Research Article - International Journal of Clinical Rheumatology (2020) Volume 15, Issue 3
Obstructive sleep apnea and fatigue in patients with inflammatory arthritis taking TNF-inhibitors
- Corresponding Author:
- Rebecca S. Overbury
Division of Rheumatology
Department of Internal Medicine, Salt Lake City
Veteran Affairs and University of Utah Medical Centers, Salt Lake City, UT, USA
E-mail: rebecca.overbury@hsc.utah.edu
Abstract
p>Background: Fatigue is common in inflammatory arthritis (IA). Obstructive sleep apnea (OSA) causes fatigue and is common in IA. OSA and IA are associated with systemic inflammation, including elevated levels of tumor necrosis factor alpha (TNFα). Tumor necrosis factor inhibitors (TNFi) reduce musculoskeletal symptoms and fatigue in IA. The effects of TNFi on OSA are unknown. Our goal was to explore the relationships between TNFi, fatigue, and OSA in IA.Methods: Consecutive patients starting TNFi for IA and high risk for OSA were enrolled. OSA was assessed with the Apnea Hypopnea Index (AHI) and percent time below 90% oxygen saturation (%time < 90%). Fatigue was assessed with the Functional Severity Scale (FSS). Sleep was assessed with the Functional Outcome of Sleep Questionnaire (FOSQ). IA was assessed with the patient global assessment of arthritis (PGA). Parameters were compared before and after TNFi. Changes in OSA, sleep, and fatigue outcomes were adjusted for changes in the PGA.
Findings: Eighteen participants completed the study between September 2011 and February of 2014. The mean age was 54 years. 72% were male. Before and after TNFi: mean AHIs were 12.5 and 13.1, respectively (p=0.97); mean %times < 90% were 29.5 and 35.4, respectively (p=0.18); and mean FSS scores were 43.1 and 41.3, respectively (p=0.08); mean FOSQ scores were 11.4 and 11.8, respectively (p=0.09).
Conclusions: OSA parameters did not improve with TNFi, but there were statistically non-significant trends toward improvement in fatigue and sleepiness with TNFi.
Keywords
tumor necrosis factor inhibitors • obstructive sleep apnea • inflammatory arthritis • fatigue • sleepiness
Abbreviations
AHI: Apnea Hypopnea Index; BMI: Body Mass Index; CPAP: Continuous Positive Airway Pressure; FOSQ: Functional Outcome of Sleep Questionnaire; FSS: Functional Severity Scale; IA: Inflammatory Arthritis; ICC: Interclass Correlation; Mg: Milligram; MSLT: Multiple Sleep Latency Test; OSA: Obstructive Sleep Apnea; PGA: Patient Global assessment of Arthritis; SD: Standard Deviation; TNF: Tumor Necrosis Alpha; TNFi: Tumor Necrosis Factor Inhibitors; VA: Veteran’s Affairs
Introduction
Fatigue and sleep disturbances are common and burdensome in patients with Inflammatory Arthritis (IA) [1,2]. Approximately 50% of patients with inflammatory arthritis experience severe fatigue, and up to 84% have poor sleep quality [3-5]. Patients with IA and sleep disturbances suffer more pain and functional limitation than patients without sleep disturbances, and IA patients consistently prioritize fatigue amongst their top goals for disease improvement [6-8]. Several IA treatments improve fatigue and sleep quality, but the magnitude of improvement has been small compared to the improvement in musculoskeletal IA, suggesting that musculoskeletal symptoms may not be the most important driver of fatigue or poor sleep in IA patients [9-11].
