Editorial - Interventional Cardiology (2009) Volume 1, Issue 1
Novel 320-slice multislice CT angiography as a gatekeeper for invasive coronary angiography
- Corresponding Author:
- Jeroen J Bax
Department of Cardiology, Leiden University Medical Center
Albinusdreef 2, 2333 ZA, Leiden, The Netherlands
Tel: +31 (0)71 526 2020
Fax: +31 (0)71 526 6809
E-mail: j.j.bax@lumc.nl
Abstract
“While diagnostic accuracy of 320‑slice CTA will most likely be similar to 64‑slice systems, the main advantages of this technique are decreased scan time, contrast administration and radiation dose. Furthermore, volumetric scanning allows for CTA in conjunction with myocardial perfusion imaging in a single examination and heart beat.”
Invasive coronary angiography (ICA) is considered to be the gold standard for the diagnosis of coronary artery disease (CAD). However, in many cases ICA is used for diagnostic evaluation only and is not followed by coronary revascularization. Consequently, these patients could benefit from a noninvasive approach to visualize the coronary arteries. To this end, use of multislice computed tomography angiography (CTA) has been proposed. Since its introduction, the technique has evolved into a promising imaging modality for noninvasive imaging of the coronary arteries. Owing to rapid technological innovations, such as increasing temporal and spatial resolution as well as an increasing number of detectors, accurate assessment of CAD has become possible. Most recently, 256- and 320‑slice systems have become available, for the first time allowing volumetric scanning of the entire heart in a single heart beat [1,2]. It is anticipated that these developments will continue to translate into superior image quality and further improvements in the evaluation of CAD. In the current article, the role of novel CTA technology in the evaluation of patients with known or suspected CAD is discussed.
CTA as gatekeeper for ICA in patients with suspected CAD
▪ Exclusion of obstructive CAD with CTA
Although the resolution of CTA remains inferior to that of ICA, high diagnostic accuracies have been reported for the detection of obstructive coronary atherosclerosis. Recently, 320‑slice CTA was introduced, allowing image acquisition of the entire heart in a single heart beat. Although the diagnostic accuracy of 320‑slice CTA has not yet been determined, initial reports have described excellent image quality of this state-of-the-art technology [2,3].
“Most recently, 256- and 320‑slice systems have become available, for the first time allowing volumetric scanning of the entire heart in a single heart beat.”
The diagnostic accuracy of 64‑slice CTA, currently the most widely used CTA system, has been well established by several multicenter studies [4–6]. In a recent prospective multicenter, multivendor trial by Meijboom and coworkers, high diagnostic accuracy of 64‑slice CTA was observed in the evaluation of 360 symptomatic patients with acute and stable angina [5]. On a per-patient basis, the sensitivity and specificity for the detection of obstructive CAD were 99 and 64%, respectively. The positive and negative predictive values were 86 and 97%, respectively. Owing to the high negative predictive value, the authors concluded that CTA is reliable for the exclusion of obstructive CAD. Similarly, in another recent prospective multicenter investigation, the Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) trial, the diagnostic accuracy of 64‑slice CTA was determined in 230 patients with an intermediate prevalence of CAD [4]. On a per-patient basis, the sensitivity, specificity, positive and negative predictive values were 95, 83, 64 and 99%, respectively. These observations further strengthened the notion that the high negative predictive value may establish CTA as a useful alternative to ICA to rule out the presence of significant CAD. Conversely, the CORE-64 trial resulted in a different outcome.
The diagnostic accuracy of 64‑slice CTA was compared with ICA in 291 patients [6]. On a patient basis, sensitivity, specificity, positive and negative predictive values were 85, 90, 91 and 83%, respectively. Accordingly, the investigators concluded that, although CTA accurately identifies the presence of severe CAD, the negative and positive predictive values indicate that CTA at present cannot replace invasive ICA.
“...initial reports have described excellent image quality of this state-of-the-art technology.”
