Review Article - Imaging in Medicine (2010) Volume 2, Issue 3
Noninvasive cardiovascular imaging in coronary artery disease
Nina Ghosh1, Ronnen Maze2, Benjamin Chow1, Carole Dennie3, Alexander Dick1 and Terrence Ruddy†1
1 Division of Cardiology, University of Ottawa Heart Institute, 40 Ruskin Street, Ottawa, ON K1Y 4W7, Canada
2Department of Medicine, University of Ottawa, ON, Canada
3Department of Radiology, University of Ottawa, ON, Canada
- *Corresponding Author:
- Terrence Ruddy
Division of Cardiology
University of Ottawa Heart Institute 40 Ruskin Street
Ottawa, ON K1Y 4W7, Canada
Tel.: +1 613 761 4085
Fax: +1 613 761 4929
E-mail: truddy@ottawaheart.ca
Abstract
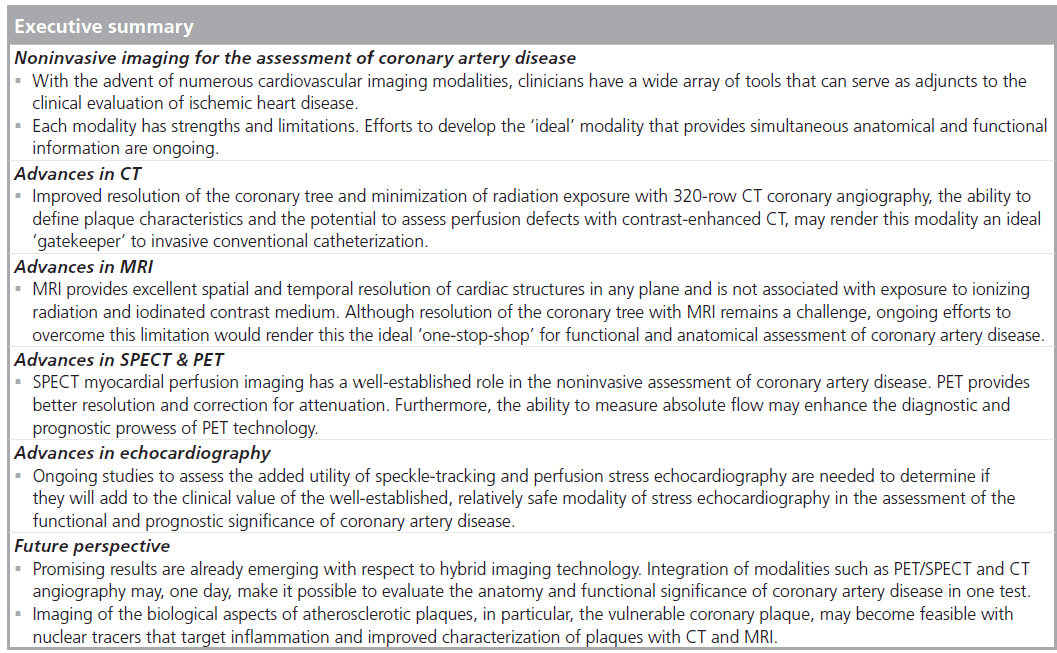
Coronary heart disease accounts for 7.2 million deaths per year worldwide. Noninvasive imaging modalities have the potential to provide important diagnostic and prognostic information while avoiding the potential risks of invasive coronary angiography. The ideal imaging modality should provide the optimal combination of patient safety, anatomical, functional, diagnostic and prognostic information (i.e., the ‘one-stop-shop’). Exciting new developments in cardiac imaging, including hybrid SPECT/PET‑CT technology, refinements in MRI angiography and vulnerable plaque imaging, may make this ‘ideal’ modality achievable. However, continued refinements and further prospective data to validate the use of such new technologies are required, particularly in the context of limited healthcare resources and economic constraints.
Keywords
cardiovascular MRI; CCTA ; CMR imaging; CT coronary angiography ; noninvasive imaging; PET; SPECT; stress echocardiography
Despite a reported reduction in age-adjusted cardiovascular disease death rates in the USA of more than 58% between 1972 and 2004, the WHO estimates that coronary heart disease (CHD) continues to account for 7.2 million deaths per year worldwide [1,101]. There continues to be an imperative to develop and incorporate innovative tools, such as noninvasive cardiovascular imaging, to prevent, diagnose and guide intervention in CHD and, in turn, to improve patient outcomes.
With the advent of myriad imaging technologies in cardiovascular medicine, clinicians, patients and healthcare systems are challenged to appreciate the clinical and cost implications of incorporating such technologies into practice. Despite the accelerating dissemination of imaging, such as SPECT‑PET perfusion scans, cardiac CT angiography (CTA), stress echocardiography and cardiovasular magnetic resonance (CMR), the relative diagnostic merits of each test, how they should be applied and how they can complement each other to improve patient outcome are only starting to be elucidated. The immense number of potential applications of these technologies to coronary artery disease (CAD) reflects the complexity of atherosclerotic heart disease itself, which encompasses several phases, including a subclinical phase of plaque build-up, where screening imaging modalities may be useful, and acute chest pain syndromes, where imaging tests used to confirm acute plaque rupture, may facilitate patient care.
In the assessment of CAD, a common algorithm includes confirmation of the diagnosis of coronary disease, prognostication with risk stratification and evaluation of therapy. This article uses an evidence-based approach to summarize the role of echocardiography, PET/SPECT, cardiac CT and CMR in the diagnosis and prognostication for both stable and unstable CAD.
Coronary calcium score
Coronary calcification is seen predominantly in the context of atherosclerosis. Within a coronary artery, the quantity of calcification is closely related to the extent of atherosclerotic plaque burden. Although the burden of coronary calcium tends to be greater in persons experiencing acute coronary syndromes (ACSs) compared with controls, coronary calcification occurs in more advanced lesions and the site of its accumulation does not seem to predict lesion vulnerability.
To detect and quantify coronary calcification by CT, high-resolution cross-sectional data of the heart are acquired without contrast enhancement. Both electron beam CT and an electrocardiogram (ECG)-gated multidetector row CT (MDCT) can be used to detect coronary artery calcium (CAC). Areas of calcification are defined as contiguous pixels (>1 mm2) with a density of 130 Hounsfield Units or more and most commonly quantified with the Agatston score. To obtain the Agatston score, the area of each calcified coronary lesion is measured and is multiplied by a coefficient from 1 to 4 (according to the highest attenuation within the lesion). The Agatston score equals the sum of all of these areas. Other scores such as volume score and mass score are alternative scoring methods that may be more accurate than Agatston scores [2]. However, the Agatston score has the most extensive and robust prognostic data to date.
Coronary calcium score for diagnosis of CAD in symptomatic & asymptomatic populations
Although CAC is not specific for obstructive coronary disease and is found in both nonobstructive and obstructive atherosclerotic plaques, evidence indicates that the extent of coronary artery calcification can predict the extent of angiographically determined CAD. One study examined 308 symptomatic patients with suspected but previously unknown CAD who were also undergoing selective coronary angiography. The study found that the total CAC scores were better independent predictors of the number of coronary segments with at least 50% stenoses than SPECT variables and risk factors defined by the National Cholesterol Education Program [3].
Coronary calcium score for prognostication in CAD
Global risk calculators use traditional risk factors, such as sex, age, blood pressure, smoking, cholesterol levels and diabetes, to estimate a patient’s risk of cardiovascular disease. However, these calculations are based on population estimates and may not directly apply to the individual being evaluated [1,4]. Coronary calcium scoring has been demonstrated to predict coronary events independently of standard risk factors and scores [4–6]. For example, Detrano et al. demonstrated that, in a population- based sample of subjects of various ethnic origins, the addition of coronary calcium score to standard risk factors significantly improved the prediction of major coronary events [5]. Similarly, in an asymptomatic population with at least one traditional cardiac risk factor excluding diabetes, the addition of CAC scores allowed better prediction of future cardiac events than the Framingham Risk Score alone [6]. Thus, the degree of CAC is useful in predicting future cardiovascular events in both asymptomatic and symptomatic patients with suspected CAD.
The diagnostic and prognostic implications of a CAC score of 0 in symptomatic or asymptomatic intermediate-risk patients are clinically relevant. A recent systematic review evaluated the prognostic relevance of the absence of CAC [7]. This included 13 studies assessing the relationship of CAC with adverse cardiovascular outcomes in 64,873 asymptomatic patients. In this cohort, 146 of 25,903 patients without CAC (0.56%) had a cardiovascular event during a mean follow-up period of 51 months.
In the seven studies assessing the prognostic value of CAC in a symptomatic population, 1.8% of patients without CAC had a cardiovascular event [7]. However, the American Heart Association (AHA)/American College of Cardiology Foundation (ACCF) guidelines suggest that there is insufficient evidence to reduce the intensification of treatment in intermediate-risk patients with a CAC score of 0. Recent data corroborate the contention that the absence of coronary calcification is not sufficient to exclude significant coronary stenosis or the need for revascularization [8]. In a substudy of the Coronary Evaluation Using Multidetector Spiral Computed Tomography Angiography Using 64 Detectors (CORE64) study in which patients underwent calcium scoring up to 30 days prior to 64-detector cardiac CT and/or conventional angiography, 72 of the 291 patients had a calcium score of 0. Of these patients, 14 (19%) had at least one or 50% stenosis. The sensitivity of a calcium score of 0 to predict the absence of at least 50% stenosis was only 45%. Furthermore, 12.5% of patients with a calcium score of 0 underwent revascularization within 30 days of calcium score testing [9]. By contrast, Budoff et al. demonstrated that a cohort of asymptomatic patients with little or no CAC are at very low risk of future cardiovascular events [10]. Minimal CAC was associated with a threefold risk of a hard CHD event [1–9,101] compared with those with no CAC [10]. However, the difference in results between the latter two studies may have been modified by the fact that the CORE64 trial had a large proportion of symptomatic patients while only asymptomatic individuals were included in the study by Budoff et al. The effect of symptomatic status on the significance of a 0 calcium score was addressed in a group of 210 consecutive patients referred for CAC scoring. Sensitivity, specificity, positive predictive value and negative predictive value of CAC in the symptomatic population for detection of obstructive CAD were 86, 42, 28 and 92%, respectively. In the asymptomatic group, sensitivity, specificity, positive predictive value and negative predictive value were 100, 32, 18 and 100%, respectively. Based on these results, a CAC score of 0 has a much better negative predictive value to exclude obstructive CAD in asymptomatic individuals than symptomatic individuals [11]. Thus, the symptomatic status of the patient must be taken into account before drawing conclusions regarding the implications of a coronary calcium score of 0.