Obstructive Sleep Apnea (OSA) is one of the most common causes of sleep disturbance and fatigue [12,13]. OSA occurs more frequently in patients with IA than controls [14,15]. In a study of rheumatoid arthritis patients, the prevalence of OSA was 47%, and in our previous research in a spondyloarthritis population, the frequency of OSA was 76% [13]. The elevated OSA risk in IA populations is independent of traditional risk factors, including sex, age, hypertension, diabetes, hyperlipidemia, ischemic heart disease, arrhythmia, asthma/chronic obstructive pulmonary disease, obesity, heart failure, chronic kidney disease, and stroke [16]. While OSA is common in IA, it is unknown if OSA is a major contributor to the fatigue and sleepiness experienced by IA patients. Systemic inflammation occurs with OSA and IA, and there is overlap in the inflammatory mediators associated with both diseases [17]. For example, IA disease activity is associated with serum and synovial TNF-α levels , and intermittent hypoxia from OSA upregulates the production of TNF-α [13,18,19]. Furthermore, successful treatment of OSA and IA reduces TNF-α levels in both diseases [1,2,16,18,19]. While inflammatory mediators, such as TNF-α, are well recognized in the pathogenesis IA, the role of systemic inflammation in OSA is inadequately understood. The goals of this study were to explore the hypotheses that 1) OSA is a major contributor to fatigue and sleepiness in IA patients, and 2) inflammatory mediators affected by TNFi are involved in the pathogenesis of OSA. To accomplish these goals, we evaluated OSA parameters, sleepiness, and fatigue before and after initiating a TNFi in patients with active IA and suspected OSA.
Methods
We enrolled consecutive patients with IA (ankylosing spondylitis, psoriatic arthritis, enteropathic arthritis or rheumatoid arthritis) for whom we anticipated starting a TNFi. These patients were recruited from rheumatology clinics at the University of Utah and Salt Lake City Veterans Affairs Medical Center between September 2011 and February of 2014. This research was conducted in compliance with the Helsinki Declaration, with the approval of the University of Utah Institutional Review Board.
Inclusion criteria
Age ≥18 years, an IA diagnosis confirmed by a rheumatologist, a positive score (≥ 3) on the STOPBANG OSA screening questionnaire (which predicts ≥ 25% risk for severe OSA), and an anticipated TNFi initiation for active IA, according to standard-of-care practice [20]. Exclusion criteria included Continuous Positive Airway Pressure (CPAP) machine use within the six months prior to study enrollment or cigarette smoking within one week prior to the anticipated sleep test (as this precludes patients from being able to participate in the home sleep study per VA policy). Patients were excluded if they had exposure to adalimumab, golimumab, infliximab, certolizumab or abatacept within the past 12 weeks; exposure to etanercept, azathioprine, mycophenolate mofetil, cyclosporine, cyclophosphamide, or glucocorticoids (at a dose higher than the prednisone equivalent of 10mg/day) within the past 28 days; or a dose change of methotrexate, sulfasalazine, hydroxychloroquine, or leflunomide within the past 12 weeks.
Prior to starting a TNFi, participants completed questionnaires including the Patient Global assessment of Arthritis (PGA Arthritis), Functional Severity Scale (FSS) (lower scores favorable), and Functional Outcomes of Sleep questionnaire (FOSQ) (higher scores favorable). Participants completed a home sleep study, for two consecutive nights, with an ApneaLinkTM testing device. Shortly after completing the questionnaires and home sleep study, participants started a TNFi. The specific TNFi was selected by the patient and rheumatologist, according to standard of care practice. Two to three weeks after the initiation of a TNFi, patients completed a second set of identical questionnaires and two-night sleep study.
OSA measurement parameters included the Apnea Hypopnea Index (AHI) and percent time below 90% oxygen saturation (%time < 90%). These sleep studies were analyzed with the automated ApneaLinkTM system. To validate the performance of these automated interpretations, a subset of these sleep studies was also interpreted by sleep specialists, in a blinded manner. The agreement between the automated and sleep specialist interpretations was determined.
Statistics
Descriptive statistics are presented as means ± SD for continuous variables, and frequency with percentage for categorical variables. Patient characteristics before and after TNFi use were compared using the Wilcoxon signed rank test (unadjusted). Linear regression was used to test the effect of TNFi with the control of the % change in patient global assessment of arthritis severity and baseline values. All P values were 2-sided and considered significant at values ≤ 0.05.
