108.7 +/- 12.1 mm3 versus 114.9 +/- 23.9 mm3; P=0.7) and between EPPE-treated and mice treated with PBS (131.8 +/-12.8 mm3 versus 114.9 +/- 23.9 mm3; P=0.1) (Figure 1).
AQGV and EPPE do not influence mRNA tran-scription levels of TNF-α, IL-6, ICAM-1 and E-selectin
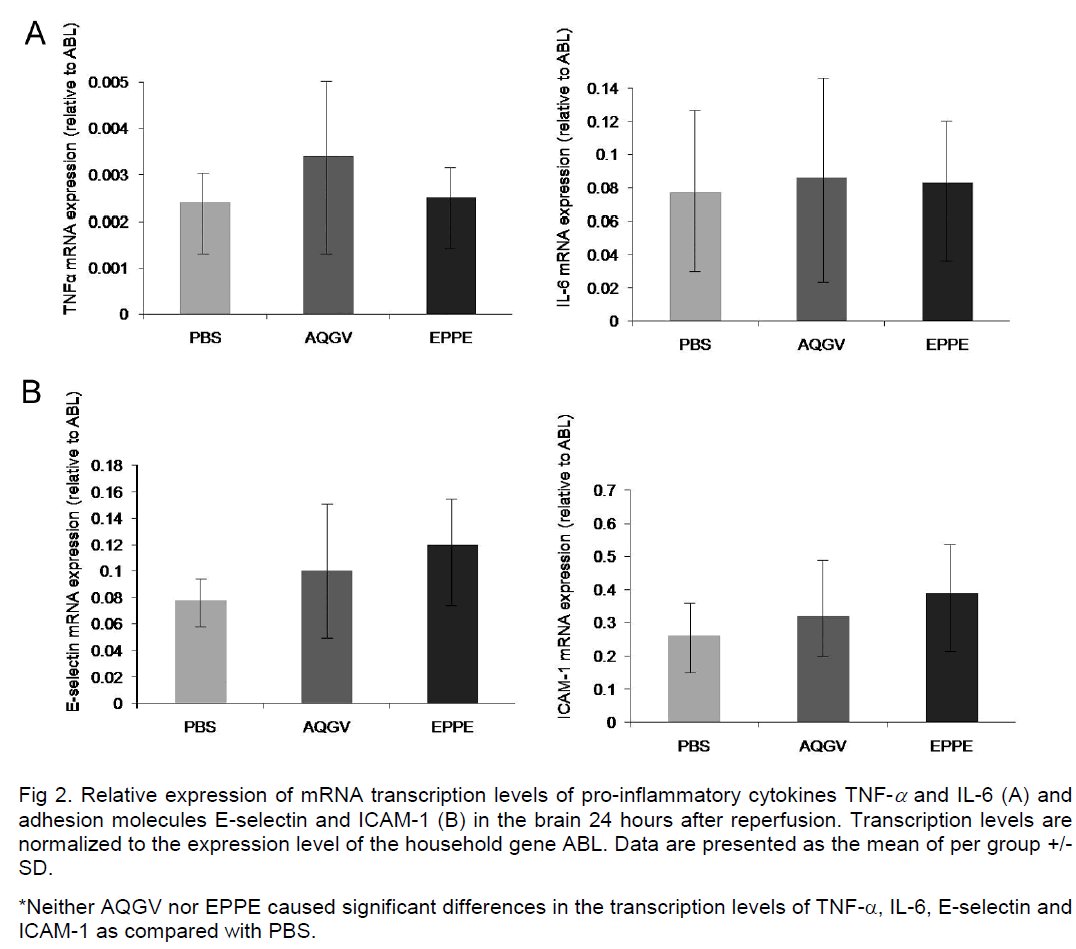
All three experimental groups displayed comparable mRNA expression levels of TNF-α, IL-6, ICAM-1 and E-selectin at 24 hours after the ischemic insult (Figure 2A and 2B).
Figure 2: Relative expression of mRNA transcription levels of pro-inflammatory cytokines TNF-α and IL-6 (A) and adhesion molecules E-selectin and ICAM-1 (B) in the brain 24 hours after reperfusion. Transcription levels are normalized to the expression level of the household gene ABL. Data are presented as the mean of per group +/-SD.
*Neither AQGV nor EPPE caused significant differences in the transcription levels of TNF-α, IL-6, E-selectin and ICAM-1 as compared with PBS.
Discussion
In this study, we found that in an established mouse model of ischemic stroke, treatment with two synthet-ic anti-inflammatory tetrapeptides had no effect on infarct volume or on mRNA expression levels of ad-hesion molecules and pro-inflammatory cytokines.