Clinical decision-making based on CAC scores
Although CAC has been found to have prognostic value in both symptomatic and asymptomatic patients [12,13], the implications of how the clinician should act on positive results, particularly in asymptomatic patients, are uncertain. Arad et al. randomized asymptomatic individuals between 50 and 70 years of age with coronary calcium scores in at least the 80th percentile for age and gender to atorvastatin 20 mg or placebo [14]. Despite a significant reduction in total cholesterol in patients randomized to atorvastatin, treatment did not reduce clinical events [14]. In another study, randomization of hypercholesterolemic postmenopausal women to aggressive versus moderate lipid-lowering therapy did not translate into reproduced progression of coronary calcification as measured by electron-beam tomography [15]. These results were confirmed in a subsequent study in which asymptomatic patients with a CAC score of at least 30 were randomized to either 80 or 10 mg of atorvastatin daily over a period of 12 months. There was no relationship between ontreatment low-density lipoprotein levels and CAC progression [16]. The results of these trials suggest that, although CAC confers prognostic value in asymptomatic individuals, the decision to employ primary preventative strategies should be based on other clinical risk factors rather than CAC alone.
Risks of coronary calcium scoring
Scans used to determine calcium scores are relatively low risk. The technique is noninvasive, requires no contrast dye and radiation exposure ranges from 1.0 to 2.0 mSv [9].
Guidelines
In 2007, the ACCF and the AHA published a consensus document on the application of CAC scoring on risk assessment and evaluation of patients with chest pain [8]. This comprehensive, evidence-based document concludes with recommendations for the utility of CAC scoring in several different patient population categories. For global risk assessment, the AHA/ACCF suggest that, in intermediate-risk, asymptomatic patients, a high CAC score adds incremental prognostic value and may allow clinicians to reclassify patients into higher risk status. On the other hand, they suggest that a CAC score of 0 in such patients should not prompt the clinician to reduce the intensity of risk reduction treatment. Furthermore, they caution against the use of this modality to screen low-risk, asymptomatic patients or in patients who are already considered at a high risk of CHD as they are candidates for intensive risk-reduction therapy. The writing committee recommend that although the use of CAC scoring has been validated in non-Hispanic, Caucasian men, more data are required to understand the implications of CAC score results with regards to white women and ethnic populations.
Cardiac CT
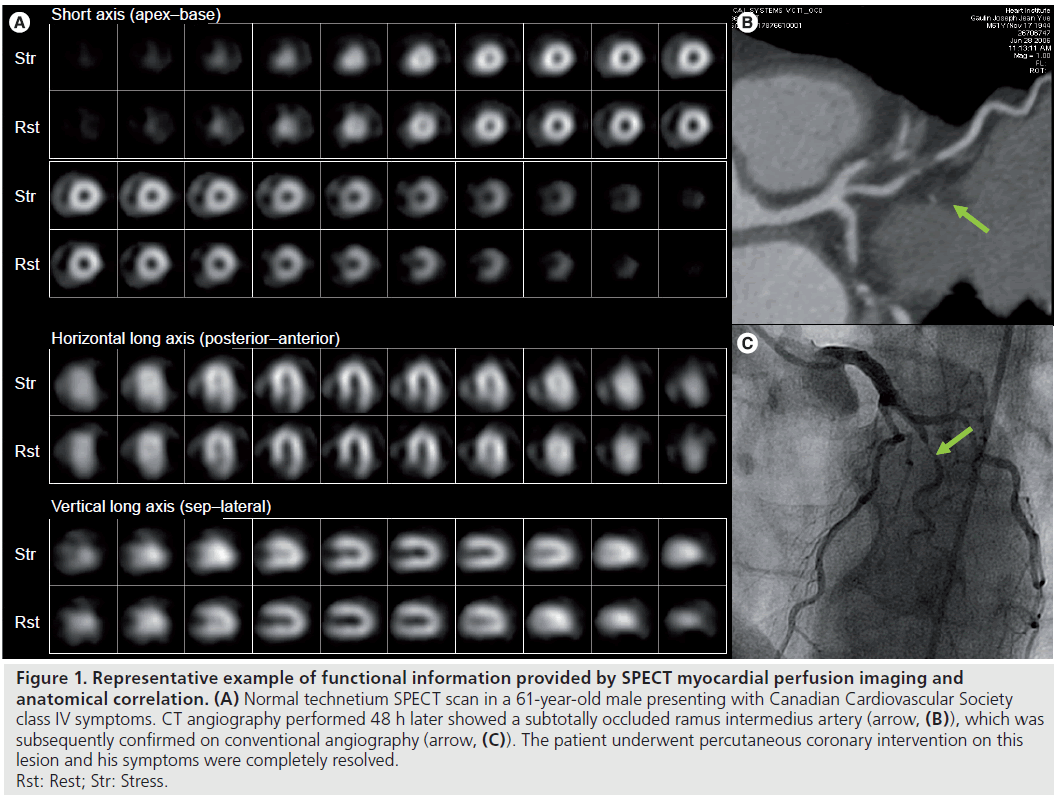
Although CAC scoring provides a low-radiation method to assess risk of future cardiovascular events, calcified plaque only represents approximately 20% of total plaque volume and may not be present early in the atherosclerotic disease process [17]. Coronary CTA (CCTA) is being increasingly used in the diagnosis of CAD (Figure 1).
Figure 1.Representative example of functional information provided by SPECT myocardial perfusion imaging and anatomical correlation. (A) Normal technetium SPECT scan in a 61-year-old male presenting with Canadian Cardiovascular Society class IV symptoms. CT angiography performed 48 h later showed a subtotally occluded ramus intermedius artery (arrow, (B)), which was subsequently confirmed on conventional angiography (arrow, (C)). The patient underwent percutaneous coronary intervention on this lesion and his symptoms were completely resolved. Rst: Rest; Str: Stress.
Two types of CT scanners – MDCT and electron beam CT – have been studied for CCTA. MDCT scanners are most widely available and clinically used.
Electron beam CT scanners, which use an electron beam in stationary tungsten targets, can acquire images at a very high imaging speed (50–100 ms) and can be ECG-triggered [18]. Electron beam CT permits quantification of calcium deposition using the Agatston method. With contrast-enhancement, electron beam CT allows visualization of the coronary artery lumen at a significantly lower spatial resolution but better temporal resolution than currently available MDCT scanner technologies.
Multidetector row CT scanners use mechanically rotating gantries that allow for acquisition of multiple slices simultaneously. Sufficient resolution without motion artifact can be achieved using MDCT if the heart rate is both regular and slow (ideally less than 65 beats per min). Many patients undergoing MDCT require oral or intravenous b‑blockers to achieve such heart rates. Temporal resolution in MDCT depends on several factors including gantry rotation times and the use of acquisition and reconstruction algorithms [19]. MDCT scanner technology is rapidly evolving and scanners that acquire 64–320 slices in one gantry rotation are now in clinical use. Dewey et al. recently demonstrated that 320‑row CT can maintain excellent diagnostic accuracy compared with cardiac catheterization while achieving a significantly smaller effective radiation dose [20].
Cardiac CT for diagnosis of CAD
Several multicenter trials have assessed the diagnostic performance of CCTA [21]. The Assessment by CCTA of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) trial prospectively evaluated 230 subjects with chest pain without known CAD in 16 different sites who underwent both CCTA and invasive coronary angiography. The prevalence of obstructive CAD in this population was 25% [21]. The sensitivity and specificity of CCTA for the detection of at least 50% stenoses were 95 and 83%, respectively. The study noted that 64‑multidetector row CCTA was particularly effective in ruling out obstructive CAD, conferring a negative predictive value of 99% for both 50% or more and 70% or more coronary stenoses on a perpatient basis. Similarly, a recent multicenter trial by Meijboom et al. with very high prevalence of CAD (68%) noted a high sensitivity and negative predictive value for diagnosis of obstructive CAD in patients without known prior CAD presenting with chest pain [22]. Miller et al. evaluated the performance of 64‑multidetector row CCTA to diagnose obstructive coronary disease (stenoses of ≥50%) in 291 patients with suspected symptomatic CAD [23]. This study population had an intermediate prevalence of CAD (56%) and found that CCTA had a sensitivity of 85% and a specificity of 90% in detecting obstructive CAD compared with conventional invasive coronary angiography. These three studies suggest that CCTA may effectively rule out significant coronary obstruction, however, variability between centers likely exists. The results also indicate that CCTA may overestimate coronary stenoses.
Several single-center studies have also evaluated the role of CCTA for triage of patients presenting to the emergency room with acute chest pain. The Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT) trial was an observational cohort study used to evaluate the value of CCTA in predicting ACS during index hospitalization and major adverse cardiac events in the following 6 months [24]. The study enrolled 368 patients presenting to the emergency department with chest pain, normal initial serum troponin levels and nondiagnostic ECGs. Prior to admission, all patients underwent 64‑slice CCTA to detect coronary plaque and stenosis greater than 50%. When CAD was absent on CCTA, sensitivity and negative predictive values for ACS were excellent (both 100%). However, specificity for the presence of plaque and stenosis for ACS was 54 and 87%, respectively. Similar results were reported in two smaller single-center studies [25,26]. Thus, in low-to-intermediate risk patients presenting with nondiagnostic biomarkers and ECG, CCTA is an excellent tool for ruling out ACS. However, in a real-world setting, specificity may be limited by the inability to determine the physiological significance of lesions of intermediate severity (<50%) and when quality images are not obtainable.
