Review Article - Interventional Cardiology (2012) Volume 4, Issue 5
Obstructive sleep apnea, coronary artery disease and continuous positive airway pressure therapy
- Corresponding Author:
- Chi-Hang Lee
Cardiac Department, National University Heart Centre Singapore
1E Kent Ridge Road, National University Health System Tower Block
Level 9, Singapore 119228
Tel: +65 6772 2493
Fax: +65 6872 2998
E-mail: mdclchr@nus.edu.sg
Abstract
Keywords
acute coronary syndrome, continuous positive airway pressure, coronary artery disease, obstructive sleep apnea
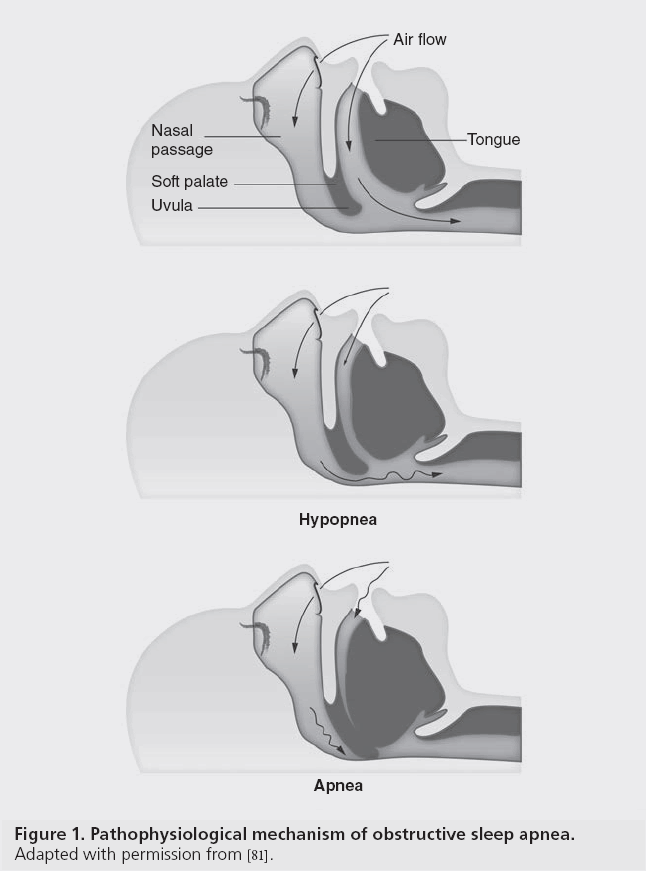
Obstructive sleep apnea (OSA), a respiratory disorder of sleep, is characterized by recurrent episodes of complete or partial upper airway obstruction, resulting in intermittent oxygen deprivation (Figure 1). The primary abnormality in patients with OSA is an anatomically small pharyngeal airway, commonly due to structural features or obesity, resulting in increased soft tissue structures. The breathing disturbances and intermittent hypoxia experienced result in sympathetic activation, surges in blood pressure, production of vasoactive substances and activation of inflammatory and procoagulant pathways. Severely affected patients have neurocognitive and neurobehavioral impairment and are at risk of adverse cardiovascular outcomes.
Figure 1: Pathophysiological mechanism of obstructive sleep apnea.
Adapted with permission from [81].
OSA is widely prevalent in the general population, with an estimated 3–7% in adult males and 2–4% in adult females [1]. Strong clinical evidence has demonstrated a clear association between OSA, hypertension and cardiovascular diseases [2], recognizing OSA as an emerging cardiovascular risk factor. In addition, an increased mortality in coronary artery disease (CAD) patients with coexisting OSA has been documented in several long-term outcome studies [3,4].
Acute coronary syndrome (ACS), one of the life-threatening manifestations of CAD, encompasses a spectrum of clinical presentations with varying degrees of progression to myocardial infarction. In recent years, the body of literature supporting a causal link between OSA and ACS has grown [5,6]. Identification of OSA in ACS patients is especially important as untreated severe OSA in cardiovascular disease can trigger worse cardiovascular outcomes.
In this review, the authors summarize the data on the relationship between OSA and CAD and on the potential role of OSA therapy in CAD using continuous positive airway pressure (CPAP) on cardiovascular outcomes.
Epidemiology studies
It is estimated that a minimum of 6% of the adult population has at least moderate OSA [7]. This figure varies with ethnicity, gender and age [8,9]. There is growing interest in the adverse cardiovascular consequences accompanying OSA. Marin et al. performed a population- based observational study comparing the incidence of cardiovascular events after a mean follow-up duration of 10 years in five groups of men: healthy men, simple snorers, patients with untreated mild or moderate OSA, patients with untreated severe OSA and patients with OSA treated with CPAP therapy [10]. Multivariate analysis with adjustments for confounding factors showed a significantly higher incidence of fatal cardiovascular events (death from myocardial infarction or stroke) and nonfatal cardiovascular events (nonfatal myocardial infarction or stroke, coronary artery bypass surgery and percutaneous transluminal coronary angiography) in patients with untreated severe OSA compared with the other groups.
This is consistent with results from the Sleep Heart Health study, which found OSA to be a significant predictor of prevalent coronary heart disease in men of 70 years of age [11]. The data also evidenced a 68% increased likelihood of developing coronary heart disease in men between 40 and 70 years of age with severe OSA (apnea– hypopnea index [AHI] 30) as compared with those without OSA (AHI <5). It is interesting to note, however, that no association between OSA and prevalent coronary heart disease in women or older men was observed in the study. It might be possible that a pathological difference in OSA response between males and females exists or that the protective effect of the female gender on cardiovascular risk may extent to OSA-related risks as well. However, a recent study found a correlation between severe OSA and cardiovascular death in women [12]. Evidence of a higher mortality from cardiovascular death in women compared with men was also previously reported [13]. Future studies to this end accounting for confounding factors are eagerly awaited.
When looking specifically at ischemic heart disease, a tightly matched case–control study on 62 acute coronary care patients confirmed OSA as an independent predictor of both angina pectoris and myocardial infarction [14]. Present reviews of OSA prevalence in ACS patients also show a much higher rate of occurrence in comparison with the general adult population.
