Review Article - Interventional Cardiology (2011) Volume 3, Issue 4
Novel stent and drug elution technologies
- Corresponding Author:
- Ian BA Menown
Craigavon Cardiac Centre, Southern Trust
Craigavon, Northern Ireland, BT63 5QQ, UK
Tel: +44 283 861 2902
Fax: +44 283 839 4493
E-mail: ian.menown@southerntrust.hscni.net
Abstract
Keywords
bioabsorbable polymer, drug-eluting balloon, drug-eluting stent, polymer-free, stent thrombosis
Over the last decade, the highly efficacious reduction of in-stent restenosis (ISR) by drugeluting stents (DES) [1,2], has revolutionized the practice of percutaneous coronary intervention (PCI), particularly in complex anatomical subsets. Central to the success of DES has been the use of a polymer coating to control the release profile of an antiprofilerative drug. However, the permanent polymers in first-generation sirolimus- and paclitaxel-eluting stent platforms used to control drug release may induce vascular hypersensitivity responses, which may increase risk of stent thrombosis [3,4]. Development of bioabsorbable polymer or polymer-free DES technology is based on the premise that removal of the polymer may lead to reduced stent thrombosis events, while aiming to retain similar control of antiproliferative drug delivery so that restenosis rates remain comparable with current generation DES.
Fully bioabsorbable DES platforms are also under active development.
Even with the high DES implantation rates seen in contemporary practice, restenosis can still occur, albeit much less frequently. Management of this phenomenon remains challenging, especially in the case of DES ISR, with rates of recurrent restenosis following target lesion revascularization (TLR) of between 15–20% [5–7]. To this end, one emerging strategy for treatment of ISR is the drug-eluting balloon (DEB), which has been awarded a class IIa indication for this purpose in recent European PCI guidelines. These devices are designed to enable drug delivery from the balloon surface to the vessel wall during the period of contact with the inflated balloon, while avoiding the need for a further layer of stent (±polymer). Paclitaxel, with its high lipophilicity enabling rapid uptake by the vessel wall, has been the most studied drug to date. DEB strategies are also currently being investigated in clinical scenarios where DES may have suboptimal long-term efficacy such as in small vessels or bifurcation lesions, and in situations where short duration of dual antiplatelet therapy is desirable due to increased bleeding risk.
In this article we provide a concise update on developments in bioabsorbable polymer stents, polymer-free stent platforms and emerging experience with DEB technology over the past 12 months including clinical trial data from EuroPCR 2010, Transcatheter Cardiovascular Therapeutics (TCT) 2010 and Cardiovascular Research Technologies (CRT) 2011.
Bioabsorbable polymer DES
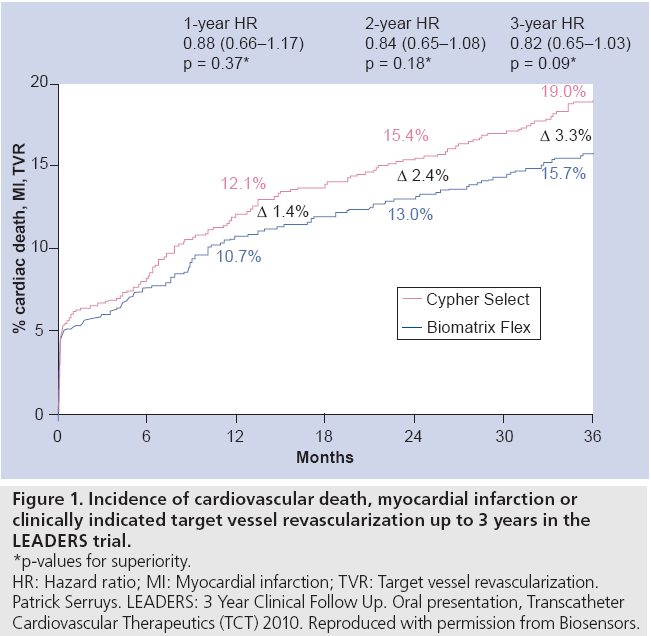
The Biomatrix Flex™ technology has been described previously [8] but in brief it comprises a 316L stainless steel stent to which a bioabsorbable polymer made up of a 50:50 matrix of polylactic acid and Biolimus A9™ (a semi-synthetic sirolimus analogue with ten times greater lipophilicity) is applied to its abluminal surface. The polylactic acid polymer is reported to be completely converted to lactic acid by 6 months and subsequently via the Krebs cycle to carbon dioxide and water between 6 and 9 months. Initial findings of the all comers LEADERS randomized study established noninferiority of the Biomatrix Flex to Cypher® Select™ sirolimuseluting stent and low rates of stent thrombosis. Three-year follow-up, recently presented at TCT2010 [9], suggested an emerging separation of Kaplan–Meier survival curves for the composite end point (cardiovascular death, myocardial infarction [MI] and clinically indicated target vessel revascularization [TVR]) with a strong trend for improved outcome with Biomatrix Flex (15.7 vs 19%; HR: 0.82; 95% CI: 0.65–1.03; p = 0.09) (Figure 1).
Figure 1: Incidence of cardiovascular death, myocardial infarction or
clinically indicated target vessel revascularization up to 3 years in the
LEADERS trial.
*p-values for superiority.
HR: Hazard ratio; MI: Myocardial infarction; TVR: Target vessel revascularization.
