Review Article - Neuropsychiatry (2019) Volume 9, Issue 3
Nutritional Epidemiology Research in the Field of Autism Spectrum Disorders A Review
Tasnime Akbaraly1,2,3,†, Stephanie MIOT2,4 and Amaria Baghdadli2,4
1Inserm U 1198, Montpellier F-34000, University Montpellier, Montpellier, F-34000, EPHE, Paris, France
2Autism Resources Centre, University Hospital of Montpellier, CHRU de Montpellier, France
3Department of Epidemiology and Public Health, London, University College London, London, United Kingdom
4Centre de Recherche en Épidémiologie et Santé des Populations, U1178, INSERM, Paris, France.
- *Corresponding Authors:
- Tasnime Akbaraly
Centre Ressources Autisme
CHU Montpellier, 39 Avenue Charles Flahaut, 34295 Montpellier cedex 05, France
Abstract
Autism Spectrum Disorders (ASD) is a heterogeneous condition with a complex and unresolved epi-genetic etiology in which the contribution of maternal diet and children’s feeding problems raise increasing interest. Nutritional epidemiology research applied to ASD offers the perspective of a better understanding of its etiology and the possibility of implementing prevention strategies.
Keywords
Autism Spectrum Disorders, Nutritional Epidemiology, dietary pattern, maternal diet, dietary behaviour disorders,
Introduction
Autism Spectrum Disorder (ASD) is defined as a neurodevelopmental disorder, characterized by the association of abnormalities in social interaction, in communication and restricted or repetitive thought and behavior patterns [1]. This particularly severe and long-lasting condition affects 1 % of the population worldwide, with a 30% prevalence increase over the last decade [2]. ASD not only places a severe emotional strain on families but is an economic burden as well. The absence of diagnostic biomarkers, its heterogeneous clinical manifestations and the high rates of comorbidity including intellectual disabilities, psychiatric and neurological disorders make identification of etiological risk factors considerably harder. To date, ASD is a highly heritable condition but current genetic studies explain only a small proportion of this heritability and generally involve multiple genes, each with a small effect. It has actually been estimated that the heritability of ASD is between 50% and 80% [3,4] leaving a substantial proportion of contribution to the non-heritable risk factors of ASD risk [5]. In the past years, possible gene environment interactions have been hypothesized to play a role in ASD through epigenetic underpinning mechanisms [6]. Epigenetics modulates gene expression by histone acetylation, DNA methylation, chromatin remodelling or micro RNAs mediated inhibition, without affecting DNA sequences [7], and is influenced by environmental factors. However its exact underpinning mechanisms in ASD pathophysiology are not identified so far [8]. All this, has enhanced an increased interest to assess the extent to which environmental factors and life style habits are associated with increased risk of ASD, its clinical phenotype and the severity of the core symptoms of ASD [9]. Amongst these non-genetic factors, we believe it of importance to further explore the role of nutrition and diet in ASD. The potential importance of prenatal diet in the aetiopathology of neurodevelopmental disorders including ASD and the high prevalence in children with ASD of dietary behaviour disorders (including food selectivity and food intolerance) linked to autistic traits lead to consider nutrition and diet as cornerstones in ASD etiology [10]. In addition, the daily exposure of dietary intakes combined to the fact that diet is a highly modifiable factoroffering therefore the possibility to implement primary and secondary prevention strategies with no or limited side effects- reinforce the urgency to understand how and when, which dietary factors impacts ASD risk and ASD clinical trajectories. However the multi-facetted aspect of diet makes it a complex exposure in relation to ASD outcomes, ranging from understanding the metabolic and biological role of nutrients in processes leading to ASD development, to the understanding of the social, cultural and clinical underpinnings of dietary behaviour and their association with ASD trajectories. Here we propose a short overview of these aspects and, based on the study-based knowledge, some suggestions for future research directions of nutritional epidemiology in the field of ASD.
Prenatal maternal dietary exposure and risk of ASD
Maternal diet is essential for fetal neurodevelopment [11-13]. Studies carried out both in animal models and humans showed associations between maternal nutrient deprivation and the development of various neuropsychiatric disorders in offspring including Attention deficit and Hyperactivity Disorder [14], schizophrenia [15], as well as anxiety and depression [16]. Nutritional deficiencies are particularly common during pregnancy due to increased metabolic demands imposed by a growing placenta, fetus and maternal tissues [17] and have been shown to influence brain development in terms of structure and function [18]. Given that ASD results from early brain development alterations and aberrant neural connectivity [19], the possibility that maternal nutrition influences ASD risk is therefore biologically possible.