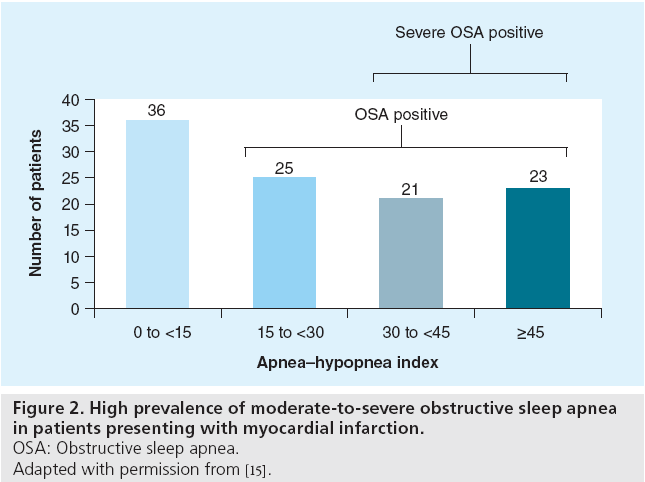
In a cohort of 120 Asian patients presenting with acute myocardial infarction, an overnight sleep study was performed between day 2 and day 5 after admission. The prevalence of previously undiagnosed moderate/severe OSA (AHI >15) was 65.7% (Figure 2) [15]. Similarly, a clinical study performed in the USA reported that 66.4% of patients had OSA (AHI >10) in a cohort of 104 ACS patients [16]. Another sleep study carried out in Portugal found an OSA prevalence of 43.1% in a group of ACS patients [17].
Figure 2: High prevalence of moderate-to-severe obstructive sleep apnea
in patients presenting with myocardial infarction.
OSA: Obstructive sleep apnea.
Adapted with permission from [15].
The above mentioned studies indicate that OSA is more common in the CAD population and an increased risk of CAD appears to be present in OSA patients. However, whether there is a differential correlation of OSA and CAD with age, ethnicity and gender has not been determined.
Pathophysiology of OSA on atherosclerosis and thrombosis
Atherosclerotic plaque accumulation and thrombosis have been recognized as the main culprits for CAD [18]. Their mechanisms, though complex and incompletely understood, can be implicated in inflammation, sympathetic nervous system overactivity, platelet reactivity and endothelial dysfunction [19]. Such cardiovascular stressors are also part of the pathophysiological components of OSA as a result of intermittent severe hypoxemia, CO2 retention, changes in intrathoracic pressure and sleep fragmentation experienced. The risk of CAD may be further heightened by the presence of comorbidities such as arterial hypertension and metabolic syndrome, which are evidently associated with OSA [20,21].
▪ Sympathetic nervous system overactivity
During recurrent apnea episodes, peripheral and central chemoreceptors are excited, eliciting increased sympathetic vasoconstrictor activity [22]. OSA can also induce an increased chemoreflex, sleep arousal and a reduction in the sensitivity of pulmonary stretch receptors, limiting their ability to inhibit central sympathetic discharge, which further contributes to sympathetic activation [23].
It has been reported that the overactivity of the sympathetic nervous system persists during daytime as well, resulting in prolonged sharp adrenergic increases in blood pressure and heart rates [24]. Increased plasma levels of norepinephrine, elevated muscle sympathetic nerve activity and altered heart rate variability are also observed in OSA patients, both at night and during wakefulness [25,26]. Elevated plasma levels of angiotensin II and aldosterone may also contribute to the blood pressure surge of chronic intermittent hypoxia in OSA patients [27]. In the presence of coronary atherosclerosis with simultaneous hypoxemia and increased myocardial oxygen demand, such responses may trigger acute nocturnal cardiac ischemia. This association has been demonstrated in several studies [28,29].
Moreover, there is evidence of a significant role for autonomic nervous activity in the clinical outcomes of patients with CAD [30]. Such data provide substantiation that adrenergic overdrive, as a result of OSA, contributes to the development of CAD.
▪ Endothelial dysfunction
Endothelial dysfunction is believed to be a key event in the pathogenesis of atherosclerosis and atherosclerotic complications [31]. Factors of endothelial dysfunction such as increased oxidative stress, reduced nitric oxide availability and systemic inflammation are evident in OSA patients. A past study demonstrated reduced endothelial nitric oxide synthase and phosphorylated endothelial nitric oxide synthase expression in OSA patients, indicative of endothelial dysfunction [32]. This is consistent with results from experimental studies showing impairment of endothelial-dependent vasodilatation and increased endothelin secretion, a potent vasoconstrictor, in response to hypoxemia [33].
▪ Inflammation
Endothelial dysfunction leads to increased macrophage activity, causing T cells to produce more cytokines and cytoxic substances. Hypoxemic stress experienced during recurrent apneas may also play a role in the activation of systemic inflammatory pathways [34]. The increased inflammatory state stimulates endothelial cells, activates more macrophages and regulates endothelial adhesion molecules [35], accelerating atherosclerosis [36]. Macrophage secretion of metalloproteinases also weakens the fibrous cap of atherosclerotic plaque, increasing the risk of plaque rupture and ACS. Multiple studies have reported an increase in the markers of systemic inflammation, such as acute phase proteins (i.e., serum amyloid A and CRP) [37,38], nuclear factor [39] and cytokines [40] in OSA patients. Widespread evidence recognizes that OSA predisposes patients to a proinflammatory state.
▪ Coagulopathy and platelet dysfunction
Increased platelet activation, abnormal platelet aggregability, fibrinogen and other markers of thrombosis risk are evident in OSA patients [41]. A review comparing multiple reports justified the presence of a hypercoaguable state in OSA, despite a lack of adequate control across studies [42]. The same conclusion was drawn by a clinical study on 110 patients, which reported a positive association between OSA, plasma fibrinogen and platelet viscosity, persisting after adjustments for cardiovascular risk factors [43]. Two other trials found that OSA was correlated with an elevated plasminogen activator inhibitor-1 level, indicative of impaired fibrinolysis [44,45].
This implicates a possible important role of OSA in the pathogenesis of atherothrombotic disease. However, the impact of OSA on platelets remains controversial, as it is difficult to exclude the effects of confounding factors such as lifestyle variables, diseases and type of drug usage. Whether there is significant hypercoagulability in patients with OSA, resulting in increased risk of CAD, remains to be established.