Although the results of these studies seem to be in disagreement, the discrepancy between the reported predictive values may be explained by a difference in disease prevalence in the study populations. As predictive values are highly dependent on disease prevalence in a study population, the diagnostic accuracy of a test should be evaluated in the population in which the test will be used. Unfortunately, many diagnostic accuracy studies, out of necessity, enrolled patients who were referred for ICA and have therefore focused on patient populations with a high prevalence of obstructive CAD. The ACCURACY study, however, was performed in a patient population with an intermediate prevalence of CAD [4], which is considered the target population for CTA. These results indicate that in this target population, 64‑slice CTA has indeed high sensitivity as well as a high negative predictive value. Therefore, in this particular patient population, CTA may serve as a triage instrument and potentially prevent unnecessary further testing, including ICA.
CTA in patients with intermediate pre-test likelihood of CAD
Owing to its high negative predictive value in intermediate pre-test likelihood individuals, the capacity to accurately rule out significant CAD is presently considered to be the major strength of CTA. Several studies have supported the use of CTA in an intermediate likelihood patient population. Meijboom and colleagues assessed the usefulness of 64‑slice CTA to detect or rule out CAD in 254 symptomatic patients with various probabilities of coronary atherosclerosis [7]. The estimated pre-test probability of CAD in high, intermediate and low groups was 87, 53 and 13%, respectively. However, the post-test probabilities of obstructive CAD were 17, 0 and 0% after a negative CTA and 96, 88 and 68% after a positive scan, respectively. These results indicate that CTA may indeed be a useful tool in the exclusion of obstructive atherosclerosis in patients with an intermediate pre-test likelihood of obstructive CAD. Importantly, the additional value of CTA in patients with a high pre-test likelihood remains limited.
“...when used in a low-to-intermediate pre-test likelihood population, CTA may not only rule out the presence of significant atherosclerosis, but may also effectively exclude the presence of any CAD in a large proportion of individuals.”
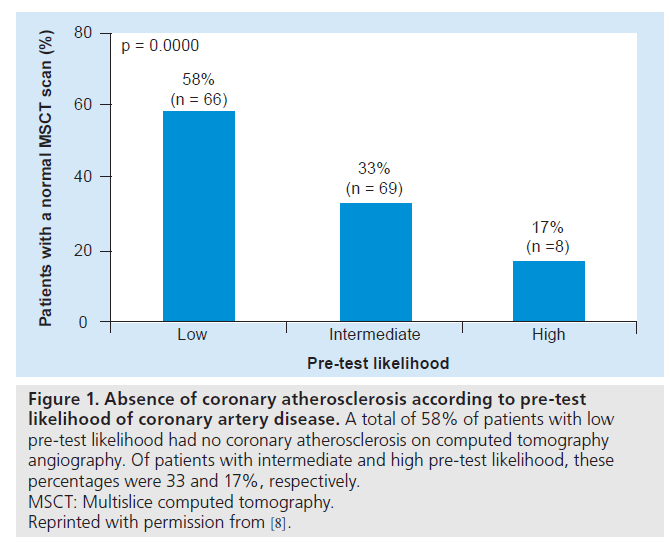
Furthermore, Henneman et al. studied the prevalence of a normal CTA (i.e., the absence of atherosclerosis) in individuals with suspected CAD [8]. Figure 1 summarizes the findings of this study, illustrating that the prevalence of a normal CTA scan significantly decreases from 58% in patients with low pre-test likelihood to 17% of patients with high pre-test likelihood of obstructive CAD. Thus, when used in a lowto- intermediate pre-test likelihood population, CTA may not only rule out the presence of significant atherosclerosis, but may also effectively exclude the presence of any CAD in a large proportion of individuals. Accordingly, patients with a normal CTA may be deferred from further testing. Nevertheless, when a stenosis is observed, stenosis severity may be overestimated by CTA [9,10]. To this end, in case of a positive CTA, further evaluation often remains required to guide patient management.
Figure 1: Absence of coronary atherosclerosis according to pre-test likelihood of coronary artery disease. A total of 58% of patients with low pre-test likelihood had no coronary atherosclerosis on computed tomography angiography. Of patients with intermediate and high pre-test likelihood, these percentages were 33 and 17%, respectively. MSCT: Multislice computed tomography. Reprinted with permission from [8].
CTA as a gatekeeper for ICA in patients with known CAD
▪ CTA following percutaneous coronary intervention
At present, percutaneous coronary intervention with coronary stent implantation is routinely used to treat symptomatic patients with obstructive CAD. Over time, however, in-stent restenosis may occur, requiring timely detection and intervention.