Results
Twenty-nine patients were enrolled. The mean age was 54 years. Eighteen (62%) of study participants were male. Ten (34%) had psoriatic arthritis, four (14%) had ankylosing spondylitis, three (10%) had rheumatoid arthritis, and one (3%) had enteropathic arthritis. The most commonly initiated TNFi was adalimumab (n=10). Four received infliximab. Four received etanercept. Ten participants reported the use of sleep medications (Table 1).
Table 1. Demographics.
| All (n=29) | Completers (n=18) | Non-Completers (n=11) | |
|---|---|---|---|
| Number or Mean (%) | Number or Mean (%) | Number or Mean (%) | |
| Mean Age | 54.9 | 54.4 | 55.6 |
| Male Sex | 18(62) | 13(72) | 5(45) |
| Mean BMI | 31.75 | 30.6 | 32.9 |
| IA Subtype | |||
| Axial Spondyloarthritis | 8(28) | 4(22) | 4(36) |
| Psoriatic Arthritis | 16(55) | 10(56) | 6(55) |
| Enteropathic Arthritis | 1(3) | 1(6) | 0 |
| Rheumatoid Arthritis | 4(14) | 3(17) | 1(9) |
| Sleep Medication Use* | 10(34) | 6(33) | 4(36) |
| Any Alcohol use | 5(17) | 4(22) | 1(9) |
| TNF-I | |||
| Adalimumab | 10(56) | 10(56) | N/A |
| Infliximab | 4(22) | 4(22) | N/A |
| Etanerecept | 4(22) | 4(22) | N/A |
*Included trazodone, zolpidem, melatonin and/or alprazolam
Twenty-nine participants completed the baseline questionnaires and sleep test. Eighteen participants completed the follow-up sleep studies and questionnaires. Of the 11 non-completers, 2 did not start a TNFi therapy and 8 were lost to follow up. One non-completer died from causes unrelated to IA or OSA. Demographics and baseline characteristics were similar between completers and non-completers.
The initial 16 sleep studies were analyzed by both the automated ApneaLink software and by sleep specialists. The Interclass Correlation (ICC) between the automated and manual interpretations were 0.94 for the baseline sleep studies and 0.96 for the follow-up sleep studies. Since these ICCs exceeded the predetermined target of 0.90, the subsequent sleeps studies were interpreted only with the automated system interpretation.
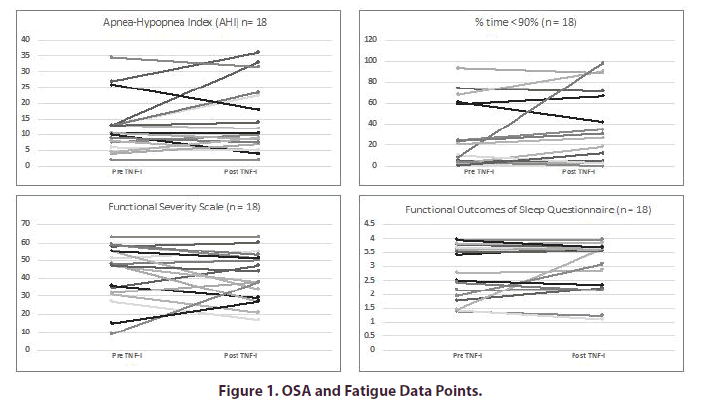
The mean AHI before and after TNFi initiation were 12.5 and 13.1 respectively (Table 2). The mean %times < 90% were 29.5 and 35.4, respectively. After adjustment for changes in PGA Arthritis, the initiation of a TNFi was not associated with changes in AHI (p=0.97) or %time < 90% (p=0.18). The mean FSS scores before and after TNFi initiation were 43.1 and 41.3, respectively. The mean FOSQ scores before and after TNFi were 11.4 and 11.8, respectively (Figure 1). After adjustment for changes in PGA, the initiation of a TNFi was not associated with changes in the FSS (p=0.08) or the FOSQ (p=0.09).
