Review Article - Interventional Cardiology (2014) Volume 6, Issue 1
Nontransfemoral access alternatives for transcatheter aortic valve replacement
- Corresponding Author:
- Michael J Reardon
Houston Methodist DeBakey Heart & Vascular Center, Houston Methodist Hospital, Houston, TX, USA
Tel: +1 713 441 5200
E-mail: mreardon@houstonmethodist.org
Abstract
Symptomatic severe aortic stenosis (AS) carries a high mortality rate if not treated. This has led to the inclusion of a class I recommendation for surgical aortic valve replacement (AVR) in the American College of Cardiology/American Heart Association guidelines for patients with symptomatic severe AS. For over 60 years, AVR has served as the treatment of choice for AS and has saved countless lives. Unfortunately, there exists a population of patients whose age or co-morbidities makes them either an extreme risk or a high risk for open AVR. This represents between 30 and 60% of the patients with symptomatic severe AS, who are as a result denied surgical intervention.
Keywords
direct aortic access, subclavian/axillary access, transapical access, transcatheter aortic valve replacement
Symptomatic severe aortic stenosis (AS) carries a high mortality rate if not treated. This has led to the inclusion of a class I recommendation for surgical aortic valve replacement (AVR) in the American College of Cardiology/American Heart Association guidelines for patients with symptomatic severe AS. For over 60 years, AVR has served as the treatment of choice for AS and has saved countless lives. Unfortunately, there exists a population of patients whose age or co-morbidities makes them either an extreme risk or a high risk for open AVR. This represents between 30 and 60% of the patients with symptomatic severe AS, who are as a result denied surgical intervention. This population increases with increasing age and will become a larger burden in the future. Transcatheter AVR (TAVR) has been developed as an alternative treatment to surgical AVR in the extreme-risk and high-risk population who are deemed inoperable or too risky to operate on. The TAVR procedure enables AVR without performing open heart surgery and cardiopulmonary bypass. The procedure involves implantation of a prosthetic heart valve within the diseased native aortic valve through a minimally invasive approach.
TAVR valve systems consist of a frame to hold the valve and to help push the native stenotic aortic valve leaflets out of the way, along with the valve itself and the delivery system. The TAVR procedure consists of delivering a device into the stenotic aortic valve, expanding the frame of the device, deploying the valve and removing the delivery system. The sine qua non of TAVR is a safe remote access to the heart and aortic annulus.
TAVR approaches
It is generally accepted that a transfemoral approach is the least invasive alternative and should be used whenever possible. Although refinements of the delivery systems are leading to development of smaller systems, the currently available transfemoral delivery systems in the USA range from 18 to 24 F, requiring minimal arterial luminal diameters of 6–8 mm. Pushing beyond the safe limits for femoral sheath and device delivery can lead to serious vascular complications and death. To avoid such vascular disasters, nonfemoral alternative access routes for TAVR have been developed and will be the subject of this paper. The ordering of the access routes discussed below is based on the current order of preference of the nonfemoral access approaches at the Houston Methodist DeBakey Heart and Vascular Center (TX, USA).
▪ Subclavian artery access
Subclavian artery access was developed for use with the Medtronic CoreValve (Medtronic Inc., MN, USA) and its 18 F delivery system. Majority of the experience gained so far is with this valve. With the availability of the Sapien XT (Edwards Lifesciences Corporation, CA, USA) with an 18 F delivery system, subclavian access with Sapien XT has also been carried out. A minimum luminal diameter of 6 mm in an uncalcified artery is necessary for safe subclavian access and increases to 7 mm in an artery with significant calcification. For implantation of the CoreValve from the left subclavian, the angle of the aorta from the horizontal should not exceed 70° and from the right subclavian, it should not exceed 30°. Angles beyond these make axial alignment and proper placement of the valve increasingly difficult. In marginal arteries, the CoreValve can be placed bareback without the sheath. With the proximity to the annulus, this allows great control. The drawback is that a valve pop out cannot be retrieved without the sheath although this is an infrequent problem.