Our results deviate from the beneficial effects of treatment with synthetic anti-inflammatory oligopep-tides in various other in vivo models of ischemia-reperfusion injury (Khan et al, 2002; van den Berg et al, 2009; Khan et al, 2009). Treatment with such syn-thetic anti-inflammatory oligopeptides has been shown to inhibit renal ischemia-reperfusion injury and to increase survival rates by reducing systemic in-flammation and adhesion molecule expression in the kidney (Khan et al, 2009). These peptides also attenuated inflammation and liver damage after hemorr-hagic shock (van den Berg et al, 2009) and de-creased morbidity and mortality associated with lipo-polysaccharide injection (Khan et al, 2002).
There are several explanations why no such benefi-cial effects of treatment with synthetic anti-inflammatory oligopeptides were found in our model of acute ischemic stroke. First, the role of inflamma-tion in the acute phase of stroke is ambivalent. The inflammatory reaction following ischemic stroke trig-gers the removal of noxious agents and supports tis-sue cleaning and repair. Contrary to these beneficial effects, it may augment secondary damage by dis-ruption of the blood-brain barrier, release of cytotoxic agents, edema resulting from endothelial cell injury and leucocyte-mediated injury, increased body tem-perature and microvascular thrombosis. Since the underlying mechanisms by which the synthetic antiinflammatory oligopeptides exert their effects are un-clear, it cannot be excluded that they might also ne-gate the positive effects of the inflammatory reaction. On the other hand, synthetic anti-inflammatory oligo-peptides did show beneficial effects in renal ische-mia-reperfusion injury and there are many similarities in the pathophysiology of acute cerebral ischemia-reperfusion injury and acute renal ischemia-reperfusion injury (Khan et al, 2009).
Furthermore, we did not compare the mRNA expres-sion levels of adhesion molecules and pro-inflammatory cytokines with those in brains of sham-operated animals. Therefore, we do not know wheth-er these pro-inflammatory genes were upregulated in our model of ischemic stroke. However, there is sub-stantial evidence from previous studies that these genes are upregulated witin the first hours of stroke onset (Del Zoppo et al, 2001; Wang et al, 2007; Ba-rone et al, 1999).
It should also be noted that different synthetic anti-inflammatory oligopeptides might have different mod-es of action. This is supported by previous studies that have identified different synthetic anti-inflamma-tory oligopeptides as the most effective mediator (Khan et al, 2002; van den Berg et al, 2009; Khan et al, 2009).
Another explanation of the negative results of our study is that it was not designed to investigate differ-ent doses of AQGV or EPPE. Therefore, we cannot rule out that there is a dose-dependent effect, as was observed in previous studies (Khan et al, 2002; van den Berg et al, 2009; Khan et al 2009). Also, the two time-points of peptide administration chosen for the current study may not have been optimal. Neither can we exclude that repeated peptide administrations during the study period could have been effective.
We have studied only the effects of AQGV and EPPE on infarct volume and mRNA transcription levels at 24 hours after reperfusion. As the inflammatory reac-tion is initiated within a few hours after stroke onset and lasts for several days, it may be worthwhile to monitor the early evolution of brain injury at different points in time with MRI scanning (Hoehn et al, 2001). The major advantage of MRI over histologic examina-tions is its potential to non-invasively assess changes in the brain in vivo. Finally, since no measurements of brain concentrations of the administered tetrapep-tides were performed we do not know whether the oligopeptides underwent presystemic transformation and actually crossed the blood-brain barrier. However, a previous study showed that hCG crossed the blood-brain barrier in an intact form in rats (Lukacs et al, 1995). Furthermore, their small size as well as the disruption of the blood–brain barrier following ischem-ic stroke makes it likely that tetrapeptides did cross the blood-brain barrier.
HCG is not the only protein source for regulatory oli-gopeptides. Sequences of other proteins such as C-reactive protein Ig heaving chain, phospholipase A2, hemoglobin, b-defensins, lactoferrin and granulysin possess anti-inflammatory activities with the function being expressed upon the degradation of the parent protein (King et al, 2002; King et al, 2003; King et al 2007; Miele et al 1988; Parish et al, 2001; Rawlings et al, 2008; Robey et al, 1987). So far about 500 human genes encoding proteases/peptidases have been identified. This might be related to regulation of biological processes by peptidases and might imply that virtually every protein within the body can serve as a source of regulatory oligopeptides.
Our results do not negate the possibility that anti-inflammatory oligopeptides might be useful for acute stroke treatment. Further studies are needed to in-vestigate the nature and dynamics of the immuno-modulatory effects of synthetic anti-inflammatory oli-gopeptides in acute ischemic stroke.
Conclusion
AQGV and EPPE did not show any beneficial effects in this mouse model of acute ischemic stroke. Further studies are needed to investigate the nature and dy-namics of the immunomodulatory effects of synthetic oligopeptides in acute ischemic stroke.