Although conventionally valued for characterizing coronary anatomy, CCTA may be useful in assessing the hemodynamic relevance of coronary artery lesions [27]. Global left ventricular function can be quantified using software capable of defining the endocardial border in the end-systolic and end-diastolic phases from short-axis reconstructions and deriving end systolic and systolic volumes by the Simpson’s method [28]. Furthermore, regional wall-motion abnormalities (defined as normokinetic, hypokinetic or akinetic/dyskinetic) may be evaluated by displaying images in cine-loop format and using a using a 17‑segment model [29]. Studies have demonstrated a good correlation between left ventricular ejection fraction (LVEF) measured using 64‑slice CT and 2D echocardiography [30] and MRI [31].
Recently, several studies have confirmed the feasibility of adenosine-induced stress CT myocardial perfusion imaging (MPI) [32,33]. Areas of hypoenhancement immediately after contrast administration correspond to perfusion defects. Conversely, delayed imaging after contrast administration may show hyperenhancement and correspond to areas of myocardial necrosis or scar [32]. Two recent studies demonstrated that adenosine stress CT can identify stress-induced myocardial perfusion defects with sensitivities and specifities similar to SPECT MPI [32,33].
CT has also demonstrated promise in the detection of microvascular obstruction postmyocardial infarction [34]. Regions of microvascular obstruction by MDCT are characterized by hypoenhancement on early imaging, despite restoration of normal flow in the infarct-related artery. In areas of microvascular obstruction, contrast material is precluded from entering the core of the damaged region owing to blockage of intramyocardial capillaries by necrosed cellular debris. Nieman et al. demonstrated that, in patients who were within 5 days of a myocardial infarction, microvascular obstruction, as detected by early hypoenhancement on MDCT, showed excellent correlation to that detected by CMR imaging [35].
Therefore, cardiac CT has become an important alternative to invasive coronary angiography for anatomical delineation of CAD, particularly under favorable conditions for imaging, including an adequately low heart rate and sufficiently low calcification, to accurately estimate stenoses. The role of adjunctive functional information derived from cardiac CT, including perfusion imaging, continues to evolve and more studies are required to further define their utility in clinical practice.
CCTA for prognostication in CAD
Recent data suggest that CTA also confers incremental prognostic value over traditional risk factors [36]. Ostrom et al. found that, in symptomatic patients without known CAD, the diagnosis of significant (≥50% luminal narrowing) stenosis on electron beam CCTA was an independent predictor of mortality in a multivariable model adjusted for age, gender, cardiac risk factors and CAC [36]. The CTA diagnosed presence of three-vessel obstructive disease independently conferred a 2.6-fold hazard ratio for death compared with those without CAD [36]. Similarly, in a group of 331 patients with suspected CAD and an intermediate pretest probability for significant CAD, van Werkhoven et al. demonstrated that multislice CCTA is useful at restratifying patients into either low or high post-test risk groups [17]. The presence of significant CAD as assessed by multislice CCTA was associated with a hazard ratio of 3.46 (p = 0.03) for a combined end point of major adverse cardiac events after controlling for other high-risk variables. Hadamitzky et al. prospectively assessed the incremental prognostic value of multislice CCTA over the Framingham risk score in 1668 patients with known CAD [37]. They reported that the rate of all cardiac events in patients without obstructive CAD was significantly lower than predicted by the Framingham risk score (p < 0.01) [37]. Thus, in the intermediate- risk patient with or without symptoms, CTA serves not only to diagnose CAD, but may also be useful in risk-stratifying patients independently of traditional risk factors. Specifically, a CTA that rules out obstructive CAD identifies a population with a very low risk of future cardiac events. Therefore, CTA may play an important role as a ‘gatekeeper’ in a strategy that aims to reserve cardiac catheterization to those who may derive the greatest benefit with the least amount of risk.
Recently, Chow et al. demonstrated that, in addition to the prognostic value of CAD severity as determined by cardiac CT, incremental prognostic value by cardiac CT could be gained by assessment of LVEF and total plaque score [38]. Importantly, this study was the first to assess the prognostic value of these parameters with 64-slice CT using hard clinical outcomes. In a population of both symptomatic and asymptomatic patients who had predominantly intermediate or high pretest probability of CAD, LVEF conferred a hazard ratio of 1.47 (p < 0.05) of major adverse cardiac events, while total plaque score provided incremental value (hazard ratio: 1.17; p < 0.05) over CAD severity and LVEF for all-cause mortality and nonfatal myocardial infarction [38].
Limitations & risks of CCTA
There are several technical considerations that may limit the utility of CCTA, especially in patients with coronary calcification, increased heart rates, arrhythmias and coronary stents.
Significant attention has recently been given to the radiation exposure associated with CTA. With the advent of 64‑slice multidetector CTA, associated radiation doses (6–8 mSv) are comparable to technetium (Tc) MPI and even lower than those associated with thallium MPI. Dewey et al. recently reported promising preliminary results with 320‑row CCTA [39]. Compared with conventional coronary angiography, they achieved a per-patient sensitivity and specificity for the diagnosis of coronary artery stenoses with at least 50% diameter obstruction of 100 and 94%, respectively, while exposing patients to much lower effective radiation doses and volumes of contrast [39]. Application of other known dose-reduction techniques have been demonstrated to be effective in reducing radiation doses from CCTA. Such techniques include minimization of the scan range, heart-rate reduction and prospectively triggered ECG gating [40]. In prospectively triggered CCTA, radiation is only administered at prespecified time points of the cardiac cycle and is ideal in patients with low and regular heart rates. Blankstein et al. demonstrated that, compared with retrospective gating, prospective ECG triggering resulted in a 73% decrease in radiation dose in selected patients [41]. Prospective ECG gating may be particularly useful in reducing radiation dosing when attempting to incorporate stress perfusion CT imaging to CCTA. One study demonstrated that a protocol involving retrospectively ECG-gated adenosine stress CT perfusion imaging with subsequent prospectively gated rest CT imaging added to CCTA resulted in improved sensitivity and specificity for the diagnosis of significant CAD. This was achieved with mean total effective radiation exposure of 11.8 ± 4.5 mSv [42].
Guidelines
In 2008, the AHA released a scientific statement on noninvasive coronary imaging with a specific focus on magnetic resonance angiography (MRA) and MDCT angiography [43]. Based on a thorough review of available evidence, the guidelines highlight that CCTA should not be used to screen for CAD in asymptomatic patients. They suggest that the utility of noninvasive coronary angiography is most likely in the assessment of symptomatic, intermediate-risk patients for initial risk stratification. Finally, CTA was not recommended for assessment of patients at high risk as they often require invasive angiography for definitive diagnosis and intervention [43].
Cardiovasular magnetic resonance
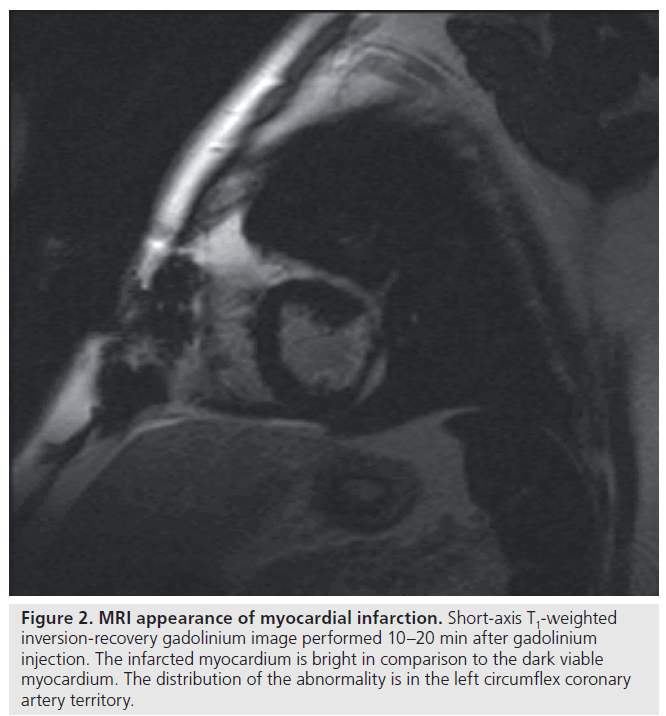
Cardiovascular magnetic resonance may play an emerging role in the diagnosis and management of both stable and unstable CAD. Factors that render this modality an attractive option is its ability to provide excellent spatial and temporal resolution of cardiac structures (Figure 2) in any plane and the lack of associated exposure to ionizing radiation and iodinated contrast medium. Since it provides quantitative measures accurately and reproducibly, MRI has been established as the reference standard in assessment of left ventricular function, mass and geometry [44]. Its unique ability to confer both functional and anatomical information and further refinements in improving resolution of the coronary tree may make this modality a ‘one-stop-shop’ for clinical decision-making in CAD. Specifically, late gadolinium enhancement, stress myocardial perfusion capabilities and free-breathing 3D gradient-echo coronary MRA have expanded the potential application of magnetic resonance in the diagnosis of ischemic heart disease.
Figure 2.MRI appearance of myocardial infarction. Short-axis T1-weighted inversion-recovery gadolinium image performed 10–20 min after gadolinium injection. The infarcted myocardium is bright in comparison to the dark viable myocardium. The distribution of the abnormality is in the left circumflex coronary artery territory.