Patrick Serruys. LEADERS: 3 Year Clinical Follow Up. Oral presentation, Transcatheter
Cardiovascular Therapeutics (TCT) 2010. Reproduced with permission from Biosensors.
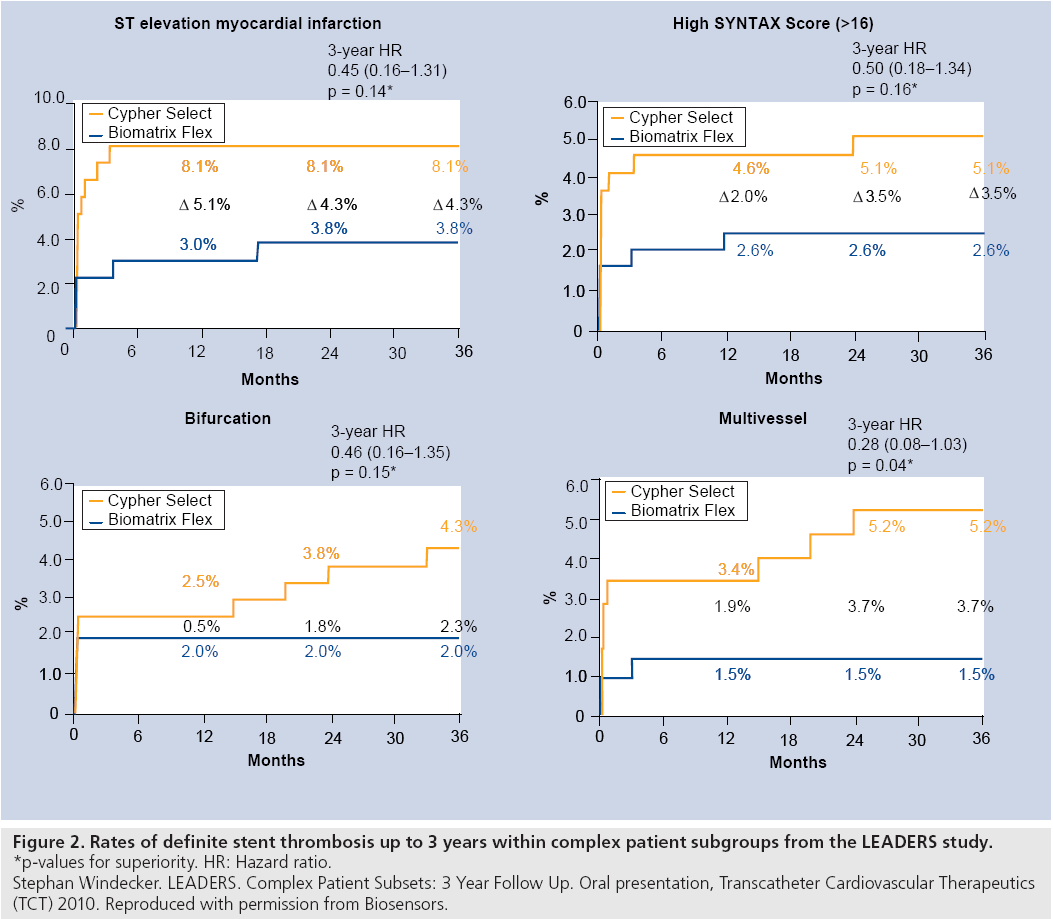
Patients with more complex anatomy showed improved clinical outcomes with Biomatrix Flex including less cardiac death (HR: 0.43; 95% CI: 0.21–0.89; p = 0.02). In addition, low rates of definite stent thrombosis remained with a trend to fewer events with Biomatrix Flex (2.2 vs 2.9%; HR: 0.78; 95% CI 0.43–1.43; p = 0.43). Indeed, there were no definite stent thrombosis events in the Biomatrix arm in year 3 of follow-up (Figure 2).
Figure 2: Rates of definite stent thrombosis up to 3 years within complex patient subgroups from the LEADERS study.
*p-values for superiority. HR: Hazard ratio.
Stephan Windecker. LEADERS. Complex Patient Subsets: 3 Year Follow Up. Oral presentation, Transcatheter Cardiovascular Therapeutics
(TCT) 2010. Reproduced with permission from Biosensors.
Also presented recently were 1-year results from the Nobori 2 Study [10]. This single-arm follow-up study to Nobori 1 (in which the Nobori™ stent was shown to be noninferior to the TAXUS Liberté™ paclitaxel-eluting permanent polymer stent with respect to late loss) evaluated the performance of the Nobori stent platform (stainless steel stent with an abluminal coating incorporating bioabsorbable polymer with Biolimus A9) in both simple and complex patient/anatomical subgroups. As expected, the primary composite end point of cardiac death, target vessel MI and TLR occurred less frequently in the ‘simple’ than complex subgroups. However, the Nobori stent also performed well in the ‘complex’ cohort with the primary end point being reached in only 4.4% of patients, driven primarily by a higher incidence of TLR (2.2% complex cohort vs 0.6% simple cohort). Performance was maintained in important subgroups including bifurcation (primary end point 4.5%), chronic occlusions (3.1%), diabetes (5.3%) and long lesions (4.9%). Importantly, definite and probable stent thrombosis at 1 year remained low in both complex and simple patients (0.6 vs 0.3%; p = ns).