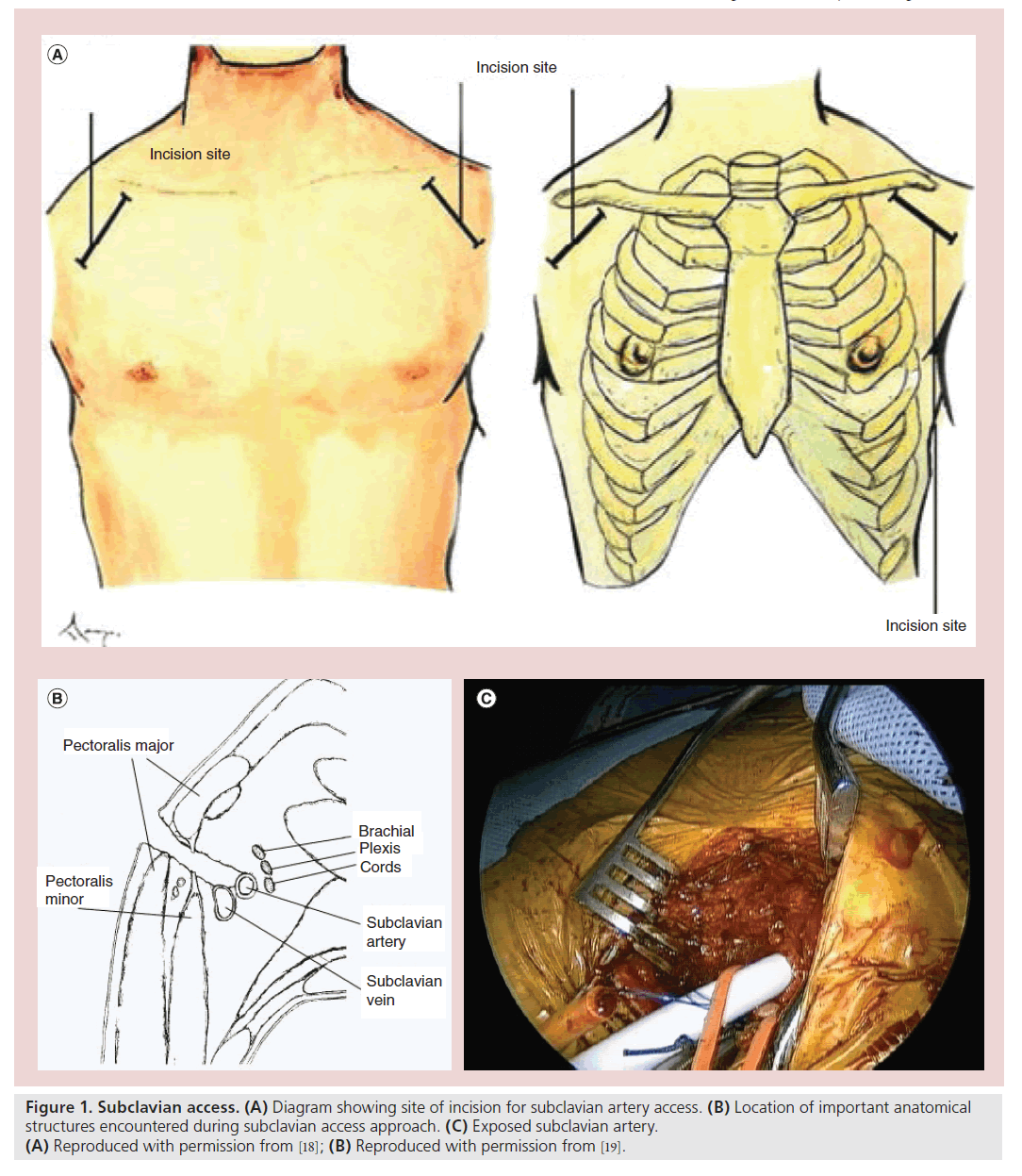
The subclavian artery exits the chest over the first rib at the thoracic inlet and travels behind the mid clavicle as it descends below the lateral clavicle and becomes the axillary artery (Figure 1). A 2–4 cm incision in the deltopectoral groove is made. The most common mistake is to make the incision too medial before the artery has descended from behind the clavicle. The fibers of the pectoralis major are split and the pectoralis minor can then be seen. This muscle can be retracted or divided to expose the subclavian artery. The artery is punctured using a standard Seldinger technique and a 6 F sheath is placed within. Through this sheath, the valve is crossed and hemodynamic data is obtained. A super stiff Amplatz™ (Boston Scientific Inc., MA, USA) wire is then placed through the ventricular pigtail and the pigtail is removed. The 18 F sheath is placed over the stiff wire. Placement over a soft wire can be done. However, it carries more risk, which we believe should be avoided. The sheath is advanced into the distal ascending aorta and the valve deployment is then performed in a standard fashion. Please refer to the ‘Subclavian access’ section in Box 1 for a step-by-step overview of the procedural technique. An advantage over femoral placement is the proximity to the area of deployment, less tension or energy stored in the system and a greater correlation between operator movement and valve movement.
Figure 1. Subclavian access. (A) Diagram showing site of incision for subclavian artery access. (B) Location of important anatomical structures encountered during subclavian access approach. (C) Exposed subclavian artery. (A) Reproduced with permission from [18]; (B) Reproduced with permission from [19].
Considerable data have begun to accumulate on subclavian access. Data from a 2-year Italian study showed that subclavian access gives comparable results to transfemoral access in matched pairs at 2 years [1]. Information has also become available to indicate that the left subclavian approach can be used safely with a patent mammary artery bypass [2]. The surgical cut down has been replaced in some centers with a percutaneous approach with initial success [3].