CMR for diagnosis of CAD
Ischemia can be detected with first-pass contrast enhanced MRI after pharmacological stress. Areas of myocardial ischemia enhance at a slower rate compared with normal myocardium and such areas may be visualized with superior spatial resolution to that of SPECT imaging [45]. A recent meta-analysis suggested excellent test characteristics with stress perfusion MRI with a sensitivity for detecting ischemia of 91% and specificity of 91% [45]. Furthermore, owing to its ability to detect diffuse subendocardial ischemia, stress MRI may be less prone to miss multivessel disease than SPECT [46]. Stress magnetic resonance perfusion imaging may also have advantages over stress echocardiography for the detection of myocardial ischemia. Dobutamine stress cine MRI allows assessment of regional wall-motion abnormalities in all segments of the left ventricle with excellent spatial resolution. In a meta-analysis, Nandalur et al. demonstrated that dobutamine stress cine MRI has a sensitivity of 83% and a specificity of 86% for the detection of significant CAD [45].
Techniques to visualize coronary anatomy with MRA are undergoing refinement. Unlike CCTA, MRI coronary angiography may allow visualization of the lumen of the coronary artery with heavy calcification. However, coronary MRA is technically challenging and is prone to motion artifacts from cardiac contraction and respiration. Owing to its slower acquisition time compared with 64-slice MDCT, it is difficult to obtain high resolution MRA images that cover the entire coronary tree with a single breath hold. The emerging technique of freebreathing 3D gradient-echo coronary MRA may circumvent the effect of motion artifacts and allow better arterial tree visualization [47–50].
Evidence suggests a potential role of CMR in the evaluation of patients presenting to the emergency department with chest pain. The utility of CMR for this purpose was evaluated in 161 subjects within 12 h of chest pain lasting at least 30 min. MRI parameters evaluated included perfusion, left ventricular function and gadolinium-enhanced myocardial infarction detection [51]. MRI had a sensitivity and specificity of 84 and 85%, respectively, for detecting ACS, compared with 80 and 61%, respectively, for abnormal ECG and 40 and 90%, respectively, for peak troponin I. The addition of T2‑weighted imaging and assessment of left ventricular wall thickness to the CMR protocol in another study was demonstrated to improve the specificity, positive predictive value and overall accuracy for the diagnosis of ACS from 84 to 96, 55 to 85, and 84 to 93%, respectively, compared with the conventional CMR protocol [52]. Further evaluation of CMR, in a greater variety of settings, is required to establish its role for the diagnosis of patients presenting with chest pain to the emergency department.
MRI has significant promise in enhancing the clinicians diagnosis, prognosis and ability to assess treatment effects in CAD. Improved accessibility and further technical improvements to allow for improved visualization of the arterial tree will hopefully allow the potential of MRI in routine clinical use to be realized.
CMR for prognosis in CAD
Recent studies have demonstrated that stress perfusion MRI may be valuable for the prognosis of CAD. For example, Jahnke et al. demonstrated that myocardial ischemia in patients with known or suspected CAD detected by stress CMR provides an incremental predictive value for cardiac death and nonfatal myocardial infarction over traditional clinical risk factors and resting wallmotion abnormalities [53]. These findings were corroborated by other studies [54,55]. As with PET, stress perfusion MRI can be performed in most patients with pharmacological stress and does not provide prognostic information based on the patient’s hemodynamic response to exercise and functional status.
CMR in assessing myocardial viability
Late gadolinium-enhanced cardiovascular MRI can be used to assess myocardial viability – information that may be important when trying to predict improvement in LVEF and survival after revascularization. Although infarcted nonviable regions enhance late after gadolinium administration, a normal pattern of late gadolinium enhancement is seen in dysfunctional but viable myocardium. As expected, low-dose dobutamine infusion improves contractility in areas of dysfunctional but viable myocardium, while areas of transmural enhancement show no contractile response to a dobutamine infusion [56]. In one study, the ability of contrast-enhanced CMR to predict whether regions of abnormal ventricular contraction would improve following revascularization, either with percutaneous coronary intervention (PCI) of coronary artery bypass surgery, was tested in a cohort of 50 patients with left ventricular dysfunction [57]. The authors found that improved contractility after revascularization decreased progressively as the degree of transmural hyperenhancement increased [57]. Similar results were seen in a group of patients exclusively undergoing coronary artery bypass surgery. In this study, there was a strong correlation between the transmural extent of hyperenhancement and recovery in regional function 6 months after bypass surgery [58]. Data demonstrating the effectiveness of late gadoliniumenhanced CMR versus standard management of patients with severe ventricular dysfunction and CAD are lacking.
Risks & limitations of CMR
Although CMR imaging is subject to less false positives or negatives caused by calcification of coronary vessels, the utility of coronary MRA is largely limited by its lower spatial resolution and the need to average data from several cardiac cycles [43]. The recent advent of 3D navigator free-breathing imaging has been demonstrated to be associated with an 82% sensitivity and 91% specificity of coronary MRA to detect angiographically significant CAD. However, MRA has limited spatial resolution and coronaries with a diameter of less than 1.5 mm are not well visualized [59]. Several factors, including motion artifacts resulting from both cardiac and respiratory motion, the limited availability of coronary MRA in nonacademic settings, the relatively high frequency of claustrophobia, and the need to exclude many patients with implanted cardiac devices, mean that coronary MRA is still very much in its developing stages and requires further refinements to improve its accessibility and to render it suitable for widespread clinical use. With continued refinements in technology and, in particular, improvements in the ability to visualize the coronary tree, MRI has the potential to provide a radiation-free method to obtain structural, functional and prognostic information with one test.
Guidelines
A consensus statement released by the AHA in 2008 emphasizes that the utility of wholeheart coronary MRA requires further, multicenter validation [43]. The guidelines also state that CTA offers a better diagnostic profile than MRA for the assessment of symptomatic, intermediate- risk patients. Recent guidelines also suggest that significant caution should be used if considering magnetic resonance examination of patients with cardiac devices, including avoidance of magnetic resonance examination in pacemaker-dependent patients and those with implantable cardioverter-defibrillators unless the benefits clearly outweigh the risks of CMR in such patients [60].
SPECT & PET
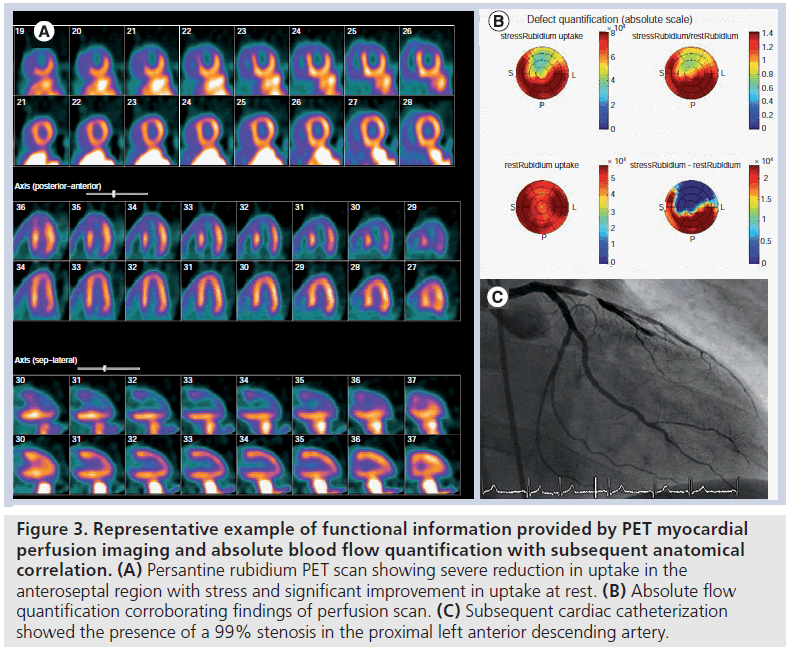
Nuclear MPI allows for the visualization of a radiopharmaceutical that is dispersed within the myocardium proportionally to coronary blood flow. Both SPECT and PET perfusion imaging are established modalities for the functional evaluation of CAD (Figure 3).
Figure 3.Representative example of functional information provided by PET myocardial perfusion imaging and absolute blood flow quantification with subsequent anatomical correlation. (A) Persantine rubidium PET scan showing severe reduction in uptake in the anteroseptal region with stress and significant improvement in uptake at rest. (B) Absolute flow quantification corroborating findings of perfusion scan. (C) Subsequent cardiac catheterization showed the presence of a 99% stenosis in the proximal left anterior descending artery.
SPECT & PET for the diagnosis of CAD
Published data suggest that the average sensitivity and specificity of SPECT for the detection of at least 50% angiographic stenosis is 87 and 73%, respectively [61]. In clinical practice, thallium-201, Tc-99m-labeled sestamibi and Tc-99m-labeled tetrofosmin are the most commonly used radiotracers in SPECT. A study randomizing 2560 patients to one of these radiotracers found no significant difference between the sensitivity and specificity for detecting significant coronary disease [62]. SPECT MPI scanning has become a mainstay of diagnostic evaluation and risk stratification of CAD in many community and academic centers.
PET MPI has been demonstrated to be very accurate in the diagnosis of CAD. In one analysis, PET provided an average sensitivity and specificity of 91 and 89%, respectively, for the detection of at least 50% angiographic stenosis [61]. In another meta-analysis that evaluated the accuracy of PET MPI, PET demonstrated a sensitivity of 92% and a specificity of 85% at the patient level, and sensitivity and specificity of 81 and 87%, respectively, at the coronary territory level [63]. The superior sensitivity and specificity of PET can be ascribed to several technical characteristics, including better resolution and attenuation correction (although some SPECT systems do incorporate attenuation correction) and higher photon energy (Figure 3). Additionally, PET is capable of flow quantification in absolute terms during stress and rest [64]. Coronary flow reserve (CFR) can, therefore, be determined by calculating the ratio of flow during stress and rest. CFR may be able to reveal the presence of multivessel obstructive CAD and detect microvascular dysfunction in the context of apparently normal perfusion imaging [65].