“...it may be concluded that CTA assessment of in-stent restenosis is feasible in stents with a large diameter (>3.0 mm). However, the number of nonevaluable stents remains high.”
With advances in CTA technology, evaluation of in-stent restenosis has improved considerably. Although early 4‑slice CTA did not allow the direct visualization of coronary instent restenosis [11], the evaluation of in-stent restenosis became feasible in a larger proportion of stented segments using 16‑slice multislice computed tomography [12,13]. Lumen interpretability depended mainly on stent diameter, with increased in-stent visualization in large diameter stents (>3 mm). With the introduction of 64‑slice CTA, the evaluation of in-stent restenosis further improved [14,15]. Cademartiri and colleagues evaluated 182 patients with previous stent implantation and reported sensitivity, specificity and positive and negative predictive values of 95, 93, 63 and 99%, respectively [15]. However, a total of 14 stented segments (7.3%) were excluded from the analysis owing to nondiagnostic CTA image quality. Moreover, even higher rates of uninterpretable stents have been reported in other studies [16].
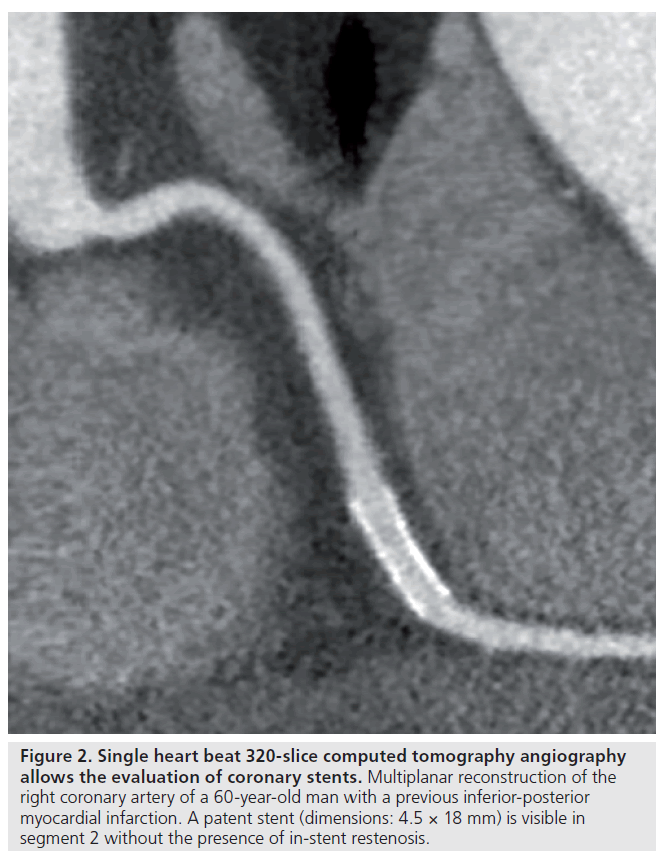
Further improvement may be achieved by newer technology. Dual-source CTA with superior temporal resolution may provide excellent diagnostic accuracy even at increased heart rates, thereby nearly eliminating the necessity for heart rate control. However, with this technology, the evaluation of in-stent restenosis in small stents (<2.75 mm) remains challenging [17]. As of yet, the diagnostic accuracy for instent evaluation using 320‑slice systems has not been reported, although further improvements are anticipated. Figure 2 shows an example of stent patency visualized using 320‑slice CTA.
Figure 2: Single heart beat 320‑slice computed tomography angiography allows the evaluation of coronary stents. Multiplanar reconstruction of the right coronary artery of a 60-year-old man with a previous inferior-posterior myocardial infarction. A patent stent (dimensions: 4.5 × 18 mm) is visible in segment 2 without the presence of in-stent restenosis.
Overall, it may be concluded that CTA assessment of in-stent restenosis is feasible in stents with a large diameter (>3.0 mm). However, the number of nonevaluable stents remains high [18].
Thus, although CTA may be a useful alternative to ICA in selected cases, technological improvements are warranted to further increase in-stent lumen visibility.