▪ Direct aortic access
Direct aortic (DA) access was developed for CoreValve insertion in patients without adequate femoral or subclavian access. DA access and transapical access, which will be discussed later, share several advantages. They are the most direct access routes for TAVR. Neither of the two methods is limited by peripheral vascular disease or sheath size. Neither is limited by aortic angle and axial alignment is easy to achieve. Both methods avoid passing the device over the arch and allow precise deployment due to the straight path and proximity of the operator to the deployment site. DA access does have some advantages over transapical access. DA access can be done without a thoracotomy, which is required for transapical access. This causes less pulmonary impairment. DA access requires no injury to the myocardium. The aorta moves less than the cardiac apex which allows the DA sheath to be sewn firmly into place while the transapical sheath is generally held by an assistant. Bleeding around the DA sheath is rarely a problem but can be an issue for transapical access. The DA approach can be used for both the CoreValve and Sapien valve. All cardiac surgeons have considerable experience with replacing the ascending aorta and perform this procedure on a routine basis. However, fewer surgeons have experience with replacing the left ventricular apex, a significantly more difficult procedure. Hence, should severe problems arise with the ascending aorta, most surgeons are likely to be more comfortable with the DA approach.
When we developed our DA access program we began by looking at the surface anatomy of the chest wall and the cardiac valves [4]. Our group also has extensive experience in using small right anterior thoracotomy for arch debranching and hybrid thoracic endovascular aortic repair work that provides similar access to the aorta [5]. Additionally, our group uses both a small right anterior thoracotomy and a mini J sternotomy for standard surgical minimally invasive AVR. We combined this with the nascent literature on DA approach led largely by Giuseppe Brushi (Niguarda Ca’ Granda Hospital, Milan, Italy) [6]. This information was applied by the CoreValve Direct Aortic Working Group to develop the DA approach for the CoreValve IDE trial in the USA.
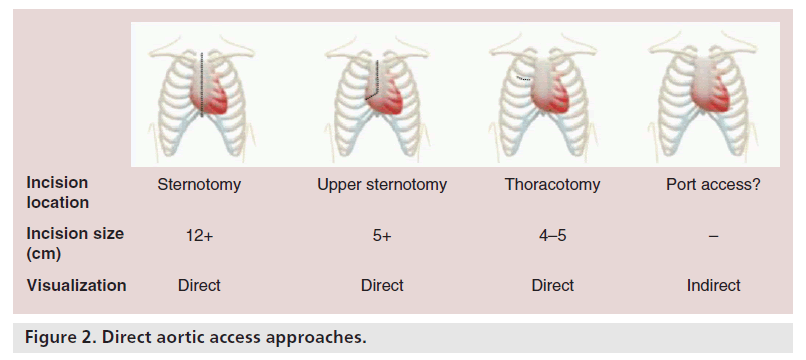
DA access can be accomplished in a number of ways (Figure 2). Full sternotomy has been used to allow DA access for TAVR combined with off-pump coronary artery bypass, although this is currently an unusual approach. The most common approaches are through a small upper right J hemisternotomy into the second or third intercostal space, or a small right anterior thoracotomy through the second intercostal space, or by removing the second rib sternal cartilage. Finally, mini sternotomy allows the potential for port access TAVR, which has recently been accomplished [7]. We will, however, focus on mini sternotomy and mini thoracotomy as the common approaches.
Mini J sternotomy
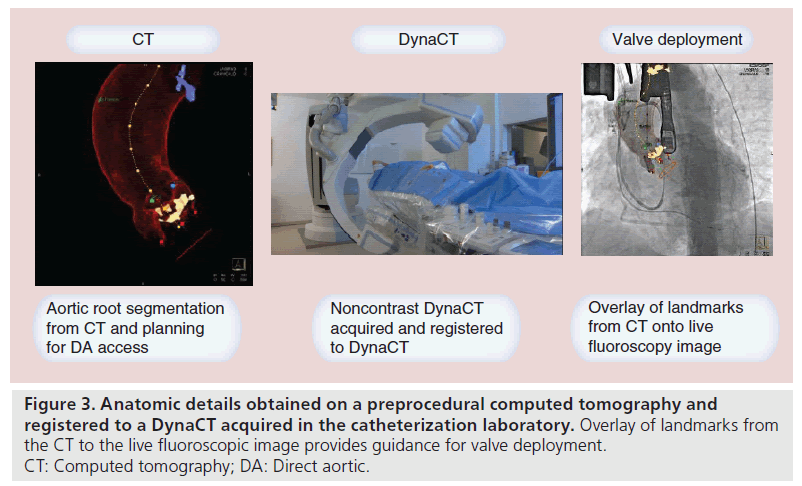
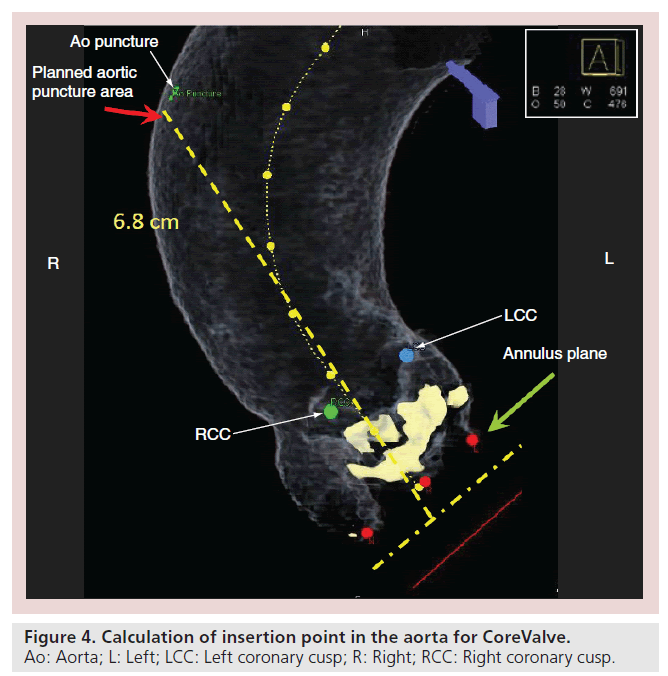
Mini J sternotomy begins with a 4 cm skin incision over the upper sternum. The sternal notch is dissected to allow access behind the sternum. The right intercostal space just below the sternal angle is dissected to allow access for the sternal saw. The sternum is opened to form a ‘J’ at the manubrium. It is divided from the sternal notch inferiorly in the midline and then angled into the right second or third intercostal space. The mediastinal fat is dissected away to reveal the innominate vein which will mark the distal extent of the ascending aorta. The takeoff of the innominate artery is generally easily identified at this point. We place a purse string of