Epidemiological studies assessing the association between prenatal diet and risk of ASD in offspring examined, whether deficiencies or excess in specific nutrients/foods were associated with an increased risk of ASD in offspring using mainly observational and case-control studies. Table 1 summarizes hypothesis and potential mechanisms underlying the association between these specific nutrients and ASD.
| Nutrients or foods whose deficiency has been hypothesized to increase the risk of ASD | |
|---|---|
| Main underlying mechanisms | |
| Folic acid | A maternal deficiency of methyl donors - such as folates and other nutrients involved in homocysteine - on DNA hypomethylation in the brains can induce a modification in gene expression controlling the fetal brain development and sustains the plausible importance of prenatal folate status on the risk of developing ASD [20,21]. |
| Vitamin D | Vitamin D’s properties to reduce neuroglial activation and neuroinflammation (by its role in up-regulating production of anti-oxidant) to contribute to DNA repair genes, and to induce T regulatory cells may have a role in reducing autoimmune conditions [22]. All of this process could therefore contribute to link vitamin D to ASD risk [23]. |
| PUFA, Omega 3/Omega 6, proxies of PUFA: fish oil, fish intakes and seafood | PUFAs (Omega 3 and 6) play an important role in various neurodevelopmental processes. The links associating PUFAs with ASD[24] involve the myelination process [25], synapse formation [26], BDNF expression levels [27] and the GABAergic transmission [28]. |
| Iron deficiencies | Iron is crucial to early neurodevelopment. In the brain, iron contributes to neurotransmitter production, myelination, and immune function dysregulation- three pathways involved in ASD [29]. |
| Nutrients or foods for whose high intakes has been hypothesized to increase the risk of ASD | |
| Main underlying mechanisms | |
| Foods containing methanol (aspartame and processed fruit juice | Congenital malformation and behavioral abnormalities were observed in children of women exposed to methanol [30] According to the Centers for Disease Control and Prevention “methanol may cause birth defects of the central nervous system in humans” [31], however the exact mechanism by which methanol induced neurological damage and might be related to ASD development remains unclear [31]. |
| Fat products (high fat diet) | Maternal obesity and high fat diet (HFD) are hypothesized to impact neural development and the regulation of offspring behavior [16]. High fat consumption during pregnancy has been associated with activation of many of the same inflammatory cytokines that are found to be elevated during gestation in mothers of children with ASD [10,16,32,33]. Maternal HFD consumption might also impact offspring neural development indirectly by modifying maternal behavior toward the infant, which has also been shown to induce changes in neural pathways critical in regulating behavior (though serotonergic [34], dopaminergic [35], and melanocortinergic pathways [36]). |
Table 1: Summary of nutrients/foods for which observational studies assessed association between their level/intake during gestational period and the risk of ASD in offspring.
A majority of studies investigated the potential etiological role of folate status in the development of ASD but the findings have been mixed [5,20,37-39]. The heterogeneity in methods estimating the levels of folates (retrospective self-report data versus blood measure, lack of information on duration, dose or exposition windows, background nutritional contexts of the countries), the quality and precision of ASD diagnosis as well as other methods limitations including study design, accounting for other nutrients involved in homocysteine pathway, the adjustment for potential confounders and presence of moderators such as carrying specific gene variants might contribute to explain the lack of consistent evidence linking prenatal folate to ASD risk. Other lines of studies examined whether prenatal PUFA were associated with ASD risk. [24,39,40] Some focused on PUFA Ω3 and PUFA Ω6 [40] others on the ratio of Ω3 on Ω6 [24], while two other studies considered proxy measures of PUFA by examining intakes of fish oil supplement or fish /seafood intakes [39,40]. Here again, the mixed findings reported might be attributable to the heterogeneity of the exposures. Despite growing evidence linking ASD with gestational iron [5,29] or vitamin D deficiencies [41,42], maternal diet with excess of high fat [43], and food rich in methanol (mainly aspartame and processed fruit juice) [31] and xenobiotics dietary exposition [44], firm conclusions on the association between levels in these nutritional compounds during pregnancy in relation to ASD risk cannot be formulated given the too small number of observational studies for each compound, and, therefore requires further investigation.