▪ Other factors
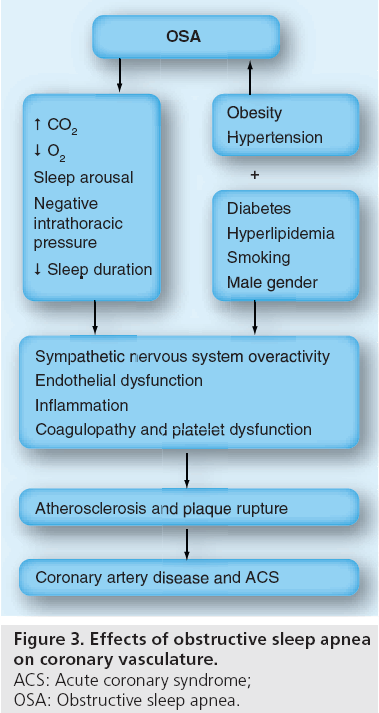
In addition to the major pathophysiological mechanisms of OSA resulting in an increased risk of CAD, several other variables may exist as well. These components, together with the previously mentioned components, are schematically presented in Figure 3.
Figure 3: Effects of obstructive sleep apnea on coronary vasculature.
ACS: Acute coronary syndrome;
OSA: Obstructive sleep apnea.
Impact of OSA on CAD
▪ Effect on time onset of myocardial ischemia
Apnea-associated hypoxemia can reduce oxygen supply to the myocardium and increase oxygen demand by labile changes in heart rates and increased cardiac afterload. Therefore, it is reasonable to suspect that nocturnal myocardial ischemia episodes may be more common in patients with OSA.
This relationship is demonstrated by a study comparing ST-segment depression episodes among patients with OSA, snoring subjects and healthy subjects [46]. It was observed that nocturnal ST-segment depression episodes were more frequent in patients with OSA than snoring and control subjects. However, the study sample size was relatively small (n = 48) and measurement values might be biased due to lack of repeated trials. A similar investigation in a randomized group of men and women (n = 226) presenting with angina pectoris who underwent coronary angiography was conducted [47]. It was observed that 70% of nocturnal ST-segment depressions were preceded by three or more apnea–hypopnea episodes and/or oxygen desaturation. Such nocturnal ST-segment depressions were significantly present in men and in people with severely disordered breathing. Another clinical observation found that 91% of patients with myocardial infarction onset between 12:00 am and 6:00 am had OSA, suggesting the role of hypoxemic episodes on triggering coronary plaque rupture [48]. This is in concordance with a reported peak in sudden cardiac death during sleeping hours in people with OSA [28].
These studies lend support to the possibility that a relationship between the time onset of myocardial ischemia or infarction and OSA exists. Together with epidemiological and pathophysiological evidence, OSA is favored as a risk factor for CAD.
▪ Effect on mortality and restenosis rate
Existing data indicate a high morbidity and mortality in patients with coexisiting OSA and CAD. A 5-year follow-up on intensive cardiac care patients presenting with angina pectoris or myocardial infarction, found untreated OSA to be independently predictive of increased cardiovascular mortality [4]. Recently, the authors demonstrated that among patients admitted with acute myocardial infarction, severe OSA (AHI >30) was an independent predictor of adverse cardiac events at 18‑month follow-up [49]. Similarly, a study on 89 ACS patients following percutaneous coronary intervention demonstrated that the presence of OSA was an independent predictor of major adverse cardiac events (cardiac death, reinfarction and target vessel revascularization). Moreover, a high OSA prevelance of 57% was documented [5].
Several studies support an association between OSA, increased late lumen loss and restenosis. Quantitative coronary angiography at 6-month follow-up found that patients with OSA had significantly greater late lumen loss (1.28 ± 0.84 vs 0.69 ± 0.81 mm) and a higher binary restenosis rate (36.5 vs 15.4%; p = 0.026) compared with patients without OSA [5]. This is in concordance with findings from another study, which demonstrated a greater incidence of angiographic restenosis and a significantly higher late lumen loss in patients with AHI >10 compared with patients with AHI <10 [6]. However, as only baremetal stents were implanted during percutaneous coronary intervention in both studies, an increased restenosis rate may not be of concern with future utilization of drug-eluting stents. Nevertheless, the higher degree of late lumen loss in patients with both OSA and CAD compared with patients without OSA can be viewed as a marker of restenosis and vessel remodeling after percutaneous intervention. This is suggestive of an increased risk of acute cardiac event reoccurrence, consistent with increased mortality in OSA patients.
▪ Effect on microvascular and macrovascular perfusion
Impaired microvascular perfusion in patients undergoing percutaneous coronary intervention is correlated with adverse clinical outcomes and poor recovery of left ventricular function [50]. As the pathophysiological effects of OSA appear to compromise microvascular integrity, it was proposed that the presence of OSA in patients with acute myocardial infarction will result in impaired microvascular perfusion after primary percutaneous coronary intervention. This hypothesis was investigated and impaired microvascular perfusion was defined as an ST-segment resolution of <70%, myocardial blush grade 0 or 1, or a corrected thrombolysis in myocardial infarction (antegrade flow scale) frame count >28. However, no relationship between OSA and impaired microvascular perfusion was observed [15].
A different result was obtained by a study examining left ventricular function recovery of 86 first acute myocardial infarction patients who underwent primary percutaneous coronary intervention. A significantly lower recovery of left ventricular ejection fraction and regional wall motion was reported in OSA patients, compared with patients without OSA [51]. This may also implicate impaired microvascular perfusion in OSA patients with acute myocardial infarction as the cause of poor recovery of left ventricular ejection fraction.
The evaluation of myocardial perfusion using real-time quantitative myocardial contrast echocardiography, as well as macrovascular and microvacular endothelial dysfunction, in the presence of OSA, was previously conducted [52]. Healthy subjects were matched with OSA subjects and it was observed that OSA subjects demonstrated significant impaired myocardial perfusion, attenuated brachial artery reactivity and cutaneous perfusion responses compared with normal subjects. Such evidence suggests the presence of macrovascular and microvascular abnormalities in OSA patients. When taken together, these studies highlight a possible role of OSA on myocardial perfusion, especially in the setting of myocardial infarction. Nonetheless, further studies are required to elucidate the exact implication of OSA in this context.
▪ Effect on central sleep apnea, heart failure and its relation to CAD
The ventilator control instability present in OSA patients can also result in central sleep apnea, depending on the direction of airway collapse. Episodes of central sleep apnea, defined by a 10-s pause in ventilation with no associated respiratory effort, are also evidenced in patients with OSA [53]. This is observed in the CAD population as well. Sleep apneas, mainly central apneas, were evidenced in patients with ACS and patients with stable angina [54]. However, no strong relationship seems to exist between them as the pathophysiological cause of OSA and central sleep apnea are markedly different.