▪ CTA following coronary artery bypass graft
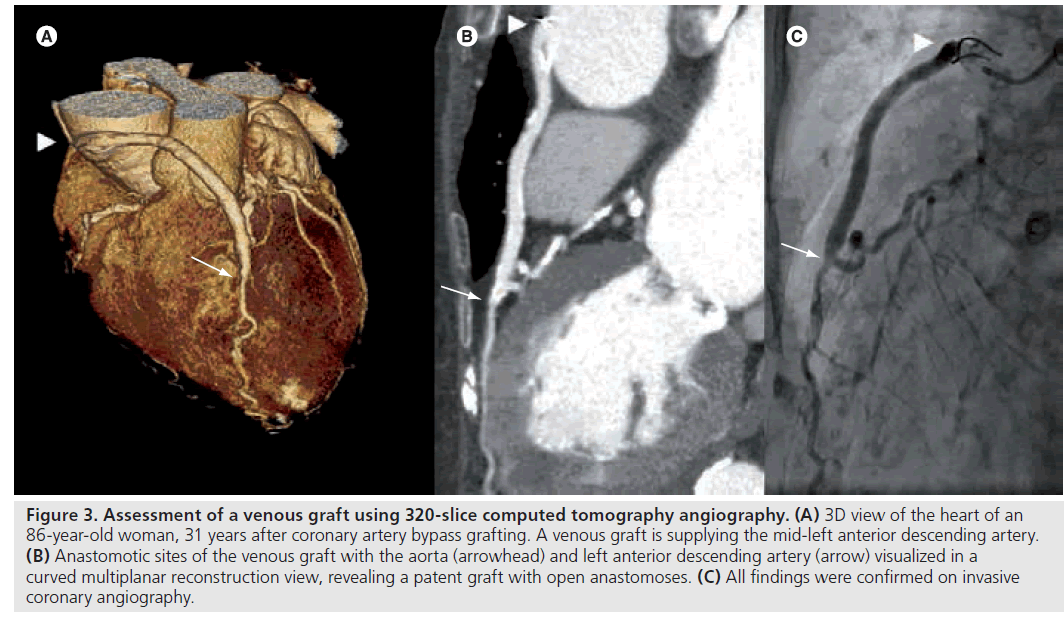
In patients with severe CAD, such as three-vessel disease, or a significant obstruction of the left main artery, coronary-artery bypass grafting (CABG) is preferred over percutaneous coronary intervention. However, graft occlusion is a common problem. As previously reported, CABG may be followed by early occlusion in up to 10% of grafts. Furthermore, 17% of arterial grafts and 59% of venous grafts may occlude within 10 years of surgery [19]. Although the diagnostic accuracy of 320‑slice CTA in the evaluation of patients with CABG has not been described, several studies have shown that 64‑slice CTA may be a valuable tool for the noninvasive evaluation of bypass grafts [20–22]. Ropers and colleagues evaluated the diagnostic accuracy of 64‑slice CTA in 50 patients with a total of 138 grafts, after a mean of 106 months after surgery. Sensitivity and specificity for the detection in patent grafts was 100 and 94%, respectively [22]. Furthermore, on a per-segment basis, the sensitivity and specificity for the evaluation of native coronary arteries and distal runoff vessels was 86 and 76%, respectively. Notably, in the presence of dense calcifications, which are common in patients with a history of CABG, accuracy of CTA significantly decreased. Overall, on a per-patient basis, sensitivity and specificity of CTA was 97 and 86%, respectively. Figure 3 illustrates the use of 320‑slice CTA in the evaluation of graft patency.
Figure 3: Assessment of a venous graft using 320-slice computed tomography angiography. (A) 3D view of the heart of an 86-year-old woman, 31 years after coronary artery bypass grafting. A venous graft is supplying the mid-left anterior descending artery. (B) Anastomotic sites of the venous graft with the aorta (arrowhead) and left anterior descending artery (arrow) visualized in a curved multiplanar reconstruction view, revealing a patent graft with open anastomoses. (C) All findings were confirmed on invasive coronary angiography.
“...technological improvements are warranted to further increase in-stent lumen visibility.”
Thus, CTA may be a useful noninvasive modality for the accurate assessment of CABG and native coronary circulation in selected patients. However, heavy calcifications as well as metallic clip artifacts are common, which significantly decrease diagnostic performance of CTA. Although follow-up imaging with CTA following CABG is presently not recommended, it may be indicated when graft visualization was unsuccessful during ICA.