The Nevo™ stent utilizes microwell technology in a cobalt chromium stent with a matrix of PLGA bioabsorbable polymer and sirolimus incorporated into the microwells, resulting in a similar drug release kinetic profile to the Cypher sirolimus-eluting stent [8]. Complete drug elution and polymer absorption occurs by 90 days. Unfortunately, difficulties have been reported with the Nevo delivery system and Cordis recently announced halting of further Nevo development. However, 12 month clinical results from the NEVO RES 1 study showed similar freedom from death/MI/TLR for Nevo and Taxus® Express™( 93.7 vs 89.4%; p = 0.107) [11]. No stent thrombosis occurred in the Nevo arm up to 12 months but one probable and one possible stent thrombosis occurred with Taxus Express between 0 and 6 months.
The Synergy™ stent, based on the same platinum chromium alloy as used in the Promus Element™ stent [12] and a similar, but thinnerstrutted, stent design, features a bioabsorbable PLGA polymer combined with everolimus applied to the abluminal stent surface. The polymer coating is reported to resorb in 3–6 months. The EVOLVE randomized, noninferiority trial has recently completed enrollment in 291 patients with results awaited (comparing the Synergy stent and standard or half doses of everolimus with the permanent polymer Promus Element stent). The primary clinical end point is target lesion failure (cardiac death, MI or TLR) at 30 days. Angiographic and intravascular ultrasound outcomes will be evaluated at 6 months.
Polymer-free DES
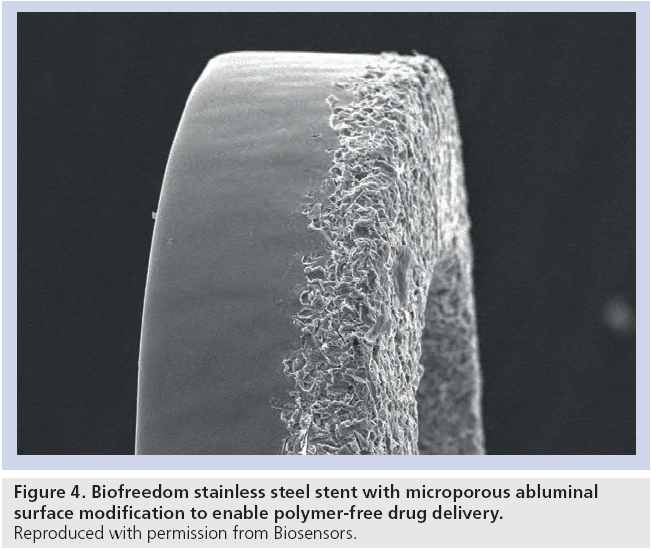
Following on from encouraging 6-month results, Grube reported 12 month outcomes for the novel Biofreedom™ polymer-free DES [13]. This stainless steel stent incorporates a microporous abluminal surface (Figure 3) to control drug release. The high lipophilicity of the Biolimus A9 drug helps maintain high levels of tissue concentrations up to 28 days postimplantation, with levels returning to near normal by day 90 (Figure 4). In this study, 107 patients were enrolled into the 12 month angiographic follow-up study randomized in a 1:1:1 ratio to Biofreedom high dose, Biofreedom low dose or TAXUS Liberté. The high-dose Biofreedom stent was noninferior to TAXUS Liberté for in-stent late loss (0.17 vs 0.35 mm; noninferiority p = 0.001). Although not powered to assess clinical outcomes, no significant differences were seen between the Biofreedom platforms and TAXUS Liberté including death, MI and TLR. As anticipated from the modest size and simple lesions, no stent thrombosis was seen in either arm of the study.
The favorable results of the ISAR-TEST 2 study, which demonstrated superiority of both a dual drug-eluting polymer-free stent (probucol and sirolimus) and the Cypher Select stent over the Endeavor® zotarolimus-eluting stent (ZES) with a primary end point of angiographic restenosis led to the ISAR-TEST 5 study investigating whether a dual drug (probucol–sirolimus) polymer- free stent was noninferior to the Endeavor Resolute DES for the composite clinical end point of TLR, target vessel MI and cardiac death [14]. In total, 3002 patients were recruited and randomized in a 2:1 ratio (dual DES: Resolute). A broad range of ‘real world’ patients were recruited resulting in almost one third of the cohort having diabetes, unstable presentation, multilesion PCI and bifurcation disease. Outcomes were identical at 12 months follow-up for the primary end point: dual DES 13.1% vs ZES 13.1%; RR: 1.01; 95% CI: 0.80–1.31; noninferiority p = 0.83. No difference in stent thrombosis was apparent between treatment arms: dual DES 1.1% vs ZES 1.2%; RR: 0.94; 95% CI: 0.45–1.84. There were also no differences across a range of higher risk subgroups including females, patients with diabetes or small vessel disease.
