Short Article - Interventional Cardiology (2011) Volume 3, Issue 5
Off-label use of medical devices in pediatric interventional cardiology: prerogative or necessity?
- Corresponding Author:
- Jamie Sutherell
Saint Louis University School of Medicine, Department of Pediatrics, 1465 South Grand Blvd, St Louis, MO, USA
E-mail: jsuthere@slu.edu
Abstract
Keywords
cardiac devices,interventional cardiology,off-label,pediatric cardiology
It is early morning in a busy pediatric intensive care unit when word comes that an undiagnosed cyanotic neonate is being transferred to the unit. Prostaglandin E1 infusion was started prior to arrival due to the cyanosis but, as of yet, the diagnosis remains uncertain. On arrival, a quick echocardiogram reveals the diagnosis of pulmonary atresia with an intact ventricular septum, and arrangements are made for transfer to the cardiac catheterization laboratory. During the catheterization procedure, the interventional cardiologist perforates the atretic pulmonary valve plate using a radiofrequency perforation wire followed by balloon pulmonary valvuloplasty, establishing antegrade pulmonary blood flow. Prostaglandin E1 infusion is discontinued in an attempt to establish if there will be ‘enough’ antegrade pulmonary blood flow to support adequate oxygenation without the aid of the patent ductus arteriosus; however, over the ensuing days, progressive cyanosis recurs and the prostaglandin E1 infusion is resumed. The child is taken back to the cardiac catheterization laboratory, where a stent is placed into the ductus arteriosus to maintain patency, and the prostaglandin E1 infusion is again stopped. The child is eventually discharged home, with stable pulmonary blood flow achieved antegradely through the perforated atretic pulmonary valve plate, in addition to flow through the patent ductus arteriosus, which is held open with an endovascular stent.
Although the diagnosis of pulmonary atresia with an intact ventricular septum is a rare diagnosis within pediatric cardiology, the previously described scenario is all too common: approved medical devices utilized for indications in pediatrics for which they were not studied and approved. The child described had two interventions performed: radiofrequency perforation of an atretic pulmonary valve plate, which used a device that was studied and approved for perforation of the atrial septum and creation of an atrial septal defect; and stenting of the ductus arteriosus, which utilized a stent that was engineered and approved for use in adults with coronary artery disease.
Children are not simply small adults. Those who work in the field of pediatrics live by this mantra. Cardiovascular physiology in the child is a dynamic process, beginning with the transition from fetal to postnatal life, and eventually progressing from adolescent to mature adult cardiac physiology. Along this continuum are profound changes in physiologic variables such as heart rate, blood pressure, and pulmonary and systemic vascular resistance, in addition to the absolute size of the organ and vasculature. However, many of the ‘cutting edge’ therapies are simply translated from adult research and adult studies for use in pediatric medicine. This extrapolation from adult data is inevitable to an extent; the ethics and availability of conducting adequately powered randomized trials in children is fraught with challenge. The end result is many medications and medical devices, which have been studied and approved for specific uses in adult patients, are applied to the clinical scenarios encountered by pediatric practitioners. While this ‘off-label use’, in which an approved medication or medical device is used for an indication outside of its labeled indication, is not unique to pediatric practice, it is simply a way of life in pediatric medicine. This is certainly within the realm of what the US FDA defines as good medical practice: ‘the interests of the patient require that the physicians use legally available drugs, biologics and devices according to their best knowledge and judgment’ [1,2]. Regardless of the device’s labeled indication, physicians have the prerogative to legally use a device to treat any condition deemed to be medically appropriate [3,4]. But is this ideal? Should pediatric practitioners be forced to adapt therapeutics studied and approved in adults for use in very different patients? This discussion will address the topic of off-label use in pediatrics, focusing on interventional cardiac devices and define the drawbacks to this practice. In addition, the strides the FDA and governing bodies have taken to remedy the situation will be discussed.
Scope of off-label use
There is extensive literature discussing and defining the off-label use of medications in pediatric patients [5–10]. The majority of medications used in pediatrics are prescribed offlabel – approximately 75% of all FDA-approved medications licensed since the early 1970s have limited or no labeling in children [11,12]. In the pediatric intensive care unit, 67% of medications administered had limited or no FDA approval for use in that setting [13]. Focusing on cardiac medications, 78% of children hospitalized with congenital and acquired heart disease received at least one medication that was used for an off-label indication [14]. By no means is this situation unique to pediatrics: 21% of all medications prescribed in an ambulatory internal medicine setting were done so for off-label indications [15]. At least one approved drug is prescribed off-label for the majority of adult cancer and AIDS patients [16]. Although a common practice in adult medicine, off-label use of medications occurs much more frequently in pediatrics.