Nevertheless, it is possible that OSA might be an indirect cause of central sleep apnea. Cheyne- Stokes respiration, a form of central sleep apnea, is induced by and occurs mainly in patients with congestive heart failure [55]. As ischemic heart disease is the primary cause of heart failure, it is hypothesized that myocardial dysfunction might be potentiated through the adverse effects of OSA on coronary vasculature. Moreover, the multiple pathophysiological mechanisms of OSA promote heart failure through increasing cardiac afterload and left ventricle wall stress. Hypoxia experienced by OSA patients may also deteriorate heart contractibility [56]. This theory is supported by a report documenting similar prevalence of central apneas and obstructive apneas (40 compared with 36%, respectively) in 700 heart failure patients [57].
Continuous positive airway pressure
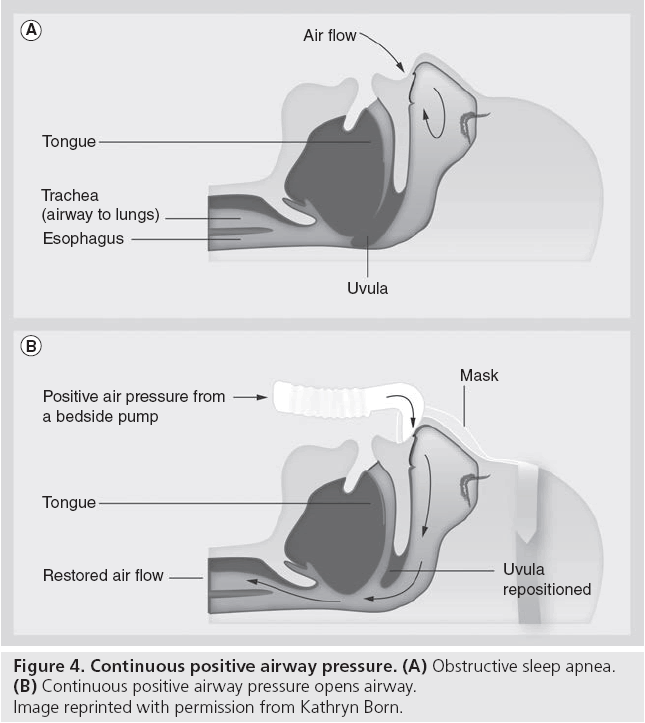
As OSA reflects counteracting forces of the upper airway dilating muscles and an imbalance of the intrapharyngeal pressure present in inspiration [58], CPAP neutralizes this by applying a continuous positive pressure through a nose mask (Figure 4). This provides an airway splint that works against the negative inspiration lung pressure, thereby preventing pharyngeal airway collapse, eliminating apnea, hypopnea and snoring episodes. Though a number of intervention approaches such as weight reduction, medical therapy or surgery are present, CPAP is the treatment of choice for all OSA patients [59]. This is because widespread evidence has recognized CPAP to be the most efficacious therapeutic option [60]. Moreover, a lack of well-designed clinical trials regarding the other treatment methods of OSA exist and present studies of other OSA treatment methods remain inconclusive [61].
Effect of CPAP on aforementioned mechanisms
The pathophysiological mechanisms of OSA arise from hypoxemia and CO2 retention experienced in apneic episodes; therefore, it is reasonable to postulate that the prevention of airway collapse through CPAP therapy would eliminate these mechanisms. This is also suggested by data showing improvement in early signs of atherosclerosis with OSA treatment using CPAP [62].
▪ Sympathetic nervous system overactivity
Present studies point to a reduction in sympathetic nervous system overactivity with the usage of CPAP. CPAP treatment in patients with myocardial ischemia and central sleep apnea or OSA demonstrated a significant lowering of sympathetic overactivity and nocturnal blood pressure surges through eliminating apnea episodes [63]. Results from a randomized controlled trial also showed diminished daytime sympathetic activation with CPAP therapy [64]. Furthermore, the usage of extended CPAP therapy has been shown to reduce muscle sympathetic nerve activity and biomarkers of sympathetic activity [65].
▪ Endothelial dysfunction
Reversal of endothelial dysfunction may be possible with CPAP treatment. A study observed an attentuation in the reduced endothelial nitric oxide synthase and phosphorylated endothelial nitric oxide synthase expression in OSA patients after 4 weeks of CPAP therapy [32]. Moreover, an in vitro study demonstrated an increased adherence of monocytes to endothelial cells in OSA patients and a decrease in adherence level after CPAP treatment, suggestive of reduced endothelial dysfunction [35]. In a clinical trial on Chinese patients, a total of 28 men with moderate/ severe OSA were randomized to CPAP or observation for 4 weeks. Subjects on CPAP had a significant increase in endothelium-dependent flow-mediated dilation as measured by Doppler ultrasound of the brachial artery, whereas those on observation had no change (4.4 vs -0.8%, difference of 5.2%; p < 0.001) [66].
When comparing markers such as VEGF and inducible nitric oxide synthase before and after 12 weeks of CPAP therapy, a significant reduction in VEGF levels and increase in nitrate–nitrate levels, suggestive of improved endothelial function, was previously reported [67]. This is consistent with results from another study demonstrating reduced endothelin levels with nasal CPAP treatment in OSA patients [68].
▪ Inflammation
A significant fall in inflammatory markers in OSA patients after effective treatment with CPAP has been widely evidenced [35,38,39]. Of note, levels of CRP and IL-6, markers of systemic inflammation, have been demonstrated to be elevated in patients with OSA but decreased with CPAP therapy [38]. Neutrophil superoxide, an important contributor to inflammatory disease and ischemia, is enhanced in OSA patients. This is diminished with CPAP therapy [69].
▪ Coagulopathy and platelet dysfunction
Although available literature on the role of CPAP therapy in coagulopathy and platelet function may be less persuasive due to the lack of randomization, control and limited sample sizes, present evidence is in favor of CPAP treatment. It was observed that the use of CPAP therapy resulted in a resolution of the increased platelet activation and aggregability in OSA patients [70,71]. Results also suggest a similar blood viscosity and platelet activity level in OSA patients using CPAP and healthy control subjects [72]. Treatment with CPAP also led to a gradual decrease in factor VII clotting activity, which was not observed in OSA subjects not receiving CPAP therapy [73].