SPECT & PET for prognostication in CAD
Nuclear MPI has been demonstrated to have an important role in prognostication after a diagnosis of CAD has been made. High-risk characteristics of MPI include transient ischemic dilatation of the left ventricle, a large defect size (>20% of the left ventricle), defects in more than one coronary territory and a decreased resting LVEF. In a study involving over 5000 patients, Hachamovitch et al. were able to demonstrate that, over an approximately 2‑year period of follow-up, patients with a normal SPECT myocardial perfusion scan had a less than 0.5% risk per year of myocardial infarction or cardiac death [66]. The investigators also determined the prognostic value conferred by the summed stress score (SSS). The SSS in their study was obtained by adding the scores of the individual myocardial segments. They used a 20‑segment model that divided the myocardium into regions based on the apical, mid and basal cuts on short axis views, and the apical region of the parasternal long axis view. An SSS under 4 was considered normal; 4–8, mildly abnormal; 9–13, moderately abnormal; and more than 13, severely abnormal. They found that patients who had an SSS greater than 13 and were revascularized had a significantly lower rate of death compared with those who had an SSS greater than 13 and were treated with medical therapy. Thus, in most circumstances, patients with a normal study or a small defect after SPECT imaging have a reassuringly excellent short-term prognosis, while those with high-risk features, including large defects should be followed carefully as they are at increased risk of subsequent cardiac death and myocardial infarction or considered revascularization.
Stress MPI with Tc-99m-labeled sestamibi has also been demonstrated to be useful in assessing the efficacy of optimal medical therapy versus PCI plus optimal medical therapy in reducing ischemic burden in patients with stable angina [67]. In the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) study, 314 patients out of 2287 underwent SPECT myocardial before and after randomization to either treatment arm. Those enrolled in the PCI plus optimal medical therapy group had a greater reduction in ischemia than the optimal medical therapy only group. Patients who had significant ischemia reduction had lower unadjusted risk for death or myocardial infarction. Although this finding was no longer statistically significant after risk-adjustment in the overall group, there was a trend towards a statistically significant risk-adjusted reduction in death or myocardial infarction in patients with moderate-to-severe baseline ischemic burden. This study raises the possibility that targeting medical and/or PCI therapy to achieve reduction in ischemic burden of at least 5% as measured by SPECT may translate into a better long-term outcome.
The ability of PET to assess CFR in addition to perfusion findings has recently been demonstrated to confer independent prognostic value over SPECT MPI [64]. Herzog et al. demonstrated that, in patients with normal perfusion, abnormal CFR (defined as a CFR <2) is independently associated with a higher annual event rate over 3 years compared with normal CFR for major adverse coronary events (1.4 vs 6.3%; p < 0.05) and cardiac death (0.5 vs 3.1%; p < 0.05) [64]. A similar independent prognostic value of CFR was reported in patients with abnormal perfusion. While gated SPECT assesses changes in left ventricular ejection function at rest and post-stress, gated PET can assess changes in LVEF from rest to peak stress. A decrease in LVEF at peak stress, even in the absence of perfusion defects, identifies multivessel or left main CAD, conferring greater sensitivity of PET to detect multivessel disease [61]. One drawback of current PET technology is that the very short half-life of the current tracers in clinical use (82-rubidium and 13N‑ammonia) precludes the use of exercise as a stressor in most clinical centers.
PET for viability imaging
Revascularization of patients with severe left ventricular systolic dysfunction can be associated with high rates of morbidity and mortality. This provides the rationale for using methods including myocardial viability imaging to determine which patients would most significantly benefit from revascularization. FDG‑PET imaging is an established modality for determining myocardial viability [68,69]. The use of FDG‑PET imaging in assessing viability is based on the principle that in the setting of chronic ischemia, segments of myocardium that show contractile dysfunction are still metabolically active and have the potential to regain contractile function upon revascularization. Since ischemia preferentially shifts myocyte metabolism from fatty acid to glucose metabolism, uptake of the glucose analog, FDG, by myocytes in an area of dysfunctional myocardium suggests viability. In PET viability imaging, segmental myocardial perfusion as assessed by 13N‑ammonia or 82‑rubidium uptake is compared with myocardial FDG uptake. Hibernating myocardium correspond to areas of mismatch between the degree of uptake of the perfusion tracer (very little uptake) and that of FDG (moderate to significant uptake) by the myocardium. Scar corresponds to myocardium showing little or no perfusion tracer and little or no FDG uptake. Beanlands et al. developed a model that demonstrated that improvement in ejection fraction with revascularization is inversely related to the extent of scar determined by FDG‑PET perfusion viability imaging [70]. They subsequently demonstrated that, when revascularization recommendations based on this FDG‑PET viability imaging model were adhered to, there was a significantly lower hazard ratio for cardiac events, including cardiac death, myocardial infarction or recurrent hospital stay for a cardiac cause [71]. In a post-hoc analysis of this study, patients with ischemic cardiomyopathy with larger amounts of mismatch were shown to have improved outcome with revascularization [72]. Additional, head-tohead studies are required to determine whether PET viability imaging-based treatment strategies lead to better patient outcomes over those based on other established modalities for myocardial viability imaging, including SPECT, dobutamine echocardiography and CMR.
Guidelines
In 2003, a consensus statement on the use of cardiac radionuclide imaging was released by the American College of Cardiology/AHA/ American Society of Nuclear Cardiology [73]. Guidelines pertaining to the assessment and management of CAD were set forth for different clinical scenarios. First, in patients presenting to the emergency room with suspected ACS, radionuclide cardiac imaging was recommended as a class I indication for assessment of myocardial risk and for the diagnosis of CAD in patients with nondiagnostic ECGs and cardiac serum biomarkers. However, radionuclide MPI was not recommended for diagnostic purposes in patients whose ECG or biomarkers were sufficient to provide the diagnosis of ACS. In the setting of non-ST‑elevation ACS, the guidelines give a class I recommendation to the use of stress MPI to identify inducible ischemia in ‘culprit’ lesions in low- and intermediate-risk patients with a diagnosis of unstable angina or non- ST‑elevation ACS.
After an ST-elevation myocardial infarction, the guidelines suggest that stress MPI with ECG-gated rest and/or stress SPECT should be used for post-thrombolytic patients who have not undergone cardiac catheterization. However, these recommendations may be less applicable in a contemporary clinical context where ST‑segment elevation myocardial infarction management standards of care encourage primary PCI and/or early cardiac catheterization post-thrombolytics.
Echocardiography
As with SPECT MPI, echocardiography has been used in combination with different forms of stress, including exercise and dobutamine, for diagnosis and prognosis in patients with suspected or known CAD. The characteristic feature of echocardiography for the purpose of diagnosing coronary disease is the inducibility of wall-thickening or motion abnormalities [74]. The incorporation of ultrasound contrast agents results in the enhancement of endocardial borders and has been demonstrated to increase the number of interpretable wall segments and to improve diagnostic accuracy [74]. In addition to its diagnostic utility, stress echocardiography can be used to assess myocardial viability prior to revascularization and to risk-stratify patients with known or suspected CAD.
Stress echocardiography for the diagnosis of CAD
Stress echocardiography can be used when baseline ECG abnormalities hinder interpretation of exercise ECG testing. Evidence suggests that, compared with SPECT MPI, both exercise and pharmacological stress echocardiography has better specificity for the diagnosis of obstructive CAD. In a meta-analysis comparing the diagnostic performance of exercise echocardiography to exercise SPECT imaging with thallium-201 or Tc-99m-labeled tracers, both modalities had similar sensitivities to detect significant CAD (85 and 87%, respectively). However, exercise echocardiography yielded a significantly greater specificity (77%) than exercise SPECT imaging (64%), which, in turn, led to a greater incremental improvement in performance for echocardiogram (3.43; 95% CI: 2.74–4.11) than for SPECT (1.49; 95% CI: 0.91–2.08) over exercise stress testing alone [75]. A meta-analysis that included 82 studies of 10,817 patients for whom angiographic data were available, found that dipyridamole and adenosine perfusion imaging conferred the highest sensitivity (89 and 90%, respectively), dipyrimadole echocardiography conferred the highest specificity (93%), while dobutamine echocardiography conferred the best combination of sensitivity and specificity (80 and 84%, respectively) [76].
Prognosis with stress echocardiography
Exercise echocardiography provides both exercise and echocardiographic variables important for prognostication. The extent and severity of exercise-induced left ventricular wall-motion abnormalities is the main echocardiographic variable that confers prognostic value. In a meta-analysis comparing the prognostic value of normal exercise MPI tests and exercise echocardiography tests, Metz et al. found that both provided similar high negative predictive values for event-free survival [77]. In an analysis that included 8008 subjects in the radionuclide MPI group and 3021 subjects in the stress echocardiography group, the negative predictive value for myocardial infarction and cardiac death was 98.8% over 36 months for radionuclide MPI and 98.4% over 33 months for echocardiography in both men and women [77]. Chua et al. followed 860 patients who had undergone dobutamine stress echocardiography over 2 years and reported that, after multivariate analysis, a model that best predicted subsequent cardiac events included clinical and stress echocardiographic data [78]. Exercise stress echocardiography has also been demonstrated to add incremental prognostic value to the assessment of patients with an interpretable baseline ECG who have a normal exercise ECG testing. In a study that included 4004 consecutive patients who underwent treadmill ECG exercise testing and did not develop chest pain or ischemic ECG abnormalities, the development of new or worsening wall motion abnormalities with exercise remained a significant independent predictor of all-cause mortality and major cardiac events. The 5‑year mortality was 6.4% in patients without inducible wall-motion abnormalities compared with 12.1% in those with inducible wall-motion abnormalities [79]. Thus, in centers with experience in both exercise and pharmacological stress echocardiography, this modality has utility in conferring prognostic information.