pledgetted 3-0 polypropylene suture in the proximal arch and place a 6 F sheath through this. A 5 F marker pigtail catheter is inserted through this sheath into the noncoronary sinus as a marker and for arteriography. At the very beginning of the procedure, we perform a noncontrast DynaCT scan which we map to the patient’s preprocedural screening CT scan (Figure 3). Our Siemens software then calculates an insertion point in the aorta that is axial and allows enough distance for insertion if we use a CoreValve (26-mm CoreValve has a deployed length of 55 mm and at least 10 mm is required for sheath insertion) (Figure 4). A double purse string of two concentric 3-0 polypropylene pledgetted sutures are placed at this spot and a 6 F sheath is inserted. The valve is crossed through this sheath and an angled pigtail catheter is placed into the ventricle, and hemodynamic readings are obtained. A precurved Amplatz super stiff wire is then placed into the ventricle and the 18 F sheath is inserted. Unfortunately, there are currently no sheaths designed specifically for transaortic insertion. One of the main issues is that the dilator is much too long, so care must be taken during insertion. In addition, with the current sheaths, there is no way to easily control insertion depth other than via manual control. Manual control is difficult to achieve as there is a release of pressure when the sheath/dilator interface pops through.
Figure 3. Anatomic details obtained on a preprocedural computed tomography and registered to a DynaCT acquired in the catheterization laboratory. Overlay of landmarks from the CT to the live fluoroscopic image provides guidance for valve deployment. CT: Computed tomography; DA: Direct aortic.
To remedy this we place a silicone ring from a standard aortic cannula 1 cm from the end of the sheath and tie a suture behind it to lock it into place (Figure 5). Once the sheath is inserted, it should be securely sutured into place as the operator needs to be focused on implanting the valve and not concerned about the stability of the sheath. This also allows the operator to remain further removed from the radiation source as radiation levels during DA and transapical access can be high due to operator proximity. Balloon aortic valvuloplasy and TAVR are then accomplished in a standard fashion with the caveat that precise deployment is greatly enhanced by operator proximity to the deployment site and the straight path of the delivery system. Once finished, the sheaths are removed and the purse strings are tied similar to a standard aortic cannulation that is used daily in cardiac surgery. A soft drain is placed in the pericardium and the chest is closed (see ‘Direct aortic access’ and ‘Mini J sternotomy’ sections in Box 1).
Mini thoracotomy
A 4-cm skin incision is made over the junction of the second rib and the sternum. The second costal cartilage is removed. The procedure can be done just through the interspace and is well tolerated but this does limit the access and removal of the costal cartilage. The internal mammary artery and vein are ligated if necessary and only a soft tissue retractor is placed. Use of a rib spreader will lead to a significant increase in pain and should be avoided if possible. The mediastinal tissue over the aorta is dissected and the pericardium sutured to the lateral wound edge to wall off the lung and provide exposure. Placement of the purse string sutures and sheaths is the same as in a mini sternotomy. It is important to line up the sheath angle with the intended approach angle for the valve and then suture the sheath securely into place. When finished, a soft pleural drain is left in the right thoracic space (see ‘Direct aortic access’ and ‘Mini thoracotomy’ sections in Box 1).