▪ Carotid intima–media thickness
In 24 patients with severe OSA who were free of comorbidities and randomized to receive no treatment (control, n = 12) or CPAP (n = 12) for 4 months, it was observed that all measurements were similar in both groups at baseline. This did not change in the control group after 4 months. By contrast, a significant decrease between baseline and 4 months of CPAP therapy occurred in carotid intima–media thickness (707 ± 105 vs 645 ± 95 mm; p = 0.04). The changes in carotid intima–media thickness were correlated with changes in catecholamine (r = 0.41; p < 0.05) [62]. Likewise, in a group of 50 symptomatic patients newly diagnosed with severe OSA, a nonrandomized, prospective study has shown that CPAP treatment (n = 28) resulted in significant reduction in carotid artery intima–media thickness compared with those who had opted for conservative treatment (n = 22) over a study period of 12 months. Most of the reduction in carotid artery intima–media thickness, when comparing CPAP against conservative treatment, appeared to have occurred within the first 6 months of treatment [74].
Effect of CPAP on cardiovascular outcomes
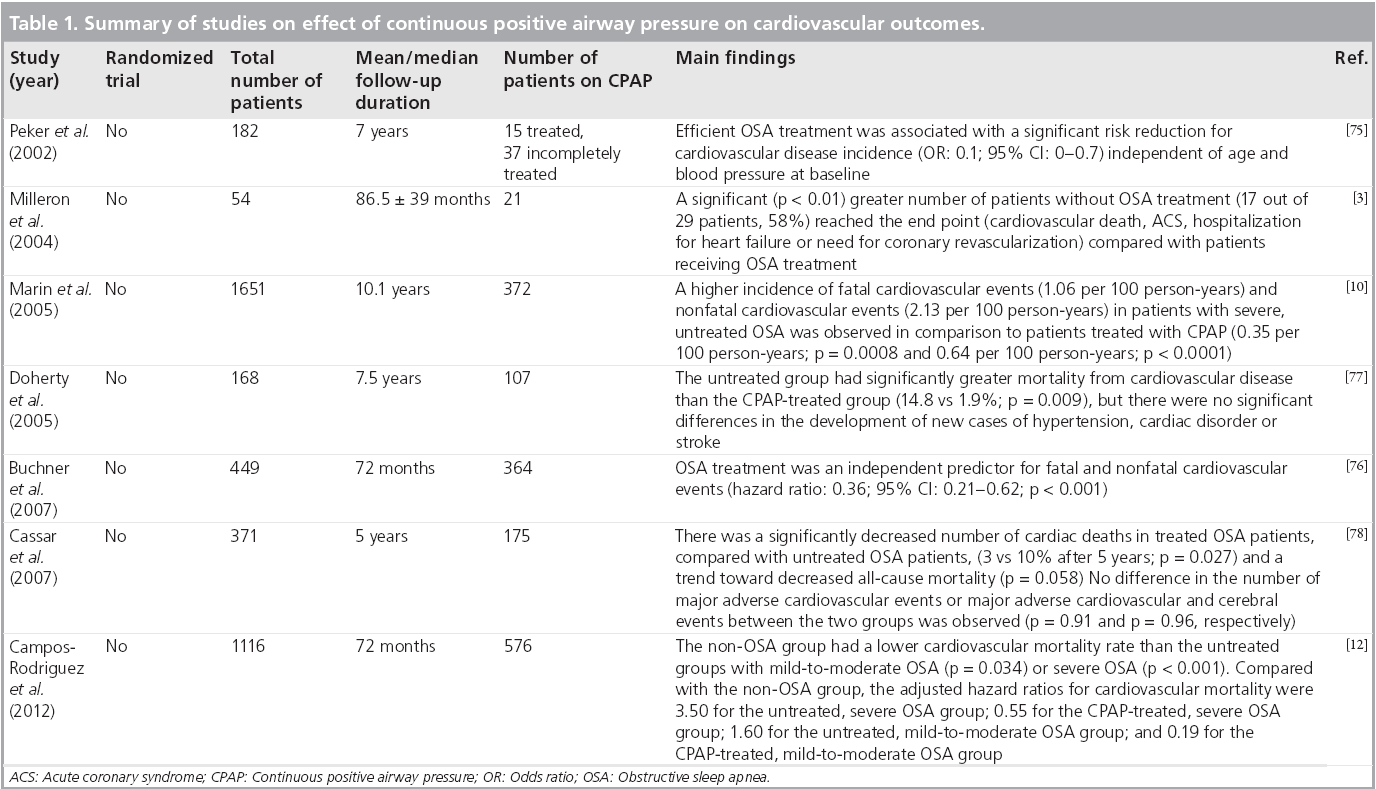
As OSA is a potential risk factor of cardiovascular diseases, treatment of OSA may alleviate cardiovascular risk. This is supported by the aforementioned reversal of the pathophysiological mechanisms of OSA with CPAP therapy. A number of moderate-to-large scale nonrandomized clinical studies have demonstrated that CPAP therapy reduces the occurrence of cardiovascular events and mortality (Table 1). In addition, OSA patients who complied with the CPAP therapy (used more than 4 h per night) were found to have lower adverse events than those who did not comply.
Table 1: Summary of studies on effect of continuous positive airway pressure on cardiovascular outcomes.
Marin et al. observed that patients treated with CPAP were found to have reduced incidence of fatal and nonfatal cardiovascular events [10]. This is consistent with results from two other studies, independent of confounding factors [75,76]. An increased incidence of death from cardiovascular disease in untreated OSA patients compared with patients using CPAP therapy (14.8 vs 1.9%, respectively; p = 0.009) was also previously reported [77]. However, no significant difference in the occurrence of major adverse cardiac events was found between the treated and untreated OSA patients.
In a recent prospective, observational cohort study conducted in Spain, 1116 female patients were classified into three groups: non-OSA, CPAP-treated OSA (adherence ≥4 h per day) and untreated OSA (adherence <4 h per day or not prescribed). Compared with the non-OSA group, the adjusted hazard ratios for cardiovascular mortality were 3.50 for the untreated, severe OSA group; 0.55 for the CPAP-treated, severe OSA group; 1.60 for the untreated, mildto- moderate OSA group; and 0.19 for the CPAPtreated, mild-to-moderate OSA group. It was concluded that adequate CPAP treatment may reduce the risk of cardiovascular death related to OSA [12]. A similar result was obtained in another Spanish trial [13].
A retrospective cohort study from the Mayo Clinic monitored the cardiovascular outcomes of 371 OSA patients who had undergone percutaneous coronary intervention [78]. The patients were stratified into two groups based on whether they had received treatment for OSA. At the 5 year follow-up, the group that had received OSA treatment showed a significantly decreased risk of cardiac deaths in comparison with the untreated group (3 vs 10%; p = 0.027). In addition, there was a trend towards lower risk of all-cause mortality (p = 0.058) favoring the treated group. Another study examined OSA treatment with CPAP on arterial restenosis rate after percutaneous transluminal coronary angiography [6]. This study demonstrated that CPAP therapy resulted in a marginally lower late lumen loss. However, this reduction was not statistically significant (0.57 ± 0.47 vs 0.99 ± 0.86 mm; p = 0.08).