Emerging techniques in stress echocardiography in the assessment of coronary disease include speckle-tracking strain echocardiography and MPI. Speckle-tracking echocardiography uses shifts in frequency of ultrasound to calculate myocardial velocity and, thus, assess myocardial motion [80]. Voigt et al. demonstrated an improvement in sensitivity and specificity for the detection of ischemic segments from 81 and 82%, respectively, for conventional dobutamine stress echocardiography, to 86 and 90%, respectively, when strain-rate imaging was added [81]. Speckle-tracking echocardiography also allows for the assessment of stress-induced diastolic dysfunction in the presence of CAD. It was recently demonstrated that detection of postischemic regional left ventricular diastolic stunning after stress testing with strain imaging has excellent sensitivity (97%) and specificity (93%) for detection of significant CAD [82]. While these techniques evolve and undergo standardization, conventional stress echocardiography continues to be an important option for the assessment of coronary disease in experienced centers and in a variety of clinical settings.
Limitations of stress echocardiography
Stress echocardiography offers some advantages over SPECT or PET MPI. The technique allows for the assessment of additional parameters, including valvular function, right ventricular systolic pressures and chamber sizes. These advantages are somewhat offset by the problem of poor image quality in a significant number of patients. Results often depend on the acquisition of good echocardiographic windows, which may be difficult to obtain in patients with chronic obstructive pulmonary disease and obese patients. Thus, the choice of stress echocardiography versus radionuclide MPI for functional assessment may depend on patient characteristics. For example, stress echocardiography may be more accurate in patients with left ventricular hypertrophy, whereas stress radionuclide MPI may be preferable in patients with a high BMI.
A potential impediment to the utility of stress echocardiography is the subjective nature of the technique as compared with radionuclide MPI, which is less prone to subjective interpretation owing to semi-automated computer quantification. However, the problem of subjectivity can be circumvented in laboratories with extensive experience where interobserver variability appears to be minimal [78,83]. Furthermore, the use of ultrasound contrast agents that enhance endocardial border clarity has been demonstrated to improve the accuracy of less experienced readers [84,85].
Guidelines
The 2007 guidelines focused update on the management of patients with chronic stable angina [86] gives a class I recommendation for the use of stress echocardiography as an initial test for diagnosis or risk stratification in patients with known or suspected CHD in a variety of scenarios. As with radionuclide MPI, exercise echocardiography is recommend to identify the severity and location of ischemia in patients who have an intermediate pretest probability of disease and ECG abnormalities that could interfere with exercise ECG test interpretation. Stress echocardiography was also given a class I recommendation to assess the functional significance of coronary lesions prior to revascularization. Dobutamine echocardiography was given a class I recommendation for diagnosis in patients with an intermediate pretest probability of CHD and for assessment of the extent, severity and location of ischemia for prognostication in patients who are unable to exercise.
Future perspective
Comparative effectiveness
The future of noninvasive imaging in diagnosis and prognostication in ischemic heart disease will be affected by the two seemingly opposing forces – the rapid development of new technologies and their implementation before evidence-based guidelines can corroborate their cost–effectiveness and the limitations posed by healthcare economic realities. The advent of a multitude of imaging modalities presents the clinician with many choices for diagnosis and prognostication in CAD without adequate concomitant data on comparative effectiveness to base these choices on. To address this issue, Hachamovitch et al. have initiated the Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in CAD (SPARC) project [87]. SPARC is a prospective, multicenter, observational registry trial that has enrolled 3019 patients who have undergone clinical SPECT, PET and CTA testing. The goal of this study is to compare posttest resource utilization in patients with an intermediate probability of CAD but without a prior history of CAD, by measuring catheterization rate differences at 90 days between the three imaging modality arms. This study will also compare the prognostic value of the three imaging modalities in the aforementioned patients and in those with a known history of CAD [87]. The Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) was developed to compare the clinical outcomes of symptomatic patients without known CAD, randomized to either an anatomic imaging strategy (CTA) or to a functional testing strategy (usual care) [102]. This study will start enrolling patients this year and is anticipated to enroll 10,000 patients with a 2‑year follow-up. The results of these studies are highly anticipated in an era of accelerating expansion of cardiovascular imaging modalities and the uncertainty regarding their respective roles in clinical practice.
Hybrid technology
Perhaps the imaging modalities of choice will provide the optimal combination of patient safety, anatomical, functional, diagnostic and prognostic information. The ideal noninvasive imaging modality would provide complementary anatomical and functional information on atherosclerosis and ischemia during a single imaging session [88]. An emerging technology for such simultaneous functional and anatomical evaluation of CAD is the hybrid technology – the integration of CTA with MPI from SPECT or PET [88]. Recent studies indicate that hybrid imaging provides added diagnostic accuracy beyond that of either modality alone in both low-risk populations and those with multivessel disease [89]. However, the actual clinical impact on treatment strategies and outcome with hybrid imaging requires further prospective, long-term studies.
Vulnerable plaque in imaging
Imaging in coronary disease has conventionally been directed at evaluating the degree of stenosis. However, vulnerable plaques (i.e., coronary plaques most prone to rupture and likely to cause the most devastating complications of CAD) are not necessarily those that are the most obstructive. Thus, a significant future challenge of CAD imaging is detection of the vulnerable coronary plaque. Since vulnerable plaques are characterized by increased inflammatory infiltrates, future imaging modalities should aim to provide insight into the biological characteristics of plaques. 18F‑FDG, a radioactively tagged glucose analog currently used to determine myocardial viability via PET, enriches in plaque macrophages. Thus, PET technology has the potential to identify inflammatory components of vulnerable plaques. CCTA has been demonstrated to provide important information regarding atherosclerotic plaque composition. One study determined plaque characteristics of culprit lesions in patients with ACS in comparison to those in patients with stable angina using 16‑slice CT [90]. The study demonstrated that culprit lesions in patients with ACS had significantly higher plaque areas and remodeling indices compared with stable lesions. Furthermore, there was a higher prevalence of noncalcified plaque in the culprit lesions compared with those in the stable lesions [90]. In a subsequent study of intermediate-risk patients with suspected significant CAD, patients with noncalcified plaques, identified by 64‑slice CT, had higher total cholesterol, low-density lipoprotein and C‑reactive protein levels [91]. Motoyama et al. evaluated the feasibility of assessing characteristics of disrupted atherosclerotic plaques in 38 patients with ACS [92]. In this study, the presence of spotty calcification, positive remodeling and noncalcified plaques was significantly more frequent than in patients with stable angina pectoris. Thus, the presence of the following plaque characteristics, as determined by contemporary contrast CCTA, are associated with adverse clinical outcomes: positive remodeling, larger plaque volume, noncalcified plaque components and mixed plaques [93]. Although high-resolution contrastenhanced MRI has been demonstrated to be useful in identifying atherosclerotic vulnerable plaque characteristics, such as a large lipid core and thin fibrous cap in carotid arteries, MRI currently lacks sufficient resolution for accurate measurement of coronary cap thickness and detailed evaluation of coronary atherosclerotic lesions [94].
Thus, several modalities, including FDG‑PET, CCTA and CMR show promise in identifying those atherosclerotic lesions that could potentially be susceptible to rupture. How to act upon these findings, both in terms of intervention and risk stratification requires further research.
Conclusion
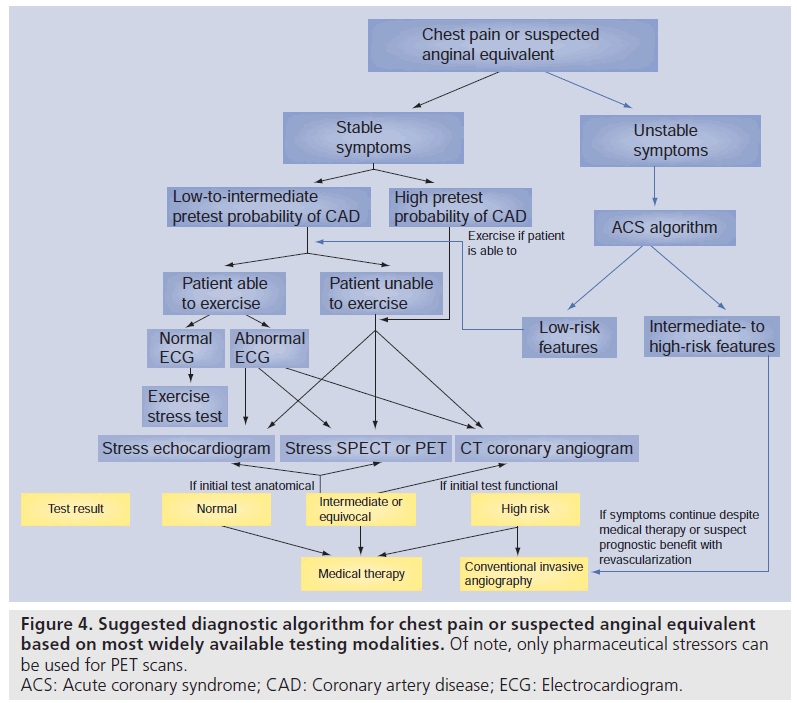
In this era of limited resources, it is crucial that ongoing research is conducted to provide insight into the incremental value of new technologies on improving outcomes. Furthermore, much of the evidence suggests that the testing characteristics of most of clinically available modalities used to diagnose and risk stratify CAD are comparable. Indeed, each modality imparts certain strengths and limitations depending on patient characteristics (see Figures 4 & 5) and the expertise of the particular institution. The use of any test continues to rely on clinical acumen and judicious use of resources, and the results of comparative effectiveness studies are eagerly awaited.
Figure 4.Suggested diagnostic algorithm for chest pain or suspected anginal equivalent based on most widely available testing modalities. Of note, only pharmaceutical stressors can be used for PET scans. ACS: Acute coronary syndrome; CAD: Coronary artery disease; ECG: Electrocardiogram.