Table 2. OSA and Fatigue Parameters.
| Before TNFI-I (Mean ± SD) | After TNF-I (Mean ± SD) | Unadjusted p value | Adjusted p value* | |
|---|---|---|---|---|
| AHI | 12.5 ± 8.5 | 13.1 ± 10.3 | 0.18 | 0.97 |
| % time<90% | 29.5 ± 30.2 | 35.4 ± 36.3 | 0.22 | 0.18 |
| FSS** | 43.1 ± 15.6 | 41.3 ± 13.5 | 0.4 | 0.08 |
| FOSQ*** | 11.4± 3.8 | 11.8 ± 3.5 | 0.79 | 0.09 |
*Adjusted for change in patient global assessment of arthritis severity
**FSS: Fatigue Severity Scale: Range 0-63. Higher values represent increased fatigue
***FOSQ: Functional Outcome of Sleep Questionnaire: Range 5-20. Higher values represent decreased sleepiness
Discussion and Limitations
There were statistically non-significant trends toward improvements in fatigue and sleepiness with TNFi after 2-3 weeks of treatment. These data are consistent with larger studies demonstrating that fatigue responds modestly to treatment with TNFi [21]. However, the trends toward improvement in fatigue and sleepiness without improvements in OSA did not support our first hypothesis that OSA is a major contributor of the fatigue and sleepiness experienced by IA patients.
Our second hypothesis that inflammatory mediators are involved in the pathogenesis of OSA was similarly unsupported by our study outcomes. There was no signal for improvements in OSA parameters with TNFi treatment. This may have occurred because the inflammatory mediators affected by TNFi are not critical to the pathogenesis of OSA. Alternatively, these inflammatory mediators may have been insufficiently altered to impact the measured OSA parameters, with the specific TNFi treatment regimens used in this study.
In contrast to our study findings, a placebo-controlled, double-blind study conducted by Vgontzas et al. in OSA patients demonstrated improvements in TNFα, IL-6, apneic events, hypopnic events, and daytime sleepiness with TNFi treatment [22]. The authors concluded that inflammation is likely part of the pathogenic mechanism leading to OSA and that both TNFα and IL-6 may mediate excessive daytime sleepiness [22]. This study differed from our study in several ways. A single TNFi (etanercept) was used in the Vgontzas study, whereas we included multiple TNFi therapies. Vgontzas’ baseline AHI was higher (55.9 ± 11.6) than our sample’s baseline of 12.5 ± 8.5. Additionally, every patient in the Vgontzas study (n=8) received therapy for a full three weeks before re-assessment, while our patients were reassessed at the 2-3-week interval. Finally, sleepiness in the Vgontzas study was assessed using Multiple Sleep Latency Test (MSLT) denoting observed sleep latency and stage of sleep, while our metrics assessed sleepiness and fatigue using the FOSQ and FSS respectively. A strength of our study was the inclusive selection criteria and real-world study design. The use of home sleep studies enabled patients to complete the sleep studies in environments that reflect their regular sleep environments. Notably, the high agreement in sleep study results between the automatic results and sleep specialists suggests accurate interpretations of the sleep studies.
A limitation of our study includes the inability to comprehensively assess OSA, sleep, and IA activity with the instruments used in this study. With the home sleep studies, we were able to measure limited aspects of sleep patterns and OSA. Similarly, the PGA Arthritis is a subjective measure of IA activity. While patients are specifically instructed to consider only their arthritis for this patient reported outcome, it is possible that changes in fatigue or energy could also have had meaningful impacts on PGA responses. Other study limitations include the small sample size and the short follow-up period. The heterogeneity of IA patients and TNFi may have precluded discovery of findings unique to a specific TNFi or IA phenotype. Furthermore, this study evaluated only the impact of IA treatment on OSA, and not the impact of OSA treatment on IA.
Conclusion
TNFi may impact fatigue and sleepiness, independently of IA activity and OSA. Also, we found no evidence that TNFi therapy affected OSA parameters. Additional research is required to understand fatigue in IA patients and the relationship between systemic inflammation and OSA.
Acknowledgments
None
Funding/Competing and conflicting interests
Rebecca S. Overbury: none. Shaobo Pei: None. Brian Breviu: None. Daniel O. Clegg: None. Jessica A. Walsh: Conflicts of interest and source of funding include Marriott Daughters Foundation, and Western Institute of Biomedical Research.