In comparison to the well-described off-label use of medications in pediatrics, relatively little literature exists detailing the frequency and nature of off-label device use within interventional pediatric cardiology. Sutherell, Hirsch, and Beekman documented the prevalence of off-label cardiac device use over a recent 3-year span (1 July 2005–30 June 2008) [17]. An approved device was utilized for an off-label indication in 63% of their 473 patients, and among a total of 595 transcatheter interventions performed during the study period, 50% of interventions used a device for an off-label indication. The data were further stratified into six device categories: dilation balloons, occlusion devices, embolization coils, stents, septostomy catheters and ‘other’ devices. ‘Other’ devices were those that were very rarely used during the study period, such as inferior vena cava filters and rotational coronary atherectomy catheters. Within these device types, virtually all stent procedures (99%) were performed for off-label indications, and the majority of dilation balloons (78%) were also utilized for off-label indications. Contrast this to the other device types, in which septostomy balloons, occlusion devices and embolization coils were generally used for on-label indications (100, 92 and 71% respectively), this confirmed what was expected: off-label use of approved medical devices is a common practice within interventional pediatric cardiology, and the data shed light onto specific areas within the field where off-label use is most common.
It should be noted that there is a fundamental difference between the labeling of medications in pediatrics versus the labeling of cardiac devices in this patient population. Approved indications for medical devices in the USA specify procedures, not patient ages; the majority of device labels do not mention use in children at all. This is in contrast to the labeling of medications in the pediatric population, in which labels almost always specify ages for approved use.
Drawbacks to off-label use of medical devices
While the practice of off-label use of medical devices within the field of pediatric interventional cardiology is common practice, it should be cautioned that the regular use of these devices for off-label indications has clear drawbacks. Consideration must be made for device safety and performance, entrepreneurial development, and educational or training issues.
▪ Safety & performance
The FDA licenses an ‘indication for use’ label for each medical device, and is responsible for overseeing the initial use and sale of all medical devices. Once a device has a specific labeled indication, the FDA generally is relegated to a limited role in overseeing the device’s ongoing use [18]. However, device malfunctions and significant adverse events require FDA notification based on the Safe Medical Devices Act [19]. In specific circumstances, the FDA may require further study of a device following marketing approval. These ‘postmarket’ studies can provide additional data regarding device safety and effectiveness for the labeled indication. Postmarket studies are only carried out for approved device indications; when a device is used off-label, the device will not be subject to the FDA’s scrutiny to ensure safety and efficacy.
Devices go through a rigorous investigational process in order to ensure safety and efficacy prior to approval. The FDA classifies medical devices on the basis of their degree of risk to the patient. Class I devices pose very little risk to patients, and often do not require prospective FDA analysis prior to marketing. Examples of class I devices are surgical instruments such as retractors. Class II devices are defined as posing moderate risk to patients, and prior to marketing the product the manufacturer is often required to submit a marketing application, or a 510(k), to the FDA. The intention of the 510(k) is to demonstrate noninferiority of the new device to a similar established device in terms of safety and efficacy. Examples of class II devices include guide wires and infusion catheters. Finally, class III devices are considered by the FDA to pose the highest risk to patients, and often require a premarket approval application that includes scientific and clinical data prior to marketing the device. Examples of class III devices are septal occluder devices and endovascular stents.
There are exceptions to the normal rigor imposed by the FDA in its approval processes. For devices that have not yet been cleared for marketing by the FDA, an investigational device exemption protocol can be created in which specific terms are defined under which that device can be legally used. For rare diseases, defined as less than 4000 patients in the USA per year, a class III device can be marketed through a humanitarian device exemption.
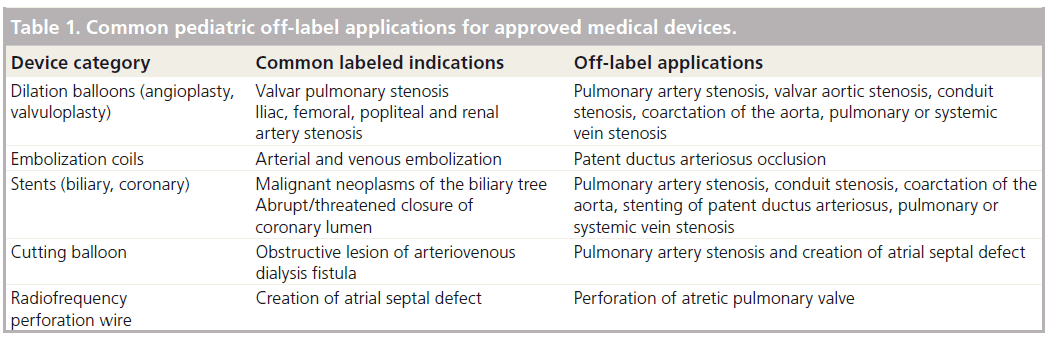
In spite of the FDA’s licensed indications, the use of an approved medical device for an off-label indication falls within the bounds of what is deemed acceptable standard of care in the USA. Table 1 lists common devices and their labeled indications, along with applications for which they are commonly utilized in an off-label fashion. However, medical devices may not have the same excellent performance characteristics and safety profiles when they are used for an indication in which they were not designed or tested. There are multiple reports of devices performing suboptimally when used off-label; two examples are stents and embolization coils, both of which are commonly used for off-label indications.