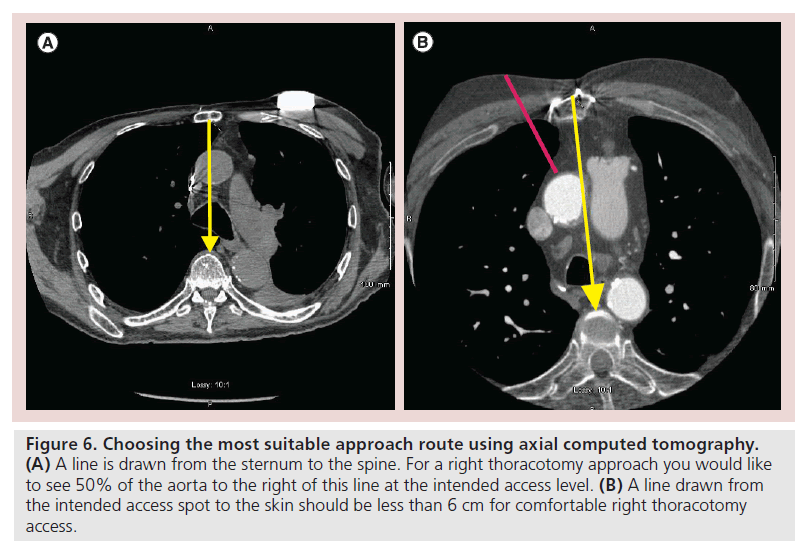
Each approach has its advantages and disadvantages. The mini sternotomy approach usually seems safer to most cardiac surgeons initially, as they are used to doing median sternotomy. While using this approach, getting out of a challenging situation can also be easier. In the virgin chest, it tends to be both quick and technically easy to perform. You do not enter the pleural space and more aortic ‘real estate’ is available for the surgeon to choose from. Achieving a straight axial approach can be more difficult than with a thoracotomy and on redo chests, as the presence of grafts behind the upper sternum can pose a risk. Mini thoracotomies can be safer in the redo chest and are generally more axial. Its disadvantage is that there is generally less room to work with and the pleural space is transgressed. Deciding which approach to use is a decision for the surgeon and the heart team to make; however, there are some general principles that are useful. On the axial CT scan at the level of planned sheath insertion, we draw a line from the mid sternum directly back to the spine (Figure 6). If at least 50% of the aorta is not to the right of this line, a thoracotomy approach may be difficult. We then draw a line from the planned sheath insertion site to the skin and if this distance is over 6 cm, then a thoracotomy approach may be difficult. The most important issue is knowledge of what is behind the sternum and the risk of injury with either of these approaches. The final decision of thoracotomy versus mini sternotomy should be a heart team decision based on what is best and safest for that individual patient [8].
Figure 6. Choosing the most suitable approach route using axial computed tomography. (A) A line is drawn from the sternum to the spine. For a right thoracotomy approach you would like to see 50% of the aorta to the right of this line at the intended access level. (B) A line drawn from the intended access spot to the skin should be less than 6 cm for comfortable right thoracotomy access.
The US data on DA access for CoreValve will not be available until after the seminal IDE trial papers are presented. Data from Europe were presented in 2012 at the 48th meeting of the Society of Thoracic Surgery in Fort Lauderdale (FL, USA). Neal Moat from the Royal Brompton in London (UK) presented the European experience for CoreValve DA access [9]. A total of 93 cases were carried out of which 49 out of 93 (52.5%) were mini thoracotomies and 44 out of 93 (47.5%) were mini sternotomies. The procedural success was 92 out of 93 (98.9%). Outcomes included a 30-day mortality of nine out of 93 (9.7%), stroke rate of three out of 93 (3.2%), life-threatening bleeds (as defined by the Valve Academic Research Consortium) in four out of 93 (4.3%) and no dissections. The incidence of major vascular injury as defined by the Valve Academic Research Consortium was four out of 93 (4.3%) and new pace maker placement was 16 out of 93 (17.2%). Notably, 21.6% of the cases had been carried out in the first 54 months and 78.4% were carried out in the last 12 months of the study, highlighting the rapid adoption and growth of this approach. The European experience with Sapien DA access was presented at this same meeting by Vinnie Bapat from St Thomas’ Hospital in London (UK) [10]. A total of 158 cases were carried out of which there were 138 mini sternotomies (87.3%), 14 mini thoracotomies (8.8%) and six full sternotomies (3.9%). Allcause mortality was 11 out of 158 (7%) and there were no strokes. Both series show very acceptable results for the contingent of critically ill patients. DA access and transapical access have been compared in an US series [11]. Both were found to have similar device success rates and safety end points such as death, myocardial infarction and stroke. The DA approach was associated with less bleeding and vascular events (27 vs 46%), shorter median intensive care unit length of stay and a better learning curve. This direct comparison represents a small early experience, which is none the less interesting.
▪ Transapical access
The transapical access for TAVR was first carried out by a Vancouver team and reported in 2006 [12]. Other investigators quickly added to the development of this approach [13–15]. Transapical and DA access share the attribute of being the most direct access methods for TAVR and the additional simplicity of being an antegrade approach. Using an antegrade approach is generally technically easier than a retrograde approach for crossing the stenotic aortic valve. Transapical access is not limited by sheath size, aortic angle or peripheral vascular disease. It avoids passing the device but not the guide wire across the aortic arch. The proximity of the operator and direct path of the delivery device allows one to one correlation between operator movement and valve movement. Disadvantages include the need for a thoracotomy in all cases, the need to penetrate the myocardium and the less stable and more mobile nature of the left ventricular apex compared with the ascending aorta.