Beyond the methodological limitations mentioned above, further explanations can be proposed as they may have contributed to inconsistencies in the evidence of a role of prenatal nutrients on ASD. First, although a potential beneficial effect of some nutrients exposure during pregnancy on the ASD development may exist, the effect of single nutrients may be too small to be detected [45]. Indeed, as people are not eating individual nutrients or individual foods, but meals which consist of complex combinations of nutrients which interact with one another [45], it appears that focusing on individual nutrients or food may provide an incomplete understanding of the relationship between diet and multi-etiological diseases such as ASD.
Accordingly, based on hypothesis that epigenetics mechanisms in intrauterine environment are associated with offspring health status, a recent systematic review of randomized case control studies showed that supplementing maternal diet with micronutrients does not affect the DNA methylation patterns in neonates [46]. However this study highlights possible strong interactions of maternal diet supplementation with body mass index -that partly reflects overall diet quality and quantity-and with smoking habits -that have been showed to be associated with dietary behaviours.
To conclude, more emphasis needs to be given to the influence of overall diet assessed through dietary patterns on ASD as it offers the possibility to assess the cumulative and synergistic effects of nutrients. While this overall diet approach has been implemented for more than a decade in the field of depression, contributing to the evidence of the importance of nutrition and diet in depression prevention [47], it is urgent to export it in the field of ASD.
Feeding disorders, putative physiopathological pathways, and their clinical implications in ASD children
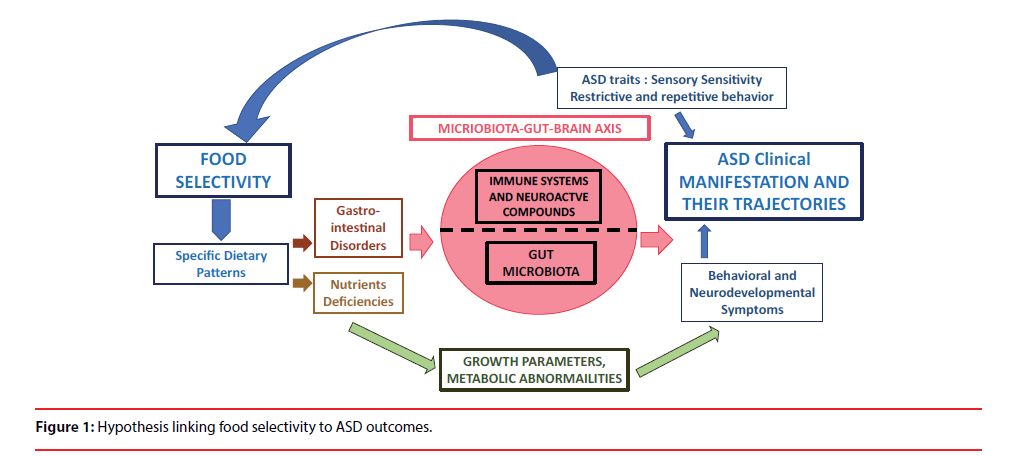
Feeding disorders are frequently reported in children with ASD. Even if its prevalence estimation varies highly across studies (from 13 to 89 %) [48, 49], a fivefold increase of the feeding disorders has been reported in children with ASD compared to those without [50]. Food selectivity is the most frequent feeding disorder [51] and encompasses all form of food refusal such as having a restricted repertoire of foods or a frequent intake of a single type of food defined as a function of their nutritional components or their sensory characteristics [52]. In ASD, food selectivity often involves (i) strong preferences for carbohydrates, snacks, and/or processed foods while rejecting fruits and vegetables [53,54]; and (ii) particular attention to texture and taste [55]. While sensory sensitivity [48,56,57] as well as repetitive and ritualistic behaviours [58]–two common ASD traits-have been proposed to explain the food selectivity in ASD children [48,56,57], the extent to which food selectivity influenced ASD clinical symptoms, their severity and their long-term trajectories remains to be further examined [55]. Despite the lack of well-phenotype prospective cohorts allowing investigating the complex diet- ASD outcomes associations, several mechanisticbased arguments support the importance of food selectivity on ASD clinical symptoms. Some involved mitochondrial dysfunctions whose higher prevalence has been described in ASD population compared to general population [59,60] but a majority involved the micro biome gut brain axis [61,62] as illustrated in Figure 1.