DEB development
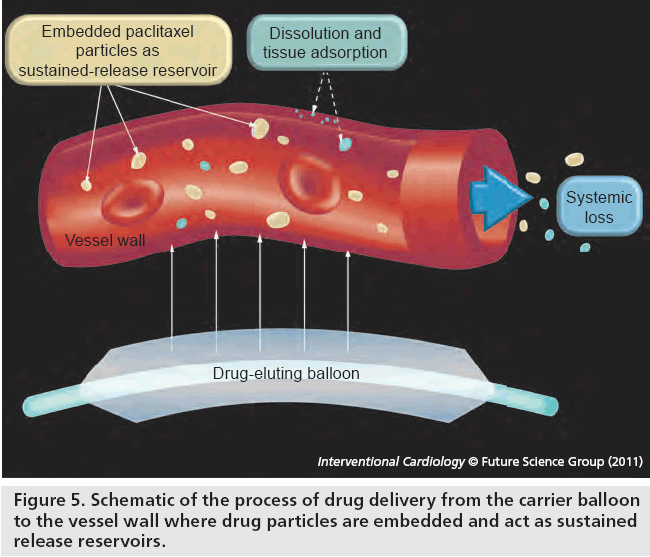
Drug-eluting balloons represent a novel method of drug delivery to the vessel wall without need for a polymer or stent (Figure 5). Paclitaxel is highly lipophilic and has been shown to reduce neointimal proliferation with only brief contact with vessel intima during paclitaxel-loaded contrast angiographic coronary injection [15,16]. An initial proof-of-concept evaluation of an early DEB platform (with paclitaxel 1.3 or 2.5 μg/mm2; plus acetone excipient) in combination with a baremetal stent (BMS) was superior to BMS alone in reducing restenosis in a swine model [17]. This study also highlighted the importance of an appropriate carrier excipient in DEB design. One arm of the study tested ethyl acetate as the excipient but this appeared ineffective at reducing restenosis. A suitable excipient must perform two functions; first, to ensure the majority of drug remains on the balloon during transit through the guide catheter and coronary artery and second, to allow rapid delivery of drug to the vessel intima within one 30–60-s balloon inflation. In this study, insertion of the acetone–paclitaxel DEB through the guide catheter and coronary artery and subsequent retraction without deployment resulted in only 6% loss of drug. Following deployment, more than 90% of drug was transferred to the vessel wall and bloodstream.
Figure 5: Schematic of the process of drug delivery from the carrier balloon to the vessel wall where drug particles are embedded and act as sustained release reservoirs.
DEB for the treatment of ISR
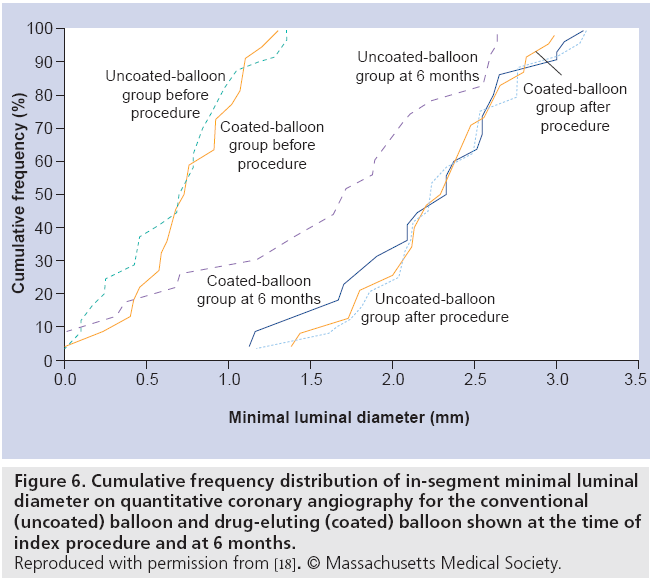
The randomized, double-blind, multicenter paclitaxel-coated balloon catheter for ISR (PACCOCATH ISR) I study was the first large-scale clinical evaluation of a DEB platform incorporating paclitaxel (3 μg/mm2) with iopromide excipient (an x-ray contrast media) to improve paclitaxel’s solubility [18]. In total, 52 patients who had clinical evidence of stable or unstable angina and a single ISR lesion were randomized to paclitaxel DEB angioplasty or conventional balloon angioplasty. The DEB strategy was superior for the primary end point of late lumen loss at 6 months (0.03 ± 0.48 mm vs 0.74 ± 0.86 mm; p = 0.002) (Figure 6). Only one of 22 DEB patients compared with ten of 23 conventional balloon patients had binary restenosis (5 vs 43%; p = 0.002). At 12 months, the rate of major adverse cardiac events was less in the DEB group (4 vs 31%; p = 0.02), mainly driven by reduced requirement for TLR.
Figure 6: Cumulative frequency distribution of in-segment minimal luminal diameter on quantitative coronary angiography for the conventional (uncoated) balloon and drug-eluting (coated) balloon shown at the time of index procedure and at 6 months. Reproduced with permission from [18]. © Massachusetts Medical Society.
The PACCOCATH ISR II study (which recruited a further 56 patients and pooled data with PACCOCATH ISR I) confirmed these positive results and also the continuing clinical safety and efficacy of the technology [19]. At 12 month follow-up, only two DEB patients compared with 20 conventional balloon patients required TLR (p = 0.001).