Nevertheless, CPAP treatment appears to reduce the risk of CAD events in OSA patients. It is conceivable that the recommendation of CPAP treatment in OSA patients, especially those at high risk of CAD, may become a standard requirement in future.
Conclusion
Many published studies have explicitly demonstrated OSA to be demonstrative of worse cardiovascular outcomes in both healthy patients and patients with CAD. As such, screening for and treating OSA in CAD patients or patients at a high risk of CAD may prove to be an effective strategy to reduce the residual risks. Meanwhile, CPAP is the recommended treatment for symptomatic OSA. The exact role of CPAP therapy in CAD patients with OSA remains to be demonstrated in randomized clinical trials.
Future perspective
Although OSA is prevalent in the CAD population, it remains underdiagnosed and undertreated. The increased occurrence of OSA in CAD patients appears to be significant enough that the possibility of OSA should be considered in any patient with CAD. However, there are several limitations to the conclusions that can be derived from current literature regarding the relationship between OSA and CAD. Limited long-term outcome studies beyond a decade in follow-up duration exist and most of the present published studies are small. A lack of a largescale evaluation of OSA in the CAD population, especially in patients presented with ACS is present. Moreover, the impact of OSA and the comorbidity of cardiovascular risk factors has not been well studied. It remains unclear whether the influence of OSA on CAD is altered in the presence of existing cardiovascular risk factors. Although most trials of CPAP on OSA patients with stable ischemia had positive results, it is less apparent whether the beneficial effects of CPAP can also be extended to patients with ACS. Evidently, it will require multiple wellexecuted large-scale prospective pathophysiological and clinical studies to give us a clear understanding in this area.
More research is required to better define the potential role of CPAP in the treatment of CAD as well. There is currently no large-scale randomized clinical trial that demonstrates whether treatment of OSA would improve clinical outcomes of patients with myocardial infarction. Therefore, the precise relationships between OSA and myocardial infarction remain unclear. In the first American Heart Association/American College of Cardiology Scientific Statement on Sleep Apnea and Cardiovascular Disease, it was emphasized that, “...although holding great promise, this general area is in need of a substantially expanded knowledge base” [2]. Furthermore, the management of asymptomatic OSA patients remains controversial and results regarding this are inconclusive. Although Barbé et al. found no significant reduction in cardiovascular events or incident hypertension in nonsleepy OSA patients using CPAP [79], preliminary results of a large randomized controlled trial found no baseline differences in prevalent CAD between symptomatic and asymptomatic OSA patients [80].
A large prospective randomized CPAP therapy trial in patients with ACS and OSA is currently being conducted (NCT01335087) [101]. This study will assess the impact of CPAP treatment on a composite end point of the rate of cardiovascular events (cardiovascular death, acute myocardial infarction, nonfatal stroke, hospital admission for heart failure and new hospitalizations) in patients with ACS and co-occurring sleep apnea. An increase in the number of such trials will help better elucidate the impact of OSA therapy on improvement of cardiovascular morbidities. Similarly, in Europe, a large prospective randomized CPAP intervention in 400 patients with CAD and OSA is currently underway (NCT00519597) [102]. This trial aims to assess the impact of CPAP treatment on a composite end point of new revascularization, myocardial infarction, stroke and cardiovascular mortality over a 3-year period in people with CAD and OSA. Future trials providing evidence to this end will guide the use of CPAP treatment for CAD in a rational, evidence-based manner.
Executive summary
Epidemiology studies
▪ Obstructive sleep apnea (OSA) has emerged as a potential risk factor for coronary artery disease (CAD).
▪ When looking specifically at ischemic heart disease, the prevalence of previously undiagnosed moderate/severe OSA (apnea–hypopnea index >15) was up to 66.4% in a group of acute coronary syndrome patients.
Pathophysiology of OSA on atherosclerosis & thrombosis
▪ At basic research and tissue levels, the pathophysiological components of OSA resulting in atherosclerosis and thrombosis can be attributed to sympathetic nervous system overactivity, endothelial dysfunction, inflammation, coagulopathy and platelet dysfunction as well as other factors.
Impact of OSA on CAD
▪ The main impacts of OSA on CAD, especially acute coronary syndrome, are on the following:
– Time onset of myocardial ischemia;
– Nocturnal myocardial ischemia, acute myocardial infarction and sudden cardiac death are correlated with peak time of apnea–hypopnea episodes and/or oxygen desaturation in OSA patients;
– Mortality and restenosis rate;
– Studies focused on acute coronary syndrome patients following percutaneous coronary intervention showed that the presence of OSA was an independent predictor of major adverse cardiac events, mortality, increased late lumen loss and restenosis;
– Microvascular and macrovascular perfusion;
– A significantly lower recovery of left ventricular ejection fraction, regional wall motion abnomalities and imparied myocardial perfusion have been evidenced in OSA patients. However, an intervention study found no relationship between OSA and impaired microvascular perfusion;
– Future studies are required to elucidate the role of OSA in this context.
Effect of continuous positive airway pressure on aforementioned mechanisms
▪ Continuous positive airway pressure treatment appears to reverse the aforementioned pathophysiological mechanism of OSA.
▪ Many studies have documented improvements in the following components: endothelial dysfunction, inflammation, coagulopathy and platelet dysfunction and carotid intima–media thickness.
Effect of continuous positive airway pressure on cardiovascular outcomes
▪ Continuous positive airway pressure treatment in OSA patients were associated with reduced incidence of fatal and nonfatal cardiovascular events compared with untreated OSA patients in moderate-to-large scale nonrandomized clinical studies.
Conclusion
▪ The increased occurrence of OSA appears to be significant enough that the possibility of OSA should be considered in any patients with CAD.
▪ Multiple well-executed large-scale prospective pathophysiological and clinical studies are required to give us a clear understanding regarding OSA and CAD.
▪ The exact role of continuous positive airway pressure therapy in CAD patients with OSA remains to be demonstrated in randomized clinical trials.
Financial and competing interests disclosure
T Hein is supported by funding from the Cardiovascular Research Institute, National University of Singapore. This project was funded by the Clinician Scientist Program, National University Health System, Singapore (grant number: R172-000-239-112). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 5(2), 136–143 (2008).