▪ Single heart beat imaging with 320‑slice CTA
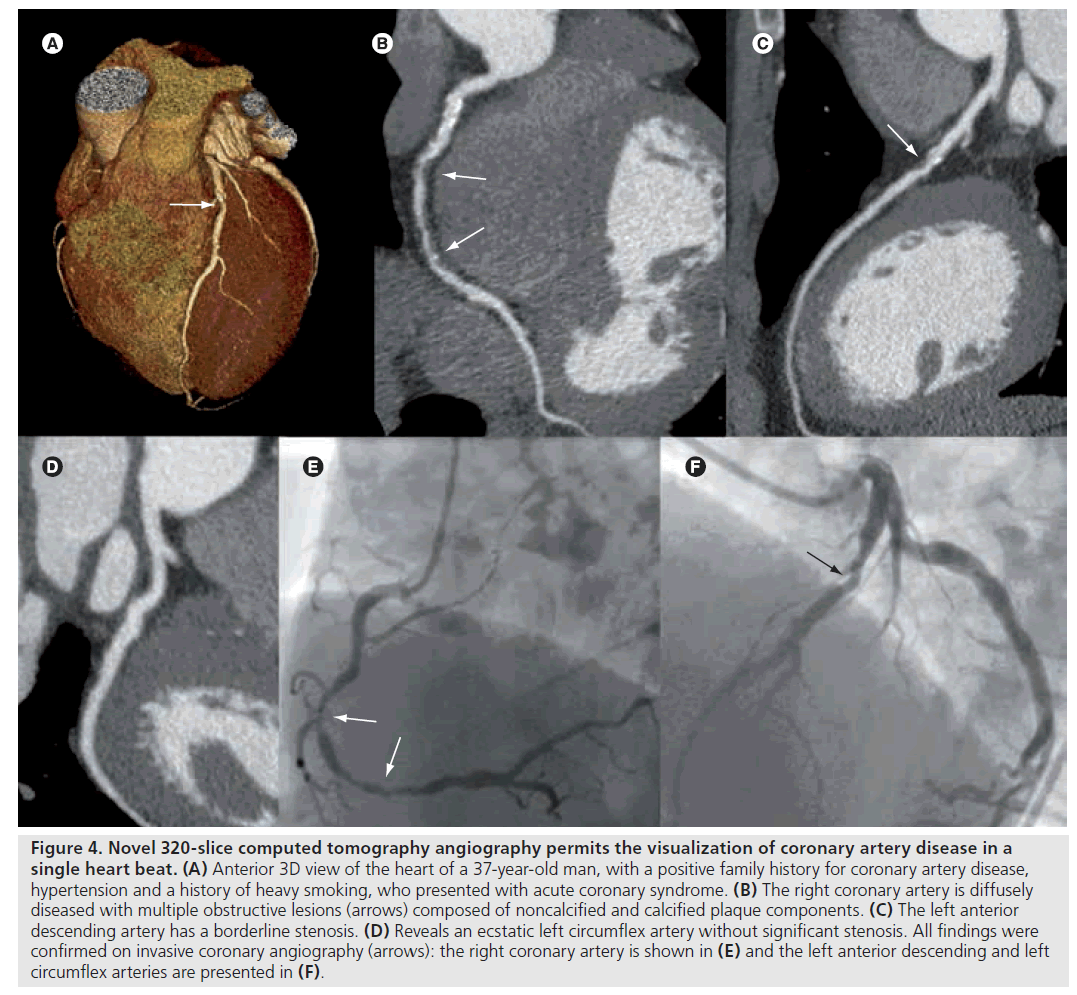
With the introduction of 320‑slice CTA with 16 cm cranio–caudal coverage in a single rotation, CTA technology has made another step forward [23]. Single heart beat scanning techniques have several advantages over helical scanning techniques used by older scanner generations. First, the entire heart can be imaged in a single gantry rotation, which significantly reduces total scan time, time of breath hold, as well as amount of contrast media. Furthermore, 320‑slice CTA in combination with prospective ECG-triggering markedly lowers patient radiation burden for a number of reasons. First, single heart beat scanning eliminates helical oversampling [24]. Second, prospective ECG-triggering allows data acquisition during a small interval of the cardiac cycle, which greatly diminishes radiation exposure [25–27]. Indeed, a recent study by Steigner et al. indicated that a phase window width of 10% may significantly decrease radiation dose, whilst still yielding diagnostic scan quality in more than 90% of scans [27]. As a result, prospectively ECG-triggered 320‑slice CTA may considerably lower patient radiation burden while maintaining image quality. Figure 4 shows an example of a patient with obstructive CAD visualized using prospectively ECG‑triggered 320‑slice CTA.
Figure 4: Novel 320-slice computed tomography angiography permits the visualization of coronary artery disease in a single heart beat. (A) Anterior 3D view of the heart of a 37-year-old man, with a positive family history for coronary artery disease, hypertension and a history of heavy smoking, who presented with acute coronary syndrome. (B) The right coronary artery is diffusely diseased with multiple obstructive lesions (arrows) composed of noncalcified and calcified plaque components. (C) The left anterior descending artery has a borderline stenosis. (D) Reveals an ecstatic left circumflex artery without significant stenosis. All findings were confirmed on invasive coronary angiography (arrows): the right coronary artery is shown in (E) and the left anterior descending and left circumflex arteries are presented in (F).
Furthermore, 320‑slice systems have a higher temporal resolution (350 ms per gantry rotation) as compared with older scanner generations, which reduces the problem of cardiac motion artifacts. Of note, dual-source CTA are equipped with even higher temporal resolution (83 ms), which significantly reduces the difficulties of cardiac motion artifacts [28]. Last, preliminary data suggest that single heart beat 320‑slice CTA also permits the use of arrhythmia-rejection software, allowing image acquisition in patients with irregular heart rates or arrhythmias such as atrial fibrillation [29]. However, currently, such arrhythmia-rejection protocols may be used at the risk of significantly increasing radiation dose, as this technology increases the total acquisition interval to cover multiple heart beats in case an arrhythmia is detected. At present, however, in patients with a low and stable sinus rhythm, the radiation doses reported for prospectively ECG-triggered CTA are approaching the radiation burden of diagnostic ICA [27]. As a result, CTA may become an acceptable alternative to diagnostic ICA in selected individuals.
▪ Myocardial perfusion imaging with CTA
Previous studies have revealed a considerable discrepancy between the presence of significant CAD on CTA and the presence of ischemia on functional testing [30]. Although CTA may confirm the presence of atherosclerosis, the hemodynamic consequences of an obstructive lesion remain uncertain using this purely anatomical approach. At present, however, therapeutic decision making, such as patient referral for ICA, is largely based on the severity of complaints as well as the extent of ischemia on functional testing. As a result, in case of a positive CTA, patients are often referred for myocardial perfusion imaging to determine the functional consequences of an obstructive lesion.
“...single heart beat 320‑slice CTA also permits the use of arrhythmia-rejection software, allowing image acquisition in patients with irregular heart rates or arrhythmias such as atrial fibrillation.”
However, CTA as well as CT myocardial perfusion imaging may be performed simultaneously in a single examination. Indeed, preliminary data suggest that this technology allows the visual or semi-quantitative assessment of myocardial perfusion differences during rest and stress. George and coworkers recently determined whether adenosine stress CT myocardial perfusion imaging with simultaneous CTA allows the detection of atherosclerosis causing myocardial perfusion abnormalities in 40 patients, using 64‑ and 256‑slice CTA [31]. Single-photon emission computed tomography served as the standard of reference. The sensitivity, specificity and positive and negative predictive values on a per-patient basis were 86, 92, 92 and 85%, respectively, suggesting that combined CTA and CT myocardial perfusion imaging permits the detection of atherosclerosis causing perfusion abnormalities. Furthermore, with the introduction of 256- and 320‑slice volumetric scanning techniques, CTA in conjunction with myocardial perfusion imaging may be performed in a single heart beat [31].
Although CT myocardial perfusion imaging is not yet commonly practiced, the prospects of simultaneous CTA and myocardial perfusion imaging are promising. Future research will determine the value of combined anatomical and functional imaging using multislice CT in symptomatic patients.
Conclusion
It seems evident that CTA is an excellent noninvasive imaging modality for detecting obstructive CAD and it is increasingly used for the evaluation of symptomatic patients. As a result of a negative predictive value approaching 100%, CTA has been proposed to serve as a gatekeeper for ICA. Particularly in patients with an intermediate pre-test likelihood of obstructive CAD, CTA may exclude obstructive atherosclerosis in the majority of patients, and avoid unnecessary testing, including ICA. Furthermore, in selected cases, CTA permits the evaluation of patients following revascularization, such as the assessment of in-stent restenosis or the evaluation of CABG and native coronary circulation. However, at present the diagnostic accuracy of in-stent restenosis as well as the assessment of grafts in the presence of metallic clips and heavy calcifications, remains limited.
“...CTA is an excellent non-invasive imaging modality for detecting obstructive CAD.”
Recently, 320‑slice systems have become available, allowing single heart beat image acquisition. While diagnostic accuracy of 320‑slice CTA will most likely be similar to 64‑slice systems, the main advantages of this technique are decreased scan time, contrast administration and radiation dose. Furthermore, volumetric scanning allows for CTA in conjunction with myocardial perfusion imaging in a single examination and heart beat. Additional research is warranted to further define and validate appropriate applications of CTA in clinical practice.
Financial & competing interests disclosure
Fleur R de Graaf is co-supported by the Dutch Technology Foundation STW (Utrecht, The Netherlands), applied science division of NWO and the Technology Program of the Ministry of Economic Affairs, grant number 10084. Joëlla E van Velzen is supported by The Netherlands Heart Foundation (The Hague, The Netherlands), grant number 2007B223. Jeroen J Bax has research grants from Medtronic (MN, USA), Boston Scientific (Natick, USA), BMS medical imaging (New York, USA), St Jude Medical (St Paul, MN, USA), GE Healthcare (Chalfont St Giles, UK), Biotronik (Berlin, Germany), and Edwards Lifesciences (Irvine, CA, USA). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Kido T, Kurata A, Higashino H et al.: Cardiac imaging using 256-detector row four-dimensional CT: preliminary clinical report. Radiat Med. 25(1), 38–44 (2007).
- Rybicki FJ, Otero HJ, Steigner ML et al.: Initial evaluation of coronary images from 320-detector row computed tomography. Int. J. Cardiovasc. Imaging 24(5), 535–546 (2008).
- Chan J, Sarwar S, Khosa F et al.: Diagnostic accuracy of 320-slice multi-detector row computed tomography to detect coronary artery disease: a direct comparison to invasive coronary angiography. Presented at: American College of Cardiology Annual Scientific Session. Orlando, FL, USA, 29–31 March 2009.
- Budoff MJ, Dowe D, Jollis JG et al.: Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 52(21), 1724–1732 (2008)
- Meijboom WB, Meijs MF, Schuijf JD et al.: Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J. Am. Coll. Cardiol. 52(25), 2135–2144 (2008).
- Miller JM, Rochitte CE, Dewey M et al.: Diagnostic performance of coronary angiography by 64-row CT. N. Engl. J. Med. 359(22), 2324–2336 (2008).
- Meijboom WB, van Mieghem CA, Mollet NR et al.: 64-slice computed tomography coronary angiography in patients with high, intermediate, or low pretest probability of significant coronary artery disease. J. Am. Coll. Cardiol. 50(15), 1469–1475 (2007).
- Henneman MM, Schuijf JD, van Werkhoven JM et al.: Multislice computed tomography coronary angiography for ruling out suspected coronary artery disease: what is the prevalence of a normal study in a general clinical population? Eur. Heart J. 29(16), 2006–2013 (2008).
- Heuschmid M, Burgstahler C, Reimann A et al.: Usefulness of noninvasive cardiac imaging using dual-source computed tomography in an unselected population with high prevalence of coronary artery disease. Am. J. Cardiol. 100(4), 587–592 (2007).
- Cury RC, Pomerantsev EV, Ferencik M et al.: Comparison of the degree of coronary stenoses by multidetector computed tomography versus by quantitative coronary angiography. Am. J. Cardiol. 96(6), 784–787 (2005).
- Kruger S, Mahnken AH, Sinha AM et al.: Multislice spiral computed tomography for the detection of coronary stent restenosis and patency. Int. J. Cardiol. 89(2–3), 167–172 (2003).
- Gilard M, Cornily JC, Pennec PY et al.: Assessment of coronary artery stents by 16 slice computed tomography. Heart 92(1), 58–61 (2006).
- Kefer JM, Coche E, Vanoverschelde JL, Gerber BL: Diagnostic accuracy of 16-slice multidetector-row CT for detection of in-stent restenosis vs detection of stenosis in nonstented coronary arteries. Eur. Radiol. 17(1), 87–96 (2007).
- van Mieghem CA, Cademartiri F, Mollet NR et al.: Multislice spiral computed tomography for the evaluation of stent patency after left main coronary artery stenting: a comparison with conventional coronary angiography and intravascular ultrasound. Circulation 114(7), 645–653 (2006).
- Cademartiri F, Schuijf JD, Pugliese F et al.: Usefulness of 64-slice multislice computed tomography coronary angiography to assess in-stent restenosis. J. Am. Coll. Cardiol. 49(22), 2204–2210 (2007).
- Manghat N, Van LR, Hewson P et al.: Usefulness of 64-detector row computed tomography for evaluation of intracoronary stents in symptomatic patients with suspected in-stent restenosis. Am. J. Cardiol. 101(11), 1567–1573 (2008).
- Pugliese F, Weustink AC, Van Mieghem C et al.: Dual source coronary computed tomography angiography for detecting in-stent restenosis. Heart 94(7), 848–854 (2008).
- Vanhoenacker PK, Decramer I, Bladt O et al.: Multidetector computed tomography angiography for assessment of in-stent restenosis: meta-analysis of diagnostic performance. BMC Med. Imaging. 8, 14 (2008).
- Bryan AJ, Angelini GD: The biology of saphenous vein graft occlusion: etiology and strategies for prevention. Curr. Opin. Cardiol. 9(6), 641–649 (1994).
- Malagutti P, Nieman K, Meijboom WB et al.: Use of 64-slice CT in symptomatic patients after coronary bypass surgery: evaluation of grafts and coronary arteries. Eur. Heart J. 28(15), 1879–1885 (2007).
- Nazeri I, Shahabi P, Tehrai M, Sharif-Kashani B, Nazeri A: Assessment of patients after coronary artery bypass grafting using 64-slice computed tomography. Am. J. Cardiol. 103(5), 667–673 (2009).
- Ropers D, Pohle FK, Kuettner A et al.: Diagnostic accuracy of noninvasive coronary angiography in patients after bypass surgery using 64-slice spiral computed tomography with 330-ms gantry rotation. Circulation 114(22), 2334–2341 (2006).
- Rybicki FJ, Otero HJ, Steigner ML et al.: Initial evaluation of coronary images from 320-detector row computed tomography. Int. J. Cardiovasc. Imaging 24(5), 535–546 (2008).
- Mori S, Endo M, Nishizawa K, Murase K, Fujiwara H, Tanada S.: Comparison of patient doses in 256-slice CT and 16-slice CT scanners. Br. J. Radiol. 79(937), 56–61 (2006).
- Earls JP, Berman EL, Urban BA et al.: Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology 246(3), 742–753 (2008).
- Husmann L, Valenta I, Gaemperli O et al.: Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur. Heart J. 29(2), 191–197 (2008).
- Steigner ML, Otero HJ, Cai T et al.: Narrowing the phase window width in prospectively ECG-gated single heart beat 320-detector row coronary CT angiography. Int. J. Cardiovasc. Imaging 25(1), 85–90 (2009).
- Achenbach S, Ropers D, Kuettner A et al.: Contrast-enhanced coronary artery visualization by dual-source computed tomography – initial experience. Eur. J. Radiol. 57(3), 331–335 (2006).
- Hirohata A, Senoh K, Murakami M et al.: Diagnostic efficacy of 320-multislice cardiac computed tomography in patients with atrial fibrillation. Presented at: American College of Cardiology Annual Scientific Session. Orlando, FL, USA, 29–31 March 2009.
- Schuijf JD, Wijns W, Jukema JW et al.: Relationship between noninvasive coronary angiography with multislice computed tomography and myocardial perfusion imaging. J. Am. Coll. Cardiol. 48(12), 2508–2514 (2006).
- George RT, Arbab-Zadeh A, Miller JM et al.: Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging. A pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ. Cardiovasc Imaging 2, 174–182 (2009).