Table 1: Common pediatric off-label applications for approved medical devices.
Stents are routinely used in interventional pediatric cardiology to open stenotic branch pulmonary arteries and stenotic conduits. The stents typically used are approved for treating malignant neoplasms of the biliary tree, a diagnosis with disparate pathophysiology from that with which pediatric cardiologists employ them. When used in great vessels, stents may result in vascular injury, be a nidus for thrombus formation, or may circumferentially fracture, which can result in stent embolization or reocclusion of the stenotic vessel [20,21].
A second example is provided by stainless steel coils that are approved for arterial and venous embolization, and for embolization of arteriovenous fistulae. Yet they are commonly used off-label to occlude a small patent ductus arteriosus, an application for which the coils were neither developed nor tested and whose pathophysiology is disparate. There are multiple reports within the literature of coils embolizing to systemic and pulmonary arteries, intravascular hemolysis and residual shunts occurring in a substantial number of patients [22–24]. Clinical studies have demonstrated that using devices specifically designed for closing the patent ductus have a significantly better performance profile than coils [25]. In spite of these data, the small patent ductus arteriosus is still commonly closed by stainless steel coils due to lack of appropriately sized approved devices to achieve closure. A device used for the indication in which it was approved has a better performance characteristic than a device used for an indication outside of which it was studied.
▪ Development
In contrast to diseases in adults, pediatric disease processes are more commonly acute than chronic, and when chronic conditions do occur in pediatric patients, they are often rare [26]. For example, the population of children with congenital heart disease is much smaller than the population of adults with acquired heart disease. In addition to being relatively uncommon, the congenital cardiac defects that afflict children are themselves diverse. As a consequence, in comparison to adults, there is a relatively small patient population available for enrollment into appropriately powered randomized clinical trials to assess device safety and efficacy for cardiac disease in children. The end result is a paucity of data available for FDA review when considering approval of devices for specific pediatric cardiovascular applications [27].
Another consequence of a small population size is a limited return on investment for entrepreneurial development from industry. In short, if there is limited market potential, then there will be limited research into the development of novel cardiac devices for children. Congenital heart disease is a prime example of a relatively uncommon group of disorders for which targeted, disease-specific therapies have been only infrequently developed.
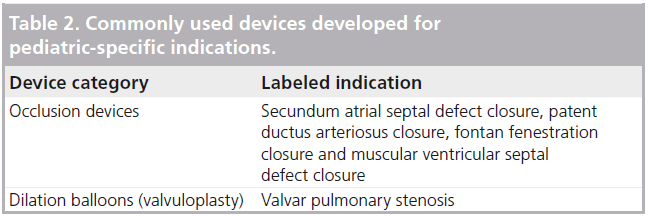
This is not to say that there have not been devices developed specifically for pediatric patients with congenital heart disease. Although limited in number, there are examples of cardiac devices designed, tested and approved in the USA specifically for treatment of congenital heart defects in pediatric patients. Table 2 lists devices that are commonly utilized in pediatric populations for their labeled indications. These include occlusion devices for closure of the secundum atrial septal defect, muscular ventricular septal defect and patent ductus arteriosus. More recently, a transcatheter pulmonary valve was approved by the FDA for use in pediatrics in 2010 [101]. Despite these advances, there remains a substantial unmet need in the review and approval of device-specific indications for pediatric conditions. With regards to device categories, this unmet need is greatest for stents and dilation balloons.
▪ Education & training
With the use of new innovative devices, educational strategies have been developed to provide training for practitioners utilizing the new technology. As part of the training program for the Cordis stent which was approved by the FDA in 2004 for the treatment of carotid artery disease, a simulation component was designed to augment device education for the utilizing physician. Concomitantly, the FDA approved the substitution of simulated cases for proctored cases in training protocols for the Cordis stent [28]. When rolling out its new product, Filterware™, Boston Scientific Corporation utilized medical simulation in the training program for over 500 physicians. Within pediatric interventional cardiology, AGA Corporation, the makers of Amplatzer® occlusion devices, designed simulator-based