Transapical access starts by identifying the left ventricular apex using fluoroscopy as its position can be highly variable. A 4–6 cm incision is made over the left ventricular apex and a soft tissue retractor is placed. A rib spreader can be used but causes much more pain post implant and should be avoided if possible. The pericardium is opened and sutured to the skin edges to expose and stabilize the apex. Two concentric purse string sutures of pledgetted 3-0 polypropylene are placed in the apex with care taken to avoid the left anterior descending coronary artery. A 6 F sheath is placed into the ventricle and a soft wire is passed antegrade across the valve and into the descending aorta. A catheter is then used to exchange this for a stiff wire and the sheath is placed. The Sapien valve has a sheath designed specifically for transapical use. Balloon aortic valvuloplasy and valve placement can then take place in a standard fashion. When removing the sheath and tying the purse string sutures, it is helpful to maintain a rapid pace to allow closure with limited ventricular pressure. The left chest is then drained and the chest is closed (see the ‘Transapical access’ section in Box 1).
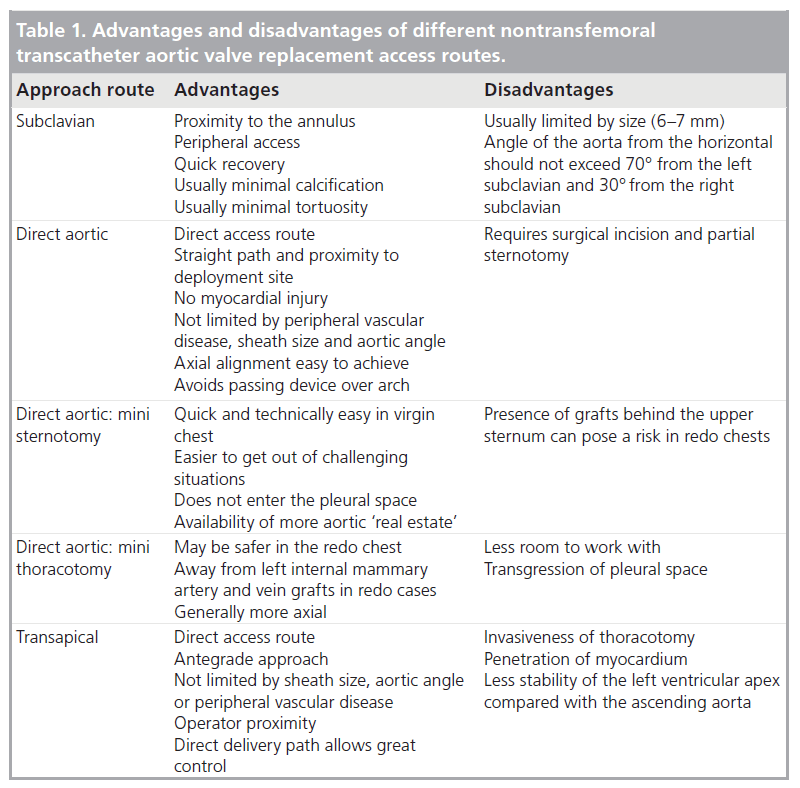
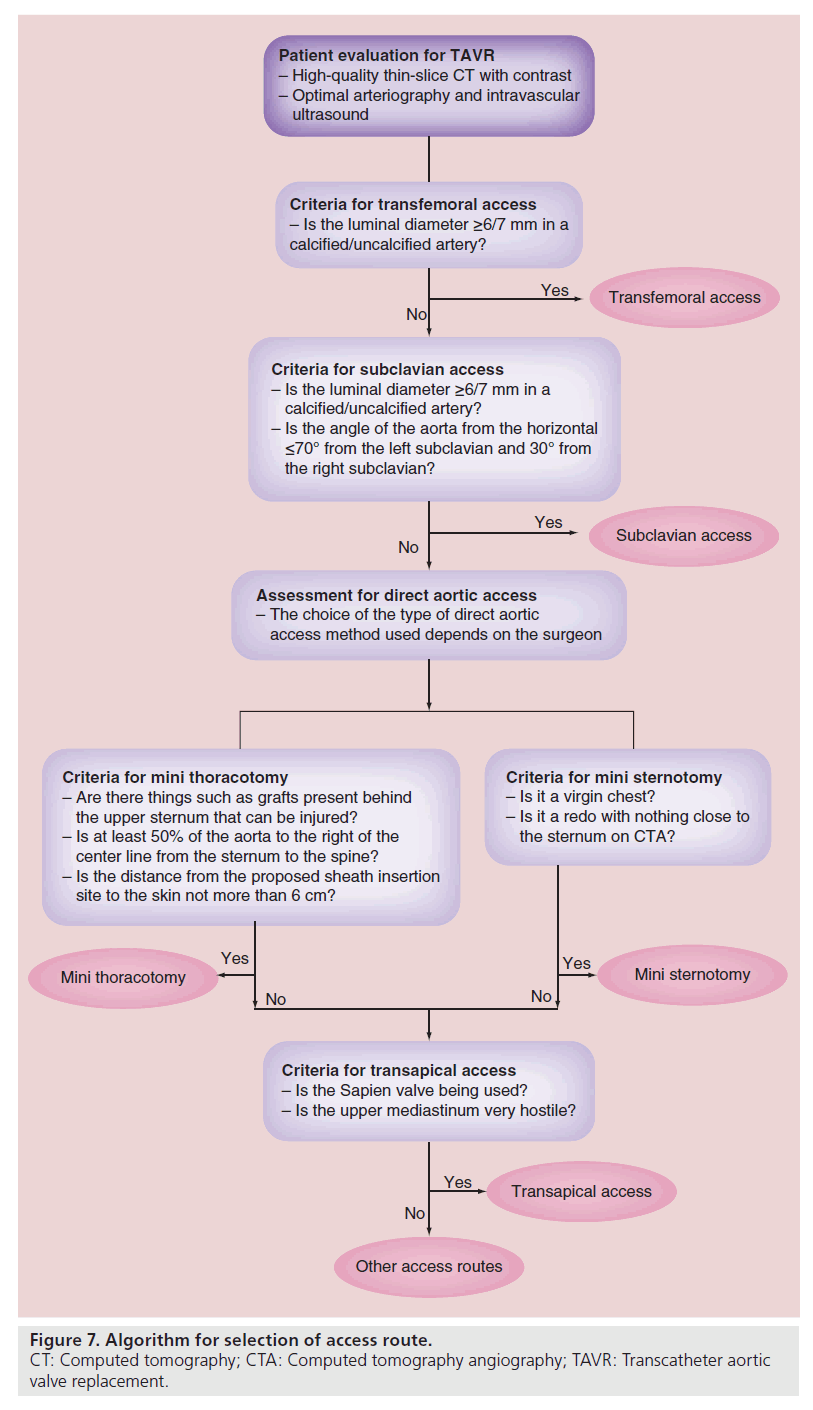
Comparing transapical to transfemoral access is difficult as you are not comparing apples to apples. By definition, transapical patients have severe peripheral vascular disease which is a known marker for risk of mortality and morbidity. Experienced centers have reported excellent results with transapical TAVR but most acknowledge a reasonably steep learning curve with this approach. With time, the use of transapical access may decrease and use of DA access may increase. However, its use will not stop because it is the most commonly used route for antegrade access and an excellent access route for the mitral valve. The main advantages and disadvantages of each of the access routes discussed in this manuscript are listed in Table 1. However, the final choice of route of access should be made by the heart team with the safety of each individual patient carefully considered. Figure 7 illustrates our algorithm for access selection in TAVR patients.
▪ Other nonfemoral access approaches
For patients who are not candidates for femoral, subclavian, DA or transapical access, transcarotid access has been pioneered by a group at Emory [16]. Even antegrade transseptal TAVR is being reconsidered [17].
Complications