- Somers VK, White DP, Amin R et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118(10), 1080–1111 (2008).
- Milleron O, Pilliere R, Foucher A et al. Benefits of obstructive sleep apnea treatment in coronary artery disease: a long term follow-up study. Eur. Heart J. 25(9), 728–734 (2004).
- Peker Y, Hedner J, Kraiczi, Loth S. Respiratory disturbance index. An independent predictor of mortality in coronary artery disease. Am. J. Respir. Crit. Care Med. 162(1), 81–86 (2000).
- Yumino D, Tsurumi Y, Takagi A, Suzuki K, Kasanuki H. Impact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndrome. Am. J. Cardiol. 99(1), 26–30 (2007).
- Steiner S, Schueller PO, Hennersdorf MG, Behrendt D, Strauer BE. Impact of obstructive sleep apnea on the occurrence of restenosis after elective percutaneous coronary intervention in ischemic heart disease. Respir. Res. 9, 50 (2008).
- Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 165(9), 1217–1239 (2002).
- Strohl K, Redline S. Recognition of obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 154(2 Pt 1), 274–289 (1996).
- Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in communitydwelling elderly. Sleep 14(6), 486–495 (1991).
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365(9465), 1046–1053 (2005).
- Shahar E, Whitney CW, Redline S et al. Sleep-disordered breathing and cardiovascular disease. Cross sectional results of the sleep heart health study. Am. J. Respir. Crit. Care Med. 163(1), 19–25 (2001).
- Campos-Rodriguez F, Martinez-Garcia M, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann. Intern. Med. 156(2), 115–122 (2012).
- Cordero-Guevara J, Teran-Santos J, Luz Alonso-Alvarez M, Castrodeza-Sanz J, Ordax-Carbajo E, Masa-Jimenez F. Effectiveness of nasal continuous positive airway pressure therapy on cardiovascular outcomes in obstructive sleep apnea-hypopnea syndrome. Curr. Res. Med. 7(2), 121–129 (2011).
- Peker Y, Kraiczi H, Hedner J, Loth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur. Respir. J. 14(1), 179–184 (1999).
- Lee CH, Khoo SM, Tai BC et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction: prevalence, predictors, and effect on microvascular perfusion. Chest 135(6), 1488–1495 (2009).
- Mehra R, Principe-Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6‑month outcome. Sleep Med. 7(6), 521–528 (2006).
- Areias V, Romero J, Cunha K et al. Sleep apnea-hypopnea syndrome and acute coronary syndrome – an association not to forget. Rev. Port. Pneumol. 18(1), 22–28 (2012).
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J. Am. Coll. Cardiol. 46(6), 937–954 (2005).
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352(16), 1685–1695 (2005).
- Kono M, Tatsumi K, Saibara T et al. Obstructive sleep apnea syndrome is associated with some components of metabolic syndrome. Chest 131(5), 1387–1392 (2007).
- Lozano L, Tovar JL, Sampol G et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J. Hypertens. 28(10), 2161–2168 (2010).
- Leuenberger UA, Brubaker D, Quraishi S, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton. Neurosci. 121(1–2), 87–93 (2005).
- Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J. Appl. Physiol. 67(5), 2101–2106 (1989).
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Invest. 96(4), 1897–1904 (1995).
- Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension; insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension 32(2), 293–297 (1998).
- Narkiewicz K, Montano N, Cogliati C, van de Borne, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation 98, 1071–1077 (1998).
- Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am. J. Hypertens. 16(4), 274–280 (2003).
- Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N. Engl. J. Med. 352(12), 1206–1214 (2005).
- Kuniyoshi FH, Garcia-Touchard A, Gami AS et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J. Am. Coll. Cardiol. 52(5), 343–346 (2008).
- Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 59(4), 256–262 (1987). .
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 115(10), 1285–1295 (2007).
- Jelic S, Padeletti M, Kawut SM et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 117(17), 2270–2278 (2008).
- Allahdadi KJ, Walker BR, Kanagy NL. Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension 45, 705–709 (2005).
- Hartmann G, Tschop M, Fischer R et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 12(3), 246–252 (2000).
- Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am. J. Respir. Crit. Care Med. 165(7), 934–939 (2002).
- Dyugovskaya L, Lavie P, Lavie L. Lymphocyte activation as a possible measure of atherosclerotic risk in patients with sleep apnea. Ann. NY Acad. Sci. 1051, 340–348 (2005).
- Svatikova A, Wolk R, Shamsuzzaman AS, Kara T, Olson EJ, Somers VK. Serum amyloid A in obstructive sleep apnea. Circulation 108(12), 1451–1454 (2003).
- Yokoe T, Minoguchi K, Matsuo H et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107(8), 1129–1134 (2003).
- Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaBdependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 174(7), 824–830 (2006).
- Sahlman J, Miettinen K, Peuhkurinen K et al. The activation of the inflammatory cytokines in overweight patients with mild obstructive sleep apnoea. J. Sleep Res. 19(2), 341–348 (2010).
- Von Känel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 131(3), 733–739 (2007).
- Von Känel R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest 124(5), 1956–1967 (2003).
- Steiner S, Jax TW, Evers S, Hennersdorf M, Schwalen A, Strauer BE. Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology 104(2), 92–96 (2005).
- Von känel R, Loredo JS, Ancoli-Israel S, Dimsdale JE. Association between sleep apnea severity and blood coagulability: treatment effects of nasal continuous positive airway pressure. Sleep Breath 10(3), 139–146 (2006).
- Ishikawa J, Hoshide S, Eguchi K et al. Increased low-grade inflammation and plasminogen-activator inhibitor-1 level in nondippers with sleep apnea syndrome. J. Hypertens. 26(6), 1181–1187 (2008).
- Alonso-Fernandez A, Garcia-Rio F et al. Cardiac rhythm disturbances and ST-segment depression episodes in patients with obstructive sleep apnea–hypopnea syndrome and its mechanisms. Chest 127(1), 15–22 (2005).
- Mooe T, Franklin KA, Wiklund U, Rabben T, Holmstrom K. Sleep-disordered breathing and myocardial ischemia in patients with coronary artery disease. Chest 117(6), 1597–1602 (2000).
- Kuniyoshi FS, Garcia-Touchard A, Gami A et al. Day–night variation of acute myocardial infarction in obstructive sleep apnea. J. Am. Coll. Cardiol. 52(5), 343–346 (2008).
- Lee CH, Khoo SM, Chan MY et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J. Clin. Sleep Med. 7(6), 616–621 (2011).