The paclitaxel-coated balloon catheter versus a paclitaxel-coated stent for the treatment of ISR (PEPCAD ISR II) study was a prospective, randomized, multicenter, two-arm Phase II pilot study conducted in Germany [20], which examined the safety and efficacy of the Sequent Please™ (B. Braun) DEB (based on PACCOCATH® technology [Bayer//Medrad]) compared with the TAXUS Liberté DES for treatment of ISR in native coronary arteries (n = 131 patients, reference diameter 2.5 mm, 3.5 mm; lesion length: ≤22 mm). At 6‑month angiographic follow up (in 89%), the DEB compared with DES strategy was associated with a significant reduction in the primary end point of in-segment late lumen loss (0.17 vs 0.38 mm; p = 0.03) and a reduction in binary restenosis (7 vs 20%; p = 0.06).
At EuroPCR 2010, in patients with sirolimus- eluting ISR, outcomes of a repeat DES strategy (paclitaxel or simolimus) during the period 2004–2009 (324 lesions) were compared with outcomes of a DEB strategy (Sequent Please) between 2008 and 2009 (88 lesions) [21]. Although nonrandomized, numerically lower binary restenosis was noted with DEB versus DES (14.3 vs 21.9%) and a significantly lower TLR (8.2 vs 19.4%; p = 0.042). These results are encouraging, especially considering the significantly lower acute gain with DEB (1.04 mm ± 0.47 vs 1.75 mm ± 0.63; p < 0.0001). It should be noted that 412 lesions were treated and included in an independent sample analysis despite representing only 369 individual patients.
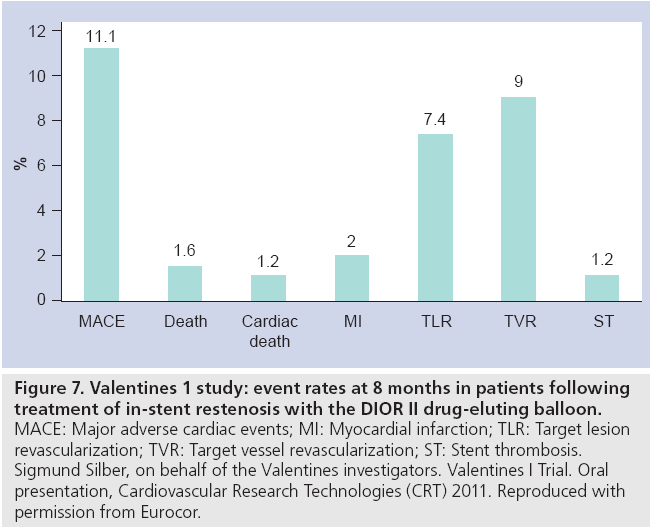
Most recently, the Valentines I registry [22] investigated the DIOR® II DEB (Eurocor; paclitaxel 3 μg/mm2 combined 1:1 with shellac excipient) in 250 patients with relatively complex BMS or DES ISR (32% diabetic, over half diffuse/proliferative/occlusive pattern, with a mean balloon diameter of 3 mm and length of 24 mm). Despite the complex patient cohort, at 8 months the observed ‘re-restenosis’ was encouragingly low with only single digit (7%) TLR (5.9% for BMS ISR and 9.8% for DES ISR) (Figure 7).
Figure 7: Valentines 1 study: event rates at 8 months in patients following treatment of in-stent restenosis with the DIOR II drug-eluting balloon. MACE: Major adverse cardiac events; MI: Myocardial infarction; TLR: Target lesion revascularization; TVR: Target vessel revascularization; ST: Stent thrombosis. Sigmund Silber, on behalf of the Valentines investigators. Valentines I Trial. Oral presentation, Cardiovascular Research Technologies (CRT) 2011. Reproduced with permission from Eurocor.
DEB for the treatment of de novo coronary artery stenosis
Given the encouraging results for ISR, the large PEPCAD III study attempted to assess DEB versus DES in the setting of native coronary disease [23], randomizing 637 patients with stable/unstable angina or documented ischemia and a single de novo lesion to a DEB (Sequent Please) plus BMS (Coroflex™; n = 312) or DES (Cypher Select sirolimus-eluting stent; n = 325). The use of DEB plus BMS represents a novel strategy for eliminating polymer exposure associated with conventional DES PCI. At 9 months, the DEB plus BMS strategy failed to meet criteria for noninferiority versus DES with respect to the primary end point of angiographic late loss (in-stent: 0.41 vs 0.16 mm; p < 0.01; in-segment: 0.20 vs 0.11 mm; p < 0.06); MI occurred in 4.6 versus 0.3% (p < 0.001) and definite/probable stent thrombosis in 2.0 versus 0.3% (p < 0.05). While the numbers of clinical events are too small to draw significant conclusions, the higher late loss with the DEB plus BMS strategy is disappointing given the promising results from PEDCAD II in ISR.
In contrast to PEPCAD III, the prospective, randomized trial evaluating a paclitaxel-eluting balloon in patients treated with endothelial progenitor cell capturing stents for de novo coronary artery disease (PERfECT STENT trial) was presented at TCT2010 [24]. This study randomized 120 patients with noncomplex de novo coronary artery disease to either the Genous™ EPC capturing stent alone or the Genous stent plus subsequent DEB (Sequent Please). Baseline characteristics including reference vessel diameter and lesion length were similar between the two groups. At 6 months follow-up (in >95% of patients), the combination of Genous plus DEB versus Genous alone reduced the primary end point of angiographic in-stent late loss (0.34 vs 0.88 mm; p < 0.001) and TLR (4.8 vs 15.5%; p = 0.05).