Indeed, several lines of studies suggested that food selectivity has been described to contribute to gastrointestinal disorders[50]-a prominent symptoms in ASD children [63,64], whose occurrence has been correlated to ASD severity [64]. While gastrointestinal tract regulates the homeostasis of its gut microbiota [10], a strong association between -gut microbiota imbalancenamed intestinal dysbiosis - and gastrointestinal disorders has been evidence on one side, and, on the other side, this intestinal dysbiosis has been described as a “fundamental mechanism linking ASD to the gut” [61,65]. Indeed it allows metabolites product by certain microbiota to cross the gut barrier leading to gut inflammation and to affect the neurodevelopment and brain function though neuroinflammation process [66]. Regarding ASD specifically, it has been shown that neuroactive compounds produced by some microbiota can (i) influence neurotransmitters regulation and oxytocin expression level [67,68] (ii) activate enteric neuron and affect brain function via the vagus nerve with a bidirectional dimension and (iii) activate the gut immune cells leading to a release of pro-inflammatory cytokines involved in ASD [61,69]. While intestinal dysbiosis has been evidenced by many studies in ASD children [61], gastro-intestinal permeability and inflammation is also suspected to be involved in ASD [70]. Children diet can influence the intestinal dysbiosis, in particular intakes of carbohydrates whose digestion is disrupted in ASD [71]. In addition microbiota is partially inherited from the mother [72]. At birth, gut microbiota is very poor. Its composition and diversity increase will depend on the birth delivery mode (vaginal birth or Caesarean section) [72], the infant term at birth [73], and also the infant alimentation mode (breast or artificial milk) [74]. It is usually admitted that microbiota is established at about 3 old.
However, studies assessing the link between food selectivity, gastrointestinal disorders, children intestinal dysbiosis, and their related effect on intestinal permeability and intestinal inflammation and maternal factors influencing post-natal microbiota composition, has never been explored in an observational framework, neither in regards to the clinical features and developmental trajectories of ASD children.
Beyond the fact that food selectivity can be a significant stress factor for families with a negative impact on quality of life [55], food selectivity and the associated dietary patterns lead to higher risks of nutritional deficiencies (including calcium, protein, vitamin D, vitamin A and vitamin B12 in ASD children[50,51,75,76] placing these children at risk for growth (bone development) [77] metabolic and neuro-developmental disorders [55] which might have deleterious impact on ASD symptoms trajectories.
This exciting field of research linking diet and nutrition to ASD has led parents and pediatricians to settle dietary interventions based on food restrictions (such gluten free casein free diet, ketogenic diet) as well as supplementation of nutrients and pro-/prebiotics targeting the microbiome-gut brain axis. Despite the little evidence supporting the effectiveness of most of these intervention [78], their use are increasingly widespread. However some authors have called attention to the possibility that some of these intervention may actually increase some nutrient deficiencies (such as calcium in dairy restricted diet) or mask an even greater risk of compromised dietary status (after a vitamin supplementation) [50].
Given the complex and multi-etiological nature of ASD, and its highly heterogeneous phenotype, a better understanding is needed on the long-term effect of dietary manipulation on nutritional and metabolic status on one hand, and, on the second hand, on biological processes and ASD outcomes trajectories.
Conclusion
In conclusion, to face the challenge of primary and secondary prevention of ASD based on intervention on diet, more knowledge is needed on maternal nutrition during pregnancy and ASD development in offspring and the impact of food disorders in children with ASD on their clinical trajectories. The accumulating evidence of the involvement of the microbiome- gutbrain axis in ASD etiology, combined with the fact that 50% of the microbial changes can be attributed to diet, strongly support enhanced holistic nutritional epidemiology research programs in the field on ASD. In addition, the key role of gene-environment interactions in ASD through epigenetics mechanisms [6], that are highly influenced by environmental factors such as nutrition [79], reinforce the need to set up research programs based on wellphenotype prospective cohorts. Those programs are required to further understand the links between food dietary behaviours (disorders and patterns) and (i) long term nutritional status, (ii) the composition and function of gut microbiota, (iii) neuroinflammation and stress oxidative processes [80], (iv) the better understanding of epigenetics mechanisms and (v) the clinical symptoms of ASD, their comorbidities and their trajectories. This approach is essential to target the setting up of efficient personalized dietary interventions to prevent ASD and its severity and to allow transition from an empirical ASD clinical medicine to an exact medical science.