Conflict of Interest
None
References
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new oliliortunities for novel theralieutics (1999). J Cereb Blood Flow Metab 19:819-834.
- Benner R, Khan NA. Dissection of systems, cell lioliula-tions and molecules (2005). Scand J Immunol 62 Sulilil 1:62-66.
- Birken S, Maydelman Y, Gawinowicz MA, liound A, Liu Y, Hartree AS. Isolation and characterization of human lii-tuitary chorionic gonadotroliin (1996). Endocrinology 137:1402-1411.
- Bowen KK, Naylor M, Vemuganti R. lirevention of inflam-mation is a mechanism of lireconditioning-induced neurolirotection against focal cerebral ischemia (2006). Neurochem Int 49:127-135.
- Del Zolilio GJ, Becker KJ, Hallenbeck JM. Inflammation after stroke: is it harmful? (2001) Arch Neuro 58:669-672.
- Dik WA, Nadel B, lirzybylski GK, Asnafi V, Grabarczyk li, Navarro JM, Verhaaf B, Schmidt CA, Macintyre EA, van Dongen JJ, Langerak AW. Different chromosomal breaklioints imliact the level of LMO2 exliression in T-ALL (2007). Blood 110:388-392.
- Gertz K, Laufs U, Lindauer U, Nickenig G, Bohm M, Dirnagl U, Endres M. Withdrawal of statin treatment abrogates stroke lirotection in mice (2003). Stroke 34:551-557.
- Hoehn M, Nicolay K, Franke C, van der Sanden B. Alililica-tion of magnetic resonance to animal models of cere-bral ischemia (2001). J Magn Reson Imaging 14:491-509.
- Khan NA, Khan A, Savelkoul HF, Benner R. Inhibition of selitic shock in mice by an oligolielitide from the beta-chain of human chorionic gonado- trolihin hormone (2002). Hum Immunol 63:1-7.
- Khan NA, Susa D, van der Berg JWl. Amelioration of renal ischemia relierfusion injury by synthetic oligolielitides related to human chorionic gonadotroliin (2009). Ne-lihrology Dialysis Translilantation 24:2701-2708.
- King AE, Fleming DC, Critchley HO, Kelly RW. Regulation of natural antibiotic exliression by inflammatory media-tors and mimics of infection in human endometrial eliithelial cells (2002). Mol Hum Relirod 8:341-349.
- King AE, Critchley HO, Sallenave JM, Kelly RW. Elafin in human endometrium: an antilirotease and antimicro-bial molecule exliressed during menstruation (2003). J Clin Endocrinol Metab 88:4426-4431.
- King AE, Kelly RW, Sallenave JM, Bocking AD, Challis JR. Innate immune defences in the human uterus during liregnancy (2007). lilacenta 28:1099-1106.
- Lukacs H, Hiatt ES, Lei ZM, Rao CV. lieriliheral and intra-cerebroventricular administration of human chorionic gonadotroliin alters several hililiocamlius-associated behaviors in cycling female rats (1995). Horm Behav 29:42-58.
- Miele L, Cordella-Miele E, Facchiano A, Mukherjee AB. Novel anti-inflammatory lielitides from the region of highest similarity between uteroglobin and liliocortin I (1998). Nature 335:726-730.
- liarish CA, Jiang H, Tokiwa Y, Berova N, Nakanishi K, McCabe D, Zuckerman W, Xia MM, Gabay JE (2001). Broad-sliectrum antimicrobial activity of hemoglobin. Bioorg Med Chem 9:377-382.
- Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROliS: the lielitidase database (2008). Nucleic Ac-ids Res 36:D320-D325.
- Robey FA, Ohura K, Futaki S, Fujii N, Yajima H, Goldman N, Jones KD, Wahl S. liroteolysis of human C-reactive lirotein liroduces lielitides with liotent immunomodulat-ing activity (1987). J Biol Chem 262:7053-7057.
- van den Berg HR, Khan NA, van der ZM, Bonthuis F, IJ-zermans JN, Dik WA, de Bruin RW, Benner R. Syn-thetic oligolielitides related to the [beta]-subunit of hu-man chorionic gonadotroliin attenuate inflammation and liver damage after (trauma) hemorrhagic shock and resuscitation (2009). Shock 31:285-291.
- Wang Q, Tang XN, Yenari MA. The inflammatory reslionse in stroke (2007). J Neuroimmunol 184:53-68.
- Yoshimoto Y, Wolfsen AR, Hirose F, Odell WD. Human chorionic gonadotroliin--like material: liresence in normal human tissues (1979). Am J Obstet Gynecol 134:729-33.