Complications from alternate access TAVR procedures can occur and are similar to most cardiac procedures. Of note, proper preoperative planning and consideration of all clinical and imaging data is essential for avoidance of access complications. The TAVR team should be familiar with all potential access techniques and offer the patient the most appropriate approach to minimize the potential for complications. Figure 7 explains our algorithm for access selection and technical details important for avoiding complications. Avoiding problem areas (excessive calcification, tortuosity, fragile tissues and grafts) is important in these often very frail patients. Meticulous surgical technique is important for minimizing bleeding, embolization and cardiovascular injury. A completely dry field is important prior to chest closure in DA approaches. Catheters and stiff wires should be carefully manipulated under direct fluoroscopy to prevent cardiac or vascular perforation.
Conclusion
TAVR has proven to be a reasonable approach for the treatment of symptomatic severe aortic stenosis in high-risk and extreme-risk patients, and is currently in randomized trials in intermediaterisk patients. All TAVR procedures require a safe, reproducible and distant access to the aortic valve for device deployment. Transfemoral access is generally accepted as the least invasive and front-line approach. For patients that do not have adequate femoral access, the heart team must know the alternative options, their finer technical points and potential complications. The final decision on the access route should always be a heart team decision.
Future Perspective
Enhanced imaging and closure devices will continue to improve the performance and safety of alternative access routes for TAVR. Specifically, novel imaging software programs can facilitate visualization of the aortic root and allow for patient-specific modeling that can guide a more precise and predictable valve deployment. Furthermore, next-generation TAVR platforms will likely become smaller in size (14 F or less) and more maneuverable, which may reduce the need for nonfemoral access. Furthermore, catheter-based valves will also undergo further modifications to minimize paravalvular leaking, heart block and migration. Similarly, nonfemoral approaches are constantly improving. Percutaneous subclavian access is currently performed by several groups in Europe. Closure devices for the left ventricular apex are under development and percutaneous transapical access is already a reality in the lab and will likely be trialed in the not too distant future in humans. Thoracoscopic port access and DA access have been accomplished in humans and could someday become a common reality. The future prospects for nonfemoral access becoming easier and safer are bright.
Acknowledgements
The authors acknowledge Maitreyi Muralidhar, employee of Houston Methodist DeBakey Heart & Vascular Center, for editorial assistance in manuscript preparation and styling as per journal requirements.