References
- Deodhar A, Braun J, Inman RD et al. Golimumab reduces sleep disturbance in patients with active ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis. Care. Res. (Hoboken). 62(9), 1266–1271 (2010).
- Katz P. Fatigue in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 19(5), 25 (2017).
- Overman CL, Kool MB, Da Silva JA et al. The prevalence of severe fatigue in rheumatic diseases: an international study. Clin. Rheumatol. 35(2), 409–415 (2016).
- Wong ITY, Chandran V, Li S et al. Sleep Disturbance in Psoriatic Disease: Prevalence and Associated Factors. J. Rheumatol. 44(9), 1369–1374 (2017).
- Loppenthin K, Esbensen BA, Jennum P et al. Sleep quality and correlates of poor sleep in patients with rheumatoid arthritis. Clin. Rheumatol. 34(12), 2029–2039 (2015).
- Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert. Rev. Clin. Immunol. 14(5), 405–417 (2018).
- Garrido-Cumbrera M, Hillmann O, Mahapatra R et al. Improving the Management of Psoriatic Arthritis and Axial Spondyloarthritis: Roundtable Discussions with Healthcare Professionals and Patients. Rheumatol. Ther. 4(2), 219–231 (2017).
- Lee YC, Chibnik LB, Lu B et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis. Res. Ther. 11(5), R160 (2009).
- Taylor-Gjevre RM, Gjevre JA, Nair BV et al. Improved Sleep Efficiency after Anti-Tumor Necrosis Factor alpha Therapy in Rheumatoid Arthritis Patients. Therapeutic advances in musculoskeletal disease. 3(5), 227–233 (2011).
- Detert J, Dziurla R, Hoff P et al. Effects of treatment with etanercept versus methotrexate on sleep quality, fatigue and selected immune parameters in patients with active rheumatoid arthritis. Clin. Exp. Rheumatol. 34(5), 848–856 (2016).
- Mease P, Walsh JA, Baraliakos X et al. Translating Improvements with Ixekizumab in Clinical Trial Outcomes into Clinical Practice: ASAS40, Pain, Fatigue, and Sleep in Ankylosing Spondylitis. Rheumatol. Ther. 6(3), 435–450 (2019).
- Matsumoto T, Chin K. Prevalence of sleep disturbances: Sleep disordered breathing, short sleep duration, and non-restorative sleep. Respiratory. Investigation. (2019).
- Walsh JA, Duffin KC, Crim J et al. Lower frequency of obstructive sleep apnea in spondyloarthritis patients taking TNF-inhibitors. J. Clin. Sleep. Med. 8(6), 643–648 (2012).
- Walsh JA, Song X, Kim G et al. Evaluation of the comorbidity burden in patients with ankylosing spondylitis treated with tumour necrosis factor inhibitors using a large administrative claims data set. J. Pharm. Health. Serv. Res. 9(2), 115–121 (2018).
- Walsh JA, Song X, Kim G et al. Evaluation of the comorbidity burden in patients with ankylosing spondylitis using a large US administrative claims data set. Clin. Rheumatol. 37(7), 1869–1878 (2018).
- Shen TC, Hang LW, Liang SJ et al. Risk of obstructive sleep apnoea in patients with rheumatoid arthritis: a nationwide population-based retrospective cohort study. BMJ. open. 6(11), e013151 (2016).
- Nadeem R, Molnar J, Madbouly EM et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J. Clin. Sleep. Med. 9(10), 1003–1012 (2013).
- Kang JH, Lin HC. Obstructive sleep apnea and the risk of autoimmune diseases: a longitudinal population-based study. Sleep. Med. 13(6), 583–588 (2012).
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 5(2), 136–143 (2008).
- Nagappa M, Liao P, Wong J et al. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS One. 10(12), e0143697 (2015).
- Leverment S, Clarke E, Wadeley A et al. Prevalence and factors associated with disturbed sleep in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a systematic review. Rheumatol. Int. 37(2), 257–271 (2017).
- Vgontzas AN, Zoumakis E, Lin HM et al. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J. Clin. Endocrinol. Metab. 89(9), 4409–4413 (2004).