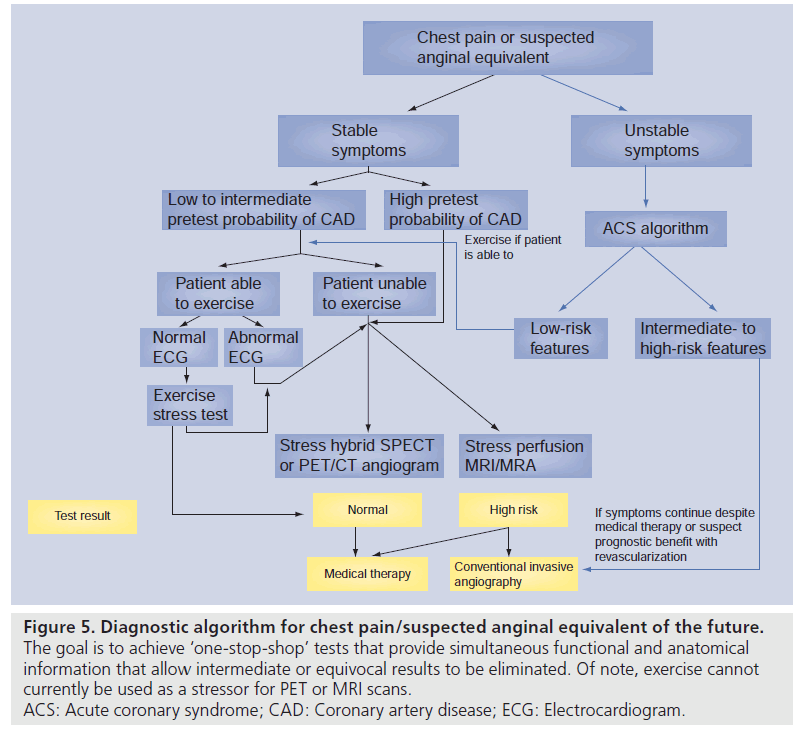
Figure 5.Diagnostic algorithm for chest pain/suspected anginal equivalent of the future. The goal is to achieve ‘one-stop-shop’ tests that provide simultaneous functional and anatomical information that allow intermediate or equivocal results to be eliminated. Of note, exercise cannot currently be used as a stressor for PET or MRI scans. ACS: Acute coronary syndrome; CAD: Coronary artery disease; ECG: Electrocardiogram.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Papers of special note have been highlighted as:
*of interest
References
- Arsenault BJ, Pibarot P, Després JP: The quest for the optimal assessment of global cardiovascular risk: are traditional risk factors and metabolic syndrome partners in crime? Cardiology 113(1), 35–49 (2009).
- Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine. (8th Edition). Libby P, Bonow R, Mann D, Zipes D, Braunwald E (Eds). Elsevier Saunders, PA, USA, 424 (2008).
- Schmermund A, Denktas AE, Rumberger JA et al.: Independent and incremental value of coronary artery calcium for predicting the extent of angiographic coronary artery disease: comparison with cardiac risk factors and radionuclide perfusion imaging. J. Am. Coll. Cardiol. 34(3), 777–786 (1999).
- Raggi P, Khan A, Arepali C, Stillman AE: Coronary artery calcium scoring in the age of CT angiography: what is its role? Curr. Atheroscler. Rep. 10(5), 438–443 (2008).
- Detrano R, Guerci AD, Carr JJ et al.: Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 358, 1336–1345 (2008).
- Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC: Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 291, 210–215 (2004).
- Rinehart S, Vazquez G, Qian Z, Voros S: Coronary plaque imaging with multi-slice computed tomographic angiography and intravascular ultrasound: a close look inside and out. J. Invasive Cardiol. 21(7), 367–372 (2009).
- Greenland P, Bonow R, Brundage BH et al.: ACCF/AHA 2007 Clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain. Circulation 115, 402–426 (2007).
- Gottlieb I, Miller JM, Arbab-Zadeh A et al.: The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J. Am. Coll. Cardiol. 55(7), 627–634 (2010).
- Budoff MJ, McClelland RL, Nasir K et al.: Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Am. Heart J. 158(4), 554–561 (2009).
- Akram K, O’Donnell RE, King S et al.: Influence of symptomatic status on the prevalence of obstructive coronary artery disease in patients with zero calcium score. Atherosclerosis 203(2), 533–537 (2009).
- Detrano R, Hsiai T, Wang S et al.: Prognostic value of coronary calcification and angiographic stenoses in patients undergoing coronary angiography. Am. Coll. Cardiol. 27(2), 285–290 (1996).
- Kondos GT, Hoff JA, Sevrukov A et al.: Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic lowto intermediate-risk adults. Circulation 107(20), 2571–2576 (2003).
- Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD: Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J. Am. Coll. Cardiol. 46(1), 166–172 (2005).
- Raggi P, Davidson M, Callister TQ et al.: Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation 112(4), 563–571 (2005).
- Schmermund A, Achenbach S, Budde T et al.: Effect of intensive versus standard lipidlowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: a multicenter, randomized, double-blind trial. Circulation 113(3), 427–437 (2006).
- van Werkhoven JM, Gaemperli O, Schuijf JD et al.: Multislice computed tomography coronary angiography for risk stratification in patients with an intermediate pretest likelihood. Heart 95(19), 1607–1611 (2009).
- Achenbach S, Moshage W, Ropers D, Nossen J, Werner DG: Value of electron-beam computed tomography for the noninvasive detection of high-grade coronary-artery stenoses and occlusions. N. Engl. J. Med. 339, 1964–1971 (1998).
- Schoepf UJ, Becker CR, Hofmann LK et al.: Multidetector-row CT of the heart. Radiol. Clin. North Am. 41(3), 491–505 (2003).
- Dewey M, Zimmermann E, Deissenrieder F et al.: Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation 120(10), 867–875 (2009).
- Budoff MJ, Dowe D, Jollis JG et al.: Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 52(21), 1724–1732 (2008).
- Meijboom WB, Meijs MF, Schuijf JD et al.: Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J. Am. Coll. Cardiol. 52(25), 2135–2144 (2008).
- Miller JM, Rochitte CE, Dewey M et al.: Diagnostic performance of coronary angiography by 64-row CT: N. Engl. J. Med. 359(22), 2324–2336 (2008). & Well-designed landmark trial showing the utility of a contemporary and widely used noninvasive imaging modality – 64-slice CT angiography – for the diagnosis of coronary artery disease.
- Hoffmann U, Bamberg F, Chae CU et al.: Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT trial. J. Am. Coll. Cardiol. 53(18), 1642–1650 (2009).
- Rubinshtein R, Halon DA, Gaspar T et al.: Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation 115(13), 1762–1768 (2007).
- Goldstein JA, Gallagher MJ, O’Neill WW, Ross MA, O’Neil BJ, Raff GL: A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J. Am. Coll. Cardiol. 49(8), 863–871 (2007).
- Emond M, Mock MB, Davis KB et al.: Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) registry. Circulation 90(6), 2645–2657 (1994).
- de Graaf FR, Schuijf JD, Delgado V et al.: Clinical application of CT coronary angiography: state of the art. Heart Lung Circ. 19(3), 107–116 (2010).
- Cerqueiras MD, Weissman NJ, Dilsizian V et al.: Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4), 539–542 (2002).
- Sugeng L, Mor-Avi V, Weinert L et al.: Quantitative assessment of left ventricular size and function: side-by-side comparison of real-time three-dimensional echocardiography and computed tomography with magnetic resonance reference. Circulation 114(7), 654–661 (2006).
- Yamamuro M, Tadamura E, Kubo S et al.: Cardiac functional analysis with multi-detector row CT and segmental reconstruction algorithm: comparison with echocardiography, SPECT, and MR imaging. Radiology 234(2), 381–390 (2005).
- Blankstein R, Shturman LD, Rogers IS et al.: Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J. Am. Coll. Cardiol. 54(12), 1072–1084 (2009).
- George RT, Arbab-Zadeh A, Miller JM et al.: Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ. Cardiovasc. Imaging 2(3), 174–182 (2009).
- Lardo AC, Cordeiro MA, Silva C et al.: Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation 113(3), 394–404 (2006).
- Nieman K, Shapiro MD, Ferencik M et al.: Reperfused myocardial infarction: contrastenhanced 64-section CT in comparison to MR imaging. Radiology 247(1), 49–56 (2008).
- Ostrom MP, Gopal A, Ahmadi N et al.: Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J. Am. Coll. Cardiol. 52(16), 1335–1343 (2008).
- Hadamitzky M, Freissmuth B, Meyer T et al.: Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc. Imaging 2(4), 404–411 (2009).
- Chow BJ, Wells GA, Chen L et al.: Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J. Am. Coll. Cardiol. 55(10), 1017–1028 (2010). & First study to assess the ability of 64-slice CT to predict clinical outcomes.
- Dewey M, Zimmermann E, Deissenrieder F et al.: Noninvasive coronary angiography by 320-row computed tomography with lower radiation exposure and maintained diagnostic accuracy: comparison of results with cardiac catheterization in a head-to-head pilot investigation. Circulation 120(10), 867–875 (2009).
- Raff GL, Chinnaiyan KM, Share DA et al.: Radiation dose from cardiac computed tomography before and after implementation of radiation dose reduction techniques. JAMA 301(22), 2340–2348 (2009). & Important randomized controlled trial that may influence clinicians on protocols and methods to reduce radiation exposure from cardiac CT.
- Blankstein R, Shah A, Pale R et al.: Radiation dose and image quality of prospective triggering with dual-source cardiac computed tomography. Am. J. Cardiol. 103(8), 1168–1173 (2009).
- Rocha-Filho JA, Blankstein R, Shturman LD et al.: Incremental value of adenosine-induced stress myocardial perfusion imaging with dual-source CT at cardiac CT angiography. Radiology 254(2), 410–419 (2010).
- Bluemke DA, Achenbach S, Budoff M et al.: Noninvasive coronary artery imaging magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation 118(5), 586–606 (2008).
- Yamamuro M, Tadamura E, Kubo S et al.: Cardiac functional analysis with multi-detector row CT and segmental reconstruction algorithm: comparison with echocardiography, SPECT, and MR imaging. Radiology 234(2), 381–390 (2005).
- Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC: Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J. Am. Coll. Cardiol. 50(14), 1343–1353 (2007).
- Schwitter J, Wacker CM, van Rossum AC et al.: MR-IMPACT: comparison of perfusion-cardiovasular magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur. Heart J. 29(4), 480–489 (2008).
- Jahnke C, Paetsch I, Nehrke K et al.: Rapid and complete coronary arterial tree visualization with magnetic resonance imaging: feasibility and diagnostic performance. Eur. Heart J. 26(21), 2313–2319 (2005).
- Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K: Detection of coronary artery stenosis with whole heart coronary magnetic resonance angiography. J. Am. Coll. Cardiol. 48(10), 1946–1950 (2006).
- Stuber M, Botnar RM, Fischer SE et al.: Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn. Reson. Med. 48(3), 425–429 (2002).
- Sommer T, Hackenbroch M, Hofer U et al.: Coronary MR angiography at 3.0 T versus that at 1.5 T: initial results in patients suspected of having coronary artery disease. Radiology 234(3), 718–725 (2005).
- Kwong RY, Schussheim AE, Rekhraj S et al.: Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation 107(4), 531–537 (2003).
- Cury RC, Shash K, Nagurney JT et al.: Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation 118(8), 837–844 (2008).
- Jahnke C, Nagel E, Gebker R et al.: Prognostic value of cardiovasular magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation 115(13), 1769–1776 (2007).
- Bodi V, Sanchis J, Lopez-Lereu MP et al.: Prognostic value of dipyridamole stress cardiovascular magnetic resonance imaging in patients with known or suspected coronary artery disease. J. Am. Coll. Cardiol. 50(12), 1174–1179 (2007).
- Bodi V, Sanchis J, Lopez-Lereu MP et al.: Prognostic and therapeutic implications of dipyridamole stress cardiovascular magnetic resonance on the basis of the ischemic cascade. Heart 95(1), 49–55 (2009).
- Gerber BL, Rochitte CE, Bluemke DA et al.: Relation between Gd-DTPA contrast enhancement and regional inotropic response in the periphery and center of myocardial infarction. Circulation 104(9), 998–1004 (2001).
- Kim RJ, Wu E, Rafael A et al.: The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 343(20), 1445–1453 (2000).
- Selvanayagam JB, Kardos A, Francis JM et al.: Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation 110(12), 1535–1541 (2004).
- Matsumoto N, Nagao K, Hirayama A, Sato Y: Non-invasive assessment and clinical strategy of stable coronary artery disease by magnetic resonance imaging, multislice computed tomography and myocardial perfusion SPECT. Circ. J. 74(1), 34–40 (2010).
- Levine GN, Gomes AS, Arai AE et al.: Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council of Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation 116(24), 2878–2891 (2007).
- Kaufmann PA, Di Carli MF: Hybrid SPECT/CT and PET/CT imaging: the next step in noninvasive cardiac imaging. Semin. Nucl. Med. 39(5), 341–347 (2009). & Excellent review outlining recent advances in hybrid imaging technology.
- Kapur A, Latus KA, Davies G et al.: A comparison of three radionuclide myocardial perfusion tracers in clinical practice: the ROBUST study. Eur. J. Nucl. Med. Mol. Imaging 29, 1608–1616 (2002).
- Nandalur KR, Dwamena BA, Choudhri AF, Nandalur SR, Reddy P, Carlos RC: Diagnostic performance of positron emission tomography in the detection of coronary artery disease: a meta-analysis. Acad. Radiol. 15(4), 444–451 (2008).
- Herzog BA, Husmann L, Valenta I et al.: Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J. Am. Coll. Cardiol. 54(2), 150–156 (2009).
- Beanlands RS, Ziadi MC, Williams K: Quantification of myocardial flow reserve using positron emission imaging the journey to clinical use. J. Am. Coll. Cardiol. 54(2), 157–159 (2009).
- Hachamovitch R, Berman DS, Shaw LJ et al.: Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: differential stratification for risk of cardiac death and myocardial infarction. Circulation 97(6), 535–543 (1998).
- Shaw LJ, Berman DS, Maron DJ et al.: Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 117(10), 1283–1291 (2008).
- Marshall RC, Tillisch JH, Phelps ME et al.: Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18F-labeled fluorodeoxyglucose and N-13 ammonia. Circulation 67(4), 766–778 (1983).
- Sawada SG, Allman KC, Muzik O et al.: Positron emission tomography detects evidence of viability in rest technetium-99m sestamibi defects. J. Am. Coll. Cardiol. 23(1), 92–98 (1994).
- Beanlands RS, Ruddy TD, deKemp RA et al.: Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J. Am. Coll. Cardiol. 40(10), 1735–1743 (2002).
- Beanlands RS, Nichol G, Huszti E et al.: F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J. Am. Coll. Cardiol. 50(20), 2002–2012 (2007). & This landmark trial was the first to show the effects of FDG-PET-guided management of patients with severe left ventricular dysfunction and coronary heart disease.
- D’Egidio G, Nichol G, Williams KA et al.: Increasing benefit from revascularization is associated with increasing amounts of myocardial hibernation: a substudy of the PARR-2 trial. JACC Cardiovasc. Imaging 2(9), 1060–1068 (2009).
- Klocke FJ, Baird MG, Lorell BH et al. ACC/ AHA/ASNC guidelines for the clinical use of radionuclide imaging – executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging. Circulation 108(11), 1404–1418 (2003).
- Dawson D, Kaul S, Peters D et al.: Prognostic value of dipyridamole stress myocardial contrast echocardiography: comparison with single photon emission computed tomography J. Am. Soc. Echocardiogr. 22(8), 954–960 (2009).
- Fleischmann KE, Hunink MG, Kuntz KM, Douglas PS: Exercise echocardiography or exercise SPECT imaging? A meta-analysis of diagnostic test performance. JAMA 280(10), 913–920 (1998).
- Kim C, Kwok YS, Heagerty P, Redberg R: Pharmacologic stress testing for coronary disease diagnosis: a meta-analysis. Am. Heart J. 142(6), 934–944 (2001).
- Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE: The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: a meta-analysis. J. Am. Coll. Cardiol. 49(2), 227–237 (2007).
- Chuah SC, Pellikka PA, Roger VL, McCully RB, Seward JB: Role of dobutamine stress echocardiography in predicting outcome in 860 patients with known or suspected coronary artery disease. Circulation 97(15), 1474–1480 (1998).
- Bouzas-Mosquera A, Peteiro J, Alvarez-Garcia N et al.: Prediction of mortality and major cardiac events by exercise echocardiography in patients with normal exercise electrocardiographic testing. J. Am. Coll. Cardiol. 53(21), 1981–1990 (2009).
- Miyatake K, Yamagishi M, Tanaka N et al.: New method for evaluating left ventricular wall motion by color-coded tissue doppler imaging: in vitro and in vivo studies. J. Am. Coll. Cardiol. 25(3), 717–724 (1995).
- Voigt JU, Exner B, Schmiedehausen K et al.: Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation 107(16), 2120–2126 (2003).
- Ishii K, Imai M, Suyama T et al.: Exerciseinduced post-ischemic left ventricular delayed relaxation or diastolic stunning: is it a reliable marker in detecting coronary artery disease? J. Am. Coll. Cardiol. 53(8), 698–705 (2009).
- Pozzoli MM, Fioretti PM, Salustri A, Reijs AE, Roelandt JR: Exercise echocardiography and technetium-99m MIBI single-photon emission computed tomography in the detection of coronary artery disease. Am. J. Cardiol. 67(5), 350–355 (1991).
- Rainbird AJ, Mulvagh SL, Oh JK et al.: Contrast dobutamine stress echocardiography: clinical practice assessment in 300 consecutive patients. J. Am. Soc. Echocardiogr. 14(5), 378–385 (2001).
- Dolan MS, Riad K, El-Shafei A et al.: Effect of intravenous contrast for left ventricular opacification and border definition on sensitivity and specificity of dobutamine stress echocardiography compared with coronary angiography in technically difficult patients. Am. Heart J. 142(5), 908–915 (2001).
- Fraker TD, Fihn SD, Gibbons RJ et al.: 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J. Am. Coll. Cardiol. 50(23), 2264–2274 (2007).
- Hachamovitch R, Johnson JR, Hlatky MA et al.: The study of myocardial perfusion and coronary anatomy imaging roles in CAD (SPARC): design, rationale, and baseline patient characteristics of a prospective, multicenter observational registry comparing PET, SPECT, and CTA for resource utilization and clinical outcomes. J. Nucl. Cardiol. 16(6), 935–948 (2009).
- Smith MF: Advances in rubidium PET and integrated imaging with CT angiography. Curr. Cardiol. Rep. 10(2), 135–141 (2008).
- Namdar M, Hany TF, Koepfli P et al.: Integrated PET/CT for the assessment of coronary artery disease: a feasibility study. J. Nucl. Med. 46(6), 930–935 (2005).
- Hoffmann U, Moselewski F, Nieman K et al.: Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J. Am. Coll. Cardiol. 47(8), 1655–1662 (2006).
- Hausleiter J, Meyer T, Hadamitzky M, Kastrati A, Martinoff S, Schomig A: Prevalence of noncalcified coronary plaques by 64-slice computed tomography in patients with an intermediate risk for significant coronary artery disease. J. Am. Coll. Cardiol. 48(2), 312–318 (2006).
- Motoyama S, Kondo T, Sarai M et al.: Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 50(4), 319–326 (2007).
- Akram K, Rinehart S, Voros S: Coronary arterial atherosclerotic plaque imaging by contrast-enhanced computed tomography: fantasy or reality? J. Nucl. Cardiol. 15(6), 818–829 (2008).
- Tianli G, Zhuo Z, Wei Y, Zhaoqi Z, Yongjun W: Atherosclerotic carotid vulnerable plaque and subsequent stroke: a highresolution MRI study. Cerebrovasc. Dis. 27(4), 345-352 (2009).