Financial & competing interests disclosure
B Ramlawi is a consultant at Atricure, Inc. M Reardon is a consultant and on the advisory board at Medtronic, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
• The transfemoral route is generally preferred but it will not be feasible in all patients due to vessel caliber, calcification or tortuosity.
• Alternative access to the aortic valve should be readily adopted and considered to minimize vascular complications and bleeding.
• Knowledge of all viable alternative, nonfemoral access routes is imperative for a successful transcatheter aortic valve replacement team and patient safety.
• This paper describes the currently used common alternative nonfemoral access routes and how they are performed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- Petronio AS, De Carlo M, Bedogni F et al. 2-year results of CoreValve implantation through the subclavian access: a propensity-matched comparison with the femoral access. J. Am. Coll. Cardiol. 60(6), 502–507 (2012).
- Modine T, Sudre A, Delhaye C et al. Transcutaneous aortic valve implantation using the left carotid access: feasibility and early clinical outcomes. Ann. Thorac. Surg. 93(5), 1489–1494 (2012).
- Schafer U, Ho Y, Frerker C et al. Direct percutaneous access technique for transaxillary transcatheter aortic valve implantation: ‘the Hamburg Sankt Georg approach’. JACC Cardiovasc. Interv. 5(5), 477–486 (2012).
- Reardon MJ, Conklin LD, Philo R, Letsou GV, Safi HJ, Espada R. The anatomical aspects of minimally invasive cardiac valve operations. Ann. Thorac. Surg. 67(1), 266–268 (1999).
- Anaya-Ayala JE, Cheema ZF, Davies MG et al. Hybrid thoracic endovascular aortic repair via right anterior minithoracotomy. J. Thorac. Cardiovasc. Surg. 142(2), 314–318 (2011).
- Bruschi G, De Marco F, Fratto P et al. Direct aortic access through right minithoracotomy for implantation of self-expanding aortic bioprosthetic valves. J. Thorac. Cardiovasc. Surg. 140(3), 715–717 (2010).
- Etienne PY, Papadatos S, Mailleux P et al. Exclusive percutaneous approach for surgical transaortic transcatheter valve replacement. Ann. Thorac. Surg. 95(5), 1812–1814 (2013).
- Ramlawi B, Anaya-Ayala JE, Reardon MJ. Transcatheter aortic valve replacement (TAVR): access planning and strategies. Methodist Debakey Cardiovasc. J. 8(2), 22–25 (2012).
- Moat N, Laborde JC, Bruschi G et al. European experience of direct aortic TAVI with a self-expanding prosthesis. Presented at: The Society of Thoracic Surgeons 48th Annual Meeting. Fort Lauderdale, FL, USA, 29 January–1 February 2012.
- Bapat V, Romano M, Etienne P et al. Transcatheter aortic valve replacement with Edwards SAPIEN valve via transaortic route. Presented at: The Society of Thoracic Surgeons 48th Annual Meeting. Fort Lauderdale, FL, USA, 29 January–1 February 2012.
- Lardizabal JA, O’Neill BP, Desai HV et al. The transaortic approach for transcatheter aortic valve replacement: initial clinical experience in the United States. J. Am. Coll. Cardiol. 61(23), 2341–2345 (2013).
- Lichtenstein SV, Cheung A, Ye J et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 114(6), 591–596 (2006).
- Grube E, Schuler G, Buellesfeld L et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J. Am. Coll. Cardiol. 50(1), 69–76 (2007).
- Walther T, Falk V, Borger MA et al. Minimally invasive transapical beating heart aortic valve implantation--proof of concept. Eur. J. Cardiothorac. Surg. 31(1), 9–15 (2007).
- Walther T, Simon P, Dewey T et al. Transapical minimally invasive aortic valve implantation: multicenter experience. Circulation 116(11 Suppl.), I240–I245 (2007).
- Guyton RA, Block PC, Thourani VH, Lerakis S, Babaliaros V. Carotid artery access for transcatheter aortic valve replacement. Catheter. Cardiovasc. Interv. 82(4), E583–E586 (2012).
- Cohen MG, Singh V, Martinez CA et al. Transseptal antegrade transcatheter aortic valve replacement for patients with no other access approach – a contemporary experience. Catheter. Cardiovasc. Interv. doi:10.1002/ ccd.25036 (2013) (Epub ahead of print).
- Ramlawi B, Anaya-Ayala JE, Reardon MJ. Transcatheter aortic valve replacement (TAVR): access planning and strategies. Methodist Debakey Cardiovasc. J. 8(2), 22–25 (2012).
- Neri E, Massetti M, Capannini G et al. Axillary artery cannulation in type a aortic dissection operations. J. Thorac. Cardiovasc. Surg. 118(2), 324–329 (1999).
• Study on alternative access transcatheter aortic valve replacement (TAVR).
• Study on alternative access TAVR.
• Study on alternative access TAVR.
•• Focuses on alternative TAVR access technique and outcomes.
•• Focuses on alternative TAVR access technique and outcomes.
•• Focuses on alternative TAVR access technique and outcomes.
• Study on alternative access TAVR.
•• Focuses on alternative TAVR access technique and outcomes.