Thus, the role of DEB in medium to larger vessels and appropriate level of lesion complexity still remains to be established. In comparison with ISR PCI (which typically is a quiescent disease state with mature neo-intima and involves relatively little vessel wall injury), de novo PCI disrupts a plaque often with the presence of necrotic tissue and involves a higher degree of vessel wall injury. It may be that just as the pathobiology of the stenosing process differs in ISR compared with de novo disease, the optimal treatment modality might also differ.
The Valentines II study, which has just completed enrollment, has evaluated the role of DEB only (without BMS) in PEPCAD III type lesions and will provide further insights into the efficacy of DEB treatment for de novo disease.
DEB for the treatment of small vessel coronary artery stenosis
Small vessel lesions are a potentially attractive target for DEB given the potentially suboptimal performance of DES in this setting. The PICCOLETTO trial (paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels) randomized 60 patients with de novo stenosis (vessel <2.75 mm diameter) to either TAXUS Liberté DES or to DEB (firstgeneration DIOR without shellac excipient) [25]. Interpretation was confounded when, despite the small sample size, the study was stopped early after TAXUS Liberté versus DEB was noted to have a lower primary end point of 6-month angiographic percentage diameter stenosis (24.3 vs 43.6%; p = 0.029), lower binary restenosis (10.3 vs 32.1%; p = 0.043) and trend to lower TLR (10.3 vs 32.1%; p = 0.15). Of importance, it should be noted that the DEB technique was unusual (only 25% received a standard balloon predilatation before DEB thus compromising homogenous vessel wall drug delivery), DEB inflation pressure was lower (7.71 vs 13.41 atmospheres; p = 0.02), end procedure minimum lumen diameter was lower (2.47 vs 2.63 mm; p = 0.009), yet bailout BMS was required in over one third of the study population.
By contrast, the Spanish multicenter DIOR registry small vessel substudy [26], with mandatory optimal predilatation with a conventional balloon prior to DEB, described markedly more favorable outcomes with mean late loss of 0.27 mm, binary restenosis of 13.3% and TLR rate of only 2.2%. Despite use of optimal predilatation, only 7.9% of patients required subsequent stents for type B/greater coronary dissection. Small vessel DEB studies are thus continuing.
DEB for the treatment of bifurcation coronary artery stenosis
Bifurcation coronary artery stenosis management represents another challenging lesion subset in which the optimal DES-based strategy has yet to be established. Accordingly, the DEBIUT trial, a prospective, randomized, multicenter study in 120 patients with bifurcation coronary disease compared: DEB (main and side branch) plus BMS (main branch) versus conventional angioplasty (main and side branch) plus BMS (main branch) versus conventional angioplasty (main and side branch) plus DES (main branch) [27]. Balloon dilatation was performed with the kissing balloon technique. At 6 months, TLR was 15% in both DEB plus BMS and conventional angioplasty plus DES arms but 27% in the conventional angioplasty plus BMS arm. The DEB plus BMS strategy allowed only 3 months dual antiplatelet therapy with 0% occurrence of stent thrombosis.
DEB for the management of chronic total occlusions
To date, only one small nonrandomized study has investigated the potential safety and efficacy of DEB in the setting of chronic total occlusion treatment. Unfortunately, the BMS plus DEB (Coroflex plus Sequent Please) strategy was supboptimal compared with a historical TAXUS Liberté DES cohort in this challenging lesion subset [28]. The BMS plus DEB strategy had numerically higher 6 month in-stent late loss (0.64 vs 0.43 mm; p = 0.14) and binary restenosis (27.7 vs 14.6%; p = 0.12), although clinical safety end points were similar. Despite this apparent set back the hypothesis examined in this study warrants further prospective investigation to determine if there is an alternative to DES in this setting.
New DEBs
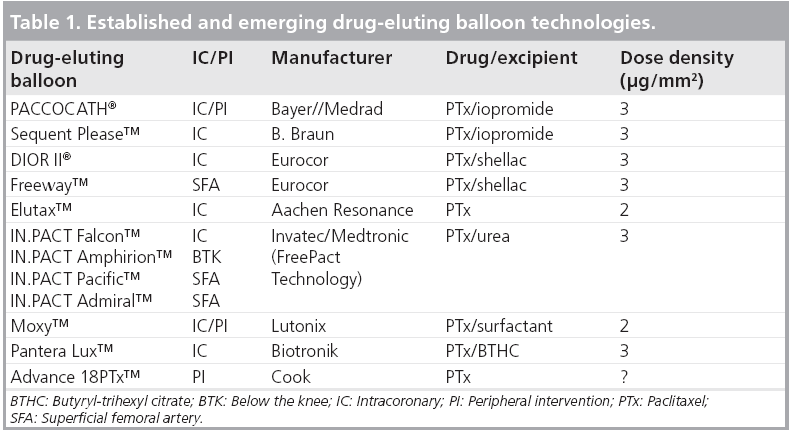
Multiple DEBs are now under development and clinical testing (Table 1) including the Invatec Falcon™ DEB (with paclitaxel 3 μg/mm2 and urea excipient) [29], the Lutonix Moxy™ DEB (paclitaxel 2 μg/mm2 plus undisclosed, nonpolymeric excipient) [30] and the Biotronik Pantera Lux™ DEB (paclitaxel 3 μg/mm2 plus butyryl-tri-hexyl citrate excipient) [31]. Preliminary results (n = 45) from the PEPPER study (paclitaxel-releasing balloon in patients presenting with ISR) [31] with the Pantera Lux DEB reported a 6-month angiographic in-stent late lumen loss of only 0.07 mm and a clinically driven TLR rate of only 5.2%, which compares well with other more widely investigated DEB platforms. The results of the ongoing 600 patient DELUX registry are thus keenly awaited.