References
- American Psychiatric A. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub (2013).
- Wingate M, Kirby RS, Pettygrove S, et al. Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, MMWR. Surveillance. Summaries 63(2), (2014).
- Gaugler T, Klei L, Sanders SJ, et al. Most genetic risk for autism resides with common variation. Nature. Genetics 46(8), 881-885 (2014).
- Sandin S, Lichtenstein P, Kuja-Halkola R, et al. The familial risk of autism. Jama 311(17), 1770-1777 (2014).
- DeVilbiss EA, Magnusson C, Gardner RM, et al. Antenatal nutritional supplementation and autism spectrum disorders in the Stockholm youth cohort: population based cohort study. BMJ 359(1), j4273 (2017).
- Grafodatskaya D, Chung B, Szatmari P, et al. Autism spectrum disorders and epigenetics. J. Am. Aca. Child. Adol. Psychiatry 49(8), 794-809 (2010).
- Delcuve GP, Rastegar M, Davie JR. Epigenetic control. J. Cell. Physiol 219(2), 243-250 (2009).
- Waye MMY, Cheng HY. Genetics and epigenetics of autism: A Review. Psych. Clin. Neurosci 72(4), 228-244 (2018).
- Schmidt RJ, Lyall K, Hertz-Picciotto I. Environment and Autism: Current State of the Science. Cutting. Edge. Psych. Prac 1(4), 21-38 (2014).
- Peretti S, Mariano M, Mazzocchetti C, et al. The keystone of autism spectrum disorder? Nut. Neurosci 1(1),1-15 (2018).
- Lindsay KL, Buss C, Wadhwa PD, et al. The Interplay Between Nutrition and Stress in Pregnancy: Implications for Fetal Programming of Brain Development. Biol. Psych (2018).
- Marques AH, O'Connor TG, Roth C, et al. The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Frontiers. Neurosci 7120 (2013).
- Monk C, Georgieff MK, Osterholm EA. Research review: maternal prenatal distress and poor nutrition - mutually influencing risk factors affecting infant neurocognitive development. J. Child. Psych. Psych. Allied. Disciplines 54(2), 115-130 (2013).
- Galera C, Heude B, Forhan A, et al. Prenatal diet and children's trajectories of hyperactivity-inattention and conduct problems from 3 to 8 years: the EDEN mother-child cohort. J. Child. Psych. Psych. Allied. Disciplines (2018).
- McGrath J, Brown A, St Clair D. Prevention and schizophrenia--the role of dietary factors. Schizophrenia. Bull 37(2), 272-283 (2011).
- Sullivan EL, Riper KM, Lockard R, et al. Maternal high-fat diet programming of the neuroendocrine system and behavior. Hormones. Behav 76153-76161 (2015).
- Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J. Nut 133(6), 1997s-2002s (2003).
- Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int. J. Epidemiol 43(2), 443-464 (2014).
- Mohammad-Rezazadeh I, Frohlich J, Loo SK, et al. Brain connectivity in autism spectrum disorder. Curr. Opin. Neurol 29(2), 137-147 (2016).
- DeVilbiss EA, Gardner RM, Newschaffer CJ, et al. Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. British. J. Nut 114(5), 663-672 (2015).
- Greenblatt JM, Huffman LC, Reiss AL. Folic acid in neurodevelopment and child psychiatry. Progress. Neuro-Psycho. Biol. Psychiatry 18(4), 647-660 (1994).
- Stubbs G, Henley K, Green J. Autism: Will vitamin D supplementation during pregnancy and early childhood reduce the recurrence rate of autism in newborn siblings? Med. hypotheses 8874-8878 (2016).
- Kocovska E, Gaughran F, Krivoy A, et al. Vitamin-D Deficiency As a Potential Environmental Risk Factor in Multiple Sclerosis, Schizophrenia, and Autism. Frontier. Psych (2017).
- Steenweg-de Graaff J, Tiemeier H, Ghassabian A, et al. Maternal Fatty Acid Status During Pregnancy and Child Autistic Traits: The Generation R Study. Am. J. Epidemiol 183(9), 792-799 (2016).
- Abbott BD. Review of the expression of peroxisome proliferator-activated receptors alpha (PPAR alpha), beta (PPAR beta), and gamma (PPAR gamma) in rodent and human development. Rep. Toxicology 27(3-4), 246-257 (2009).