- Poli A, Fetiveau R, Vandoni P et al. Integrated analysis of myocardial blush and ST-segment elevation recovery after successful primary angioplasty: real-time grading of microvascular reperfusion and prediction of early and late recovery of left ventricular function. Circulation 106, 313–318 (2002).
- Nakashima H, Katayama T, Takagi C et al. Obstructive sleep apnoea inhibits the recovery of left ventricular function in patients with acute myocardial infarction. Eur. Heart J. 27(19), 2317–2322 (2006).
- Butt M, Khair O, Dwivedi G, Shantsila A, Shantsila E, Lip GY. Myocardial perfusion by myocardial contrast echocardiography and endothelial dysfunction in obstructive sleep apnea. Hypertension 58(3), 417–424 (2011).
- White DP. Pathogenesis of obstructive and central sleep apnea. Am. J. Respir. Crit. Care Med. 172(11), 1363–1370 (2005).
- Moruzzi P, Sarzi-Braga S, Rossi M, Contini M. Sleep apnoea in ischaemic heart disease: differences between acute and chronic coronary syndromes. Heart 82(3), 343–347 (1999).
- Bradley TD, Floras JS. Sleep apnea and heart failure part II: central sleep apnea. Circulation 107, 1822–1826 (2003).
- Zamarron C, Matias FDC, Egea CJ. Obstructive sleep apnea syndrome: implications in cardiovascular disease. Curr. Respir. Med. Rev. 5, 242–262 (2009).
- Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Topfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Heart Fail. 9(3), 251–257 (2007).
- Deegan PC, McNicholas WT. Pathophysiology of obstructive sleep apnea. Eur. Respir. J. 8, 1161–1178 (1995).
- Kohnlein T, Welte T, Tan LB, Elliott MW. Central sleep apnea syndrome in patients with chronic heart disease: a critical review of the current literature. Thorax 57(6), 547–554 (2002).
- Engleman HM, Douglas NJ. Sleep 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax 59(7), 618–622 (2004).
- Morgenthaler TI, Kapen S, Lee-Chiong T et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep 29(8), 1031–1035 (2006).
- Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 176(7), 706–712 (2007).
- Heitmann J, Ehlenz K, Penzel T et al. Sympathetic activity is reduced by nCPAP in hypertensive obstructive sleep apnoea patients. Eur. Respir. J. 23(2), 255–262 (2004).
- Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davision DE, Somers WK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 100, 2332–2335 (1999).
- Ciftci TU, Kokturk O, Demirtas S, Gulbahar O, Bukan N. Consequences of hypoxiareoxygenation phenomena in patients with obstructive sleep apnea syndrome. Ann. Saudi Med. 31(1), 14–18 (2011).
- Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am. J. Respir. Crit. Care Med. 169(3), 348–353 (2004).
- Ohike Y, Kozaki K, Iijima K et al. Amelioration of vascular endothelial dysfunction in obstructive sleep apnea syndrome by nasal continuous positive airway pressure – possible involvement of nitric oxide and asymmetric NG, NG-dimethylarginine. Circ. J. 69(2), 221–226 (2005).
- Lavie L, Kraiczi H, Hefetz A et al. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am. J. Respir. Crit. Care Med. 165(12), 1624–1628 (2002).
- Schulz R, Mahmoudi S, Hattar K et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am. J. Respir. Crit. Care Med. 162(2 Pt 1), 566–570 (2000).
- Hui DS, Ko FW, Fok JP et al. The effects of nasal CPAP on platelet activation in obstructive sleep apnea syndrome. Chest 125(5), 1768–1775 (2004).
- Rangemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep 18(3), 188–194 (1995).
- Reinhart WH, Oswald J, Walter R, Kuhn M. Blood viscosity and platelet function in patients with obstructive sleep apnea syndrome treated with nasal continuous positive airway pressure. Clin. Hemorheol. Microcirc. 27(3–4), 201–207 (2002).
- Chin K, Kita H, Noguchi T et al. Improvement of factor VII clotting activity following long-term NCPAP treatment in obstructive sleep apnea syndrome. QJM 91(9), 627–633 (1998).
- Hui DS, Shang Q, Ko FW et al. A prospective cohort study of the long-term effects of CPAP on carotid artery intima-media thickness in obstructive sleep apnea syndrome. Respir. Res. 13(1), 22 (2012).
- Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7‑year follow-up. Am. J. Respir. Crit. Care Med. 166(2), 159–165 (2002).
- Buchner NJ, Sanner M, Borgel J, Rump LC. Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am. J. Respir. Crit. Care Med. 176(12), 1274–1280 (2007).
- Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest 127(6), 2076–2084 (2005).
- Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J. Am. Coll. Cardiol. 5(14)0, 1310–1314 (2007).
- Barbé F, Duran-Cantolla J, Sanchez-de-la- Torre M et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 307(20), 2161–2168 (2012).
- Peker Y, Glantz H, Thunström E et al. Baseline characteristics of the revascularized patients with coronary artery disease and obstructive sleep apnoea in the RICCADSA trial. Presented at: 20th Annual Congress of the European Society of Respirology. Barcelona, Spain, 18–22 September 2010.
- Hahn PY, Somers VK. Comprehensive Hypertension. Lip GYH, Hall JE (Eds). Elsevier Inc., Oxford, UK (2007).
▪ Pioneering study examining angiographic outcomes in acute coronary syndrome patients.
▪ Large-scale study evidencing cardiovascular outcomes in men with obstructive sleep apnea (OSA) and effects of continuous positive airway pressure treatment.
▪ Quantitative study showing the effects of OSA and continuous positive airway pressure treatment in women.
▪ Pioneering case–controlled study that adjusted for confounding factors, evidencing an independent association between OSA and coronary artery disease.
▪ Pioneering study observing microvascular perfusion in OSA patients with acute myocardial infarction.
▪ Pioneering study investigating the effects of OSA on time onset of sudden death.
▪ Pioneering study evidencing OSA effects on microvascular reperfusion.
▪ Large-scale randomized controlled trial investigating continuous positive airway pressure treatment in nonsleepy OSA patients.
▪ Websites
- Continuous Positive Airway Pressure (CPAP) in Patients With Acute Coronary Syndrome and Obstructive Sleep Apnea (OSA) (ISAACC). http://clinicaltrials.gov/ct2/show/ NCT01335087
- Continuous Positive Airway Pressure (CPAP) Treatment in Coronary Artery Disease and Sleep Apnea (RICCADSA). http://clinicaltrials.gov/ct2/show/ NCT00519597