Conclusion
This article has described the latest technology developments in bioabsorbable polymer and polymer-free DES technology which offer the potential to improve safety outcomes of patients with stable and unstable coronary disease. Impressive medium-term follow-up results are now being seen with the Biomatrix Flex bioabsorbable polymer stent and the dual drug polymer-free Yukon® stent in large-scale randomized trials. It is hoped that, as planned follow-up continues in these studies, the value of minimization of polymer exposure will be realized with reduction in very late stent thrombosis while retaining low levels of late loss.
The increasing interest in DEB technology across a variety of complex coronary lesions is notable. In particular, late loss with DEB when compared with DES is encouraging, notwithstanding the theoretical attractiveness of avoiding an additional stent layer and risk of side branch occlusion. Further research is needed to confirm the efficacy of DEB treatment for ISR and to clarify its role in de novo lesions including small vessel PCI and bifurcation treatment.
Future perspective
Bioabsorbable polymers or polymer-free technology are likely to become the preferred strategy for drug delivery from stent platforms over the next 2–3 years particularly in patients where short duration dual antiplatelet therapy is desirable or in patients with a previous history of stent thrombosis. Fully bioabsorbable DES platforms are also under active development and, provided current limitations during acute deployment and medium-term scaffolding can be overcome, may be preferable to permanent alloy stents in many clinical scenarios. Stent thrombosis with modern generation permanent polymer stents is now relatively infrequent. Thus, future trials testing the hypothesis that bioabsorbable polymers or polymer-free technology might reduce stent thrombosis appear best undertaken by all-comers patient inclusion and/or large registries rather than the conventional approach of a randomized trial in selected ‘on-label’ (typically low risk) patients. Given the results of PEPCAD ISR II and similar trials, DEB treatment may emerge as a preferred first-line treatment for the management of DES restenosis since it avoids multiple stent layers (reducing risk of side branch occlusion) and double polymer exposure. Paclitaxel is the most widely studied antiproliferative drug utilized in current DEB technology. Other antiproliferative drugs, ideally with high lipophilicity, and development of novel excipients to optimize and prolong local tissue drug exposure, are being tested. The wider value of standalone DEB therapy in de novo disease, small (2.0 mm) vessels, bifurcations and chronic total occlusions, and the use of DEB for drug delivery prior to BMS deployment remains to be established.
Financial and competing interests disclosure
Ian BA Menown has received speaker’s honoraria, conference attendance sponsorship and/or research grants from Boston Scientific, Biosensors, Biotronik, Eurocor and Medtronic. JA Shand has received conference attendance sponsorship from Boston Scientific and Biosensors. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Over the last decade, reduction of in-stent restenosis (ISR) by drug-eluting stents (DES) has revolutionized the practice of percutaneous coronary intervention (PCI), particularly in complex anatomical subsets.
▪ First-generation permanent polymers used to control drug release may induce vascular hypersensitivity responses potentially increasing the risk of stent thrombosis, thus bioabsorbable polymer and polymer-free strategies have been developed to enable similar early antiproliferative drug delivery, but with the aim to reduce the risk of late stent thrombosis.
▪ 3-year follow-up of the LEADERS trial (Biomatrix Flex stent with bioabsorbable polymer vs Cypher Select with permanent polymer) showed a trend to emerging separation of event curves for the composite clinical outcome, particularly in more complex clinical anatomy and, although numbers were small in both arms, a possible reduction in very late stent thrombosis.
▪ The ISAR-TEST 5 study demonstrated that the dual drug (probucol–sirolimus) polymer-free stent was noninferior to the Endeavor Resolute permanent polymer DES for the composite clinical end point at 1 year.
▪ The Biofreedom stent eluting biolimus A9 via microporous surface modification technology was noninferior to TAXUS Liberté for in-stent late loss at 1 year (0.17 vs 0.35 mm; noninferiority p = 0.001).
▪ Drug-eluting balloons (DEBs) represent a novel approach to polymer-free drug delivery. Current devices utilize paclitaxel given its high lipophilicity facilitating local tissue delivery/retention.
▪ DEB efficacy has been most studied in the setting of in-stent restenosis where avoiding a further layer of stent is attractive to reduce the risk of side branch occlusion and has been awarded a class IIa indication for this purpose in recent European PCI guidelines.
▪ PEPCAD ISR II found the Sequent Please™ DEB compared with the TAXUS Liberté DES for the treatment of ISR to be associated with a significant reduction in the primary end point of in-segment late lumen loss and a reduction in binary restenosis at 6-month angiographic follow-up.
▪ Encouraging clinical data are emerging for new DEBs including Dior II and Pantera Lux. The role of DEB therapy in other clinical indications such as de novo disease, small (2.0 mm) vessels, bifurcations, chronic total occlusions or prior to bare-metal stenting, remains to be established.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 346(23), 1773 (2002).
- Colombo A. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation 108(7), 788–794 (2003).
- Finn AV, Kolodgie FD, Harnek J et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation 112(2), 270–278 (2005).
- Virmani R. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation 109(6), 701–705 (2004).
- Byrne R, Mehilli J, Tiroch K, Kastrati A. Prospective, randomised trial of paclitaxel versus sirolimus eluting stents for the treatment of coronary restenosis in sirolimus eluting stents. Presented at: Transcatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 21–25 September 2009.
- Cosgrave J, Melzi G, Corbett S et al. Repeated drug-eluting stent implantation for drug-eluting stent restenosis: the same or a different stent. Am. Heart J. 153(3), 354–359 (2007).
- Garg S, Smith K, Torguson R et al. Treatment of drug-eluting stent restenosis with the same versus different drug-eluting stent. Catheter. Cardiovasc. Interv. 70(1), 9–14 (2007).
- Shand JA, Menown IB. Drug-eluting stents: the next generation. Int. Cardiol. 2(3), 341–350 (2010).
- Serruys P, Buszman P, Linke A, Windecker S. LEADERS: a prospective randomised non-inferiority trial comparing biolimus eluting stnet with biodegradable polymer versus sirolimus eluting stent with durable polymer. 3 year clinical follow up. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Chevalier B. Nobori DES – clinical update. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Mauri L. Nevo Res-Elution 1 Trial: 12 month results and subset analysis. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Menown IBA, Noad R, Garcia EJ, Meredith I. The platinum chromium element stent platform: from alloy, to design, to clinical practice. Adv. Ther. 27(3), 129–141 (2010).
- Grube E. Biofreedom: a prospective randomized trial of polymer free Biolimus A9 eluting stents and paclitaxel eluting stents in patients with coronary artery disease. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Mehilli J. ISAR TEST 5: a prospective randomised trial of polymer free sirolimusprobucol eluting stents compared to zotarolimus eluting stents in patients with coronary artery disease. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Scheller B, Speck U, Schmitt A, Böhm M, Nickenig G. Addition of paclitaxel to contrast media prevents restenosis after coronary stent implantation. J. Am. Coll. Cardiol. 42(8), 1415–1420 (2003).
- Scheller B, Speck U, Romeike B et al. Contrast media as carriers for local drug delivery. Eur. Heart J. 24(15), 1462–1467 (2003).
- Scheller B, Speck U, Abramjuk C, Bernhardt U, Bohm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 110(7), 810–814 (2004).
- Scheller B, Hehrlein C, Bocksch W et al. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N. Engl. J. Med. 355(20), 2113–2124 (2006).
- Scheller B, Hehrlein C, Bocksch W et al. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin. Res. Cardiol. 97(10), 773–781 (2008).
- Unverdorben M, Vallbracht C, Cremers B et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation 119(23), 2986–2994 (2009).
- Habara S, Yamamoto H, Hosogi S. Paclitaxel eluting balloon catheter versus repeat drug eluting stents for the treatment of Sirolimus eluting stent restenosis. Presented at: EuroPCR. Paris, France, 17–20 May 2010.
- Silber S. The Valentines Trial. Presented at: Cardiovascular Research Technologies. Washington DC, DC, USA, 27 Febuary–1 March 2011.
- Hamm C. PEPCAD III: a randomised trial comparing a pacitaxel-coated balloon-stent system. Presented at: American Heart Association Scientific Sessions. Orlando, FL, USA 14–18 November 2009.
- Wohrle J. A prospective randomised trial evaluating a paclitaxel eluting balloon in patients treated with endothelial progenitor cell capturing stents for de novo coronary artery disease. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Cortese B, Micheli A, Picchi A et al. Paclitaxelcoated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO Study. Heart 96(16), 1291–1296 (2010).
- Serra A, Vaquerizo B, Miranda F. Dior eluting balloon: The Spanish DIOR registry: Focus on small vessels. Presented at: EuroPCR. Paris, France, 17–20 May 2010.
- Stella P. Drug eluting balloons in coronary bifurcations: the drug eluting balloon in bifurcation trial. Presented at: EuroPCR. Paris, France, 17–20 May 2010.
- Werner G. PEPCAD-CTO: A prospective non-randomised study of paclitaxel eluting balloons after BMSplacement in successfully recanalised chronic total occlusions. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Scheller B. The Invatec IN-PACT Falcon paclitaxel DEB: device description and clinical studies. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Mauri L. The MOXY drug-coated PTCA balloon catheter for the treatment of in-stent restenosis within bare metal stents. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
- Hehrlein C. The Pantera Lux paclitaxel DEB – device description and clinical studies. Presented at: Transcatheter Cardiovascular Therapeutics. Washington DC, DC, USA, 21–25 September 2010.
▪▪ Landmark randomized controlled trial demonstrating the noninferiority of a bioabsorbable polymer drug-eluting stent when compared to a first-generation sirolimus-eluting drug-eluting stent.
▪ Animal model demonstrating the superiority of the drug-eluting balloon (DEB) over bare-metal stenting for reducing restenosis.
▪▪ First-in-man investigation highlighting the efficacy of local drug delivery via balloons to treat in-stent restenosis (ISR).
▪ Of note for the high rate of bailout stenting in the DEB arm, a finding not seen in DEB randomized controlled trial in ISR, highlighting the difference in pathological substrate between ISR and de novo disease.