- Delorme R, Ey E, Toro R, et al. Progress toward treatments for synaptic defects in autism. Nat. Med 19(6), 685-694 (2013).
- Saghazadeh A, Rezaei N. Brain-Derived Neurotrophic Factor Levels in Autism: A Systematic Review and Meta-Analysis. J. Autism. Dev. Dis 47(4),1018-1029 (2017).
- Morgese MG, Trabace L. Maternal Malnutrition in the Etiopathogenesis of Psychiatric Diseases: Role of Polyunsaturated Fatty Acids. Brain. Sci 6(3) (2016).
- Schmidt RJ, Tancredi DJ, Krakowiak P, et al. Maternal intake of supplemental iron and risk of autism spectrum disorder. Am. J. Epidemiol 180(9), 890-900 (2014).
- Taranenko LA, Malutina NN. [Congenital abnormalities in children whose parents were exposed to methanol and formaldehyde]. Meditsina. Truda. i. promyshlennaia. ekologiia (12), 33-35 (2012).
- Walton RG, Monte WC. Dietary methanol and autism. Med. hypoth 85(4), 441-446 (2015).
- Ashwood P, Krakowiak P, Hertz-Picciotto I, et al. Altered T cell responses in children with autism. Brain. Behav. Immunity 25(5), 840-849 (2011).
- Ashwood P, Krakowiak P, Hertz-Picciotto I, et al. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol 232(1-2), 196-199 (2011).
- Sullivan EL, Grayson B, Takahashi D, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J. Neurosci 30(10), 3826-3830 (2010).
- Vucetic Z, Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Inter. Discip. Rev. Syst. Biol. Med 2(5), 577-593 (2010).
- Grayson BE, Levasseur PR, Williams SM, et al. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151(4), 1622-1632 (2010).
- Schmidt RJ. Maternal folic acid supplements associated with reduced autism risk in the child. Evidence-Based. Med 18(6), e53 (2013).
- Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am. J. Clin. Nutrition 96(1), 80-89 (2012).
- Suren P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. Jama 309(6), 570-577 (2013).
- Lyall K, Munger KL, O'Reilly EJ, et al. Maternal dietary fat intake in association with autism spectrum disorders. Am. J. Epidemiol 178(2), 209-220 (2013).
- Vinkhuyzen AAE, Eyles DW, Burne THJ, et al. Gestational vitamin D deficiency and autism-related traits: the Generation R Study. Mol. Psych 23(2), 240-246 (2018).
- Whitehouse AJ, Holt BJ, Serralha M, et al. Maternal vitamin D levels and the autism phenotype among offspring. J. Autism Dev. Disorders 43(7), 1495-1504 (2013).
- Sullivan EL, Nousen EK, Chamlou KA, et al. The Impact of Maternal High-Fat Diet Consumption on Neural Development and Behavior of Offspring. Int. J. Obesity. Supp 2S7-s13 (2012).
- Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psych 4e360 (2014).
- Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr. Opin. Lipidol 13(1), 3-9 (2002).
- Andraos S, de Seymour JV, O'Sullivan JM, et al. The Impact of Nutritional Interventions in Pregnant Women on DNA Methylation Patterns of the Offspring: A Systematic Review. Mol. Nut. Food. Res e1800034 (2018).
- Lassale C, Batty GD, Baghdadli A, et al. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol. Psych (2018).
- Cermak SA, Curtin C, Bandini LG. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Diet. Assoc 110(2), 238-246 (2010).
- Ledford JR, Gast DL. Feeding Problems in Children With Autism Spectrum Disorders:A Review. Focus. Autism. Other. Dev. Disab 21(3), 153-166 (2006).
- Sharp WG, Berry RC, McCracken C, et al. Feeding problems and nutrient intake in children with autism spectrum disorders: a meta-analysis and comprehensive review of the literature. J. Autism. Dev. Dis 43(9), 2159-2173 (2013).
- Bandini LG, Anderson SE, Curtin C, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J. Ped 157(2), 259-264 (2010).
- Mari-Bauset S, Zazpe I, Mari-Sanchis A, et al. Food selectivity in autism spectrum disorders: a systematic review. J. Child. Neurol 29(11), 1554-1561 (2014).
- Ahearn WH, Castine T, Nault K, et al. An assessment of food acceptance in children with autism or pervasive developmental disorder-not otherwise specified. J. Autism Dev. Dis 31(5), 505-511 (2001).
- Schreck KA, Williams K, Smith AF. A comparison of eating behaviors between children with and without autism. J. Autism. Dev. Dis 34(4), 433-438 (2004).
- Postorino V, Sanges V, Giovagnoli G, et al. Clinical differences in children with autism spectrum disorder with and without food selectivity. Appetite 92126-92132 (2015).
- Mazurek MO, Vasa RA, Kalb LG, et al. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. J. Abn. Child. Psych 41(1), 165-176 (2013).
- Suarez MA, Nelson NW, Curtis AB. Longitudinal follow-up of factors associated with food selectivity in children with autism spectrum disorders. Autism. Int. J. Res. Practice 18(8), 924-932 (2014).
- Johnson CR, Turner K, Stewart PA, et al. Relationships between feeding problems, behavioral characteristics and nutritional quality in children with ASD. J. Autism. Dev. Dis 44(9), 2175-2184 (2014).
- Hollis F, Kanellopoulos AK, Bagni C. Mitochondrial dysfunction in Autism Spectrum Disorder: clinical features and perspectives. Cur. Opin. Neurobiol 45178-45187 (2017).
- Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol. Psych 17(3), 290-314 (2012).
- Li Q, Han Y, Dy ABC, Hagerman RJ. The Gut Microbiota and Autism Spectrum Disorders. Frontiers. Cell. Neurosci 11120 (2017).
- van De Sande MM, van Buul VJ, Brouns FJ. Autism and nutrition: the role of the gut-brain axis. Nut. Res. Rev 27(2), 199-214 (2014).
- Coury DL, Ashwood P, Fasano A, et al. Gastrointestinal conditions in children with autism spectrum disorder: developing a research agenda. Pediatrics 130(1), 2S160-168 (2012).
- McElhanon BO, McCracken C, Karpen S, et al. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 133(5), 872-883 (2014).
- Sandhu KV, Sherwin E, Schellekens H, et al. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl. Res. J. Labor. Clin. Med 179223-179244 (2017).
- Rea K, Dinan TG, Cryan JF. The microbiome: A key regulator of stress and neuroinflammation. NeurobioL. Stress 423-433 (2016).
- Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol 595(2), 489-503 (2017).
- Buffington SA, Di Prisco GV, Auchtung TA, et al. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 165(7), 1762-1775 (2016).
- Masi A, Quintana DS, Glozier N, et al. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol. Psych 20(4), 440-446 (2015).
- de Magistris L, Familiari V, Pascotto A, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J. Pedia. Gastroent. Nut 51(4), 418-424 (2010).
- Williams BL, Hornig M, Buie T, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PloSone 6(9), e24585 (2011).
- Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Nat. Acad. Sci. USA 107(26), 11971-11975 (2010).
- Barrett E, Guinane CM, Ryan CA, et al. Microbiota diversity and stability of the preterm neonatal ileum and colon of two infants. MicrobiologyOpen 2(2), 215-225 (2013).
- Hinde K, Lewis ZT. MICROBIOTA. Mother's littlest helpers. Science 348(6242), 1427-1428 (2015).
- Sharp WG, Postorino V, McCracken CE, et al. Dietary Intake, Nutrient Status, and Growth Parameters in Children with Autism Spectrum Disorder and Severe Food Selectivity: An Electronic Medical Record Review. J. Acad. Nutr. Diet 118(10), 1943-1950 (2018).
- Zimmer MH, Hart LC, Manning-Courtney P, et al. Food variety as a predictor of nutritional status among children with autism. J. Autism. Dev. Dis 42(4), 549-556 (2012).
- Hediger ML, England LJ, Molloy CA, et al. Reduced bone cortical thickness in boys with autism or autism spectrum disorder. J. Autism. Dev. Dis 38(5), 848-856 (2008).
- Sathe N, Andrews JC, McPheeters ML, et al. Nutritional and Dietary Interventions for Autism Spectrum Disorder: A Systematic Review. Pediatrics 139(6) (2017).
- Stover PJ, James WPT, Krook A, et al. Emerging concepts on the role of epigenetics in the relationships between nutrition and health. J. Int. Med 284(1), 37-49 (2018).
- Rose S, Melnyk S, Pavliv O, et al. Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl. Psych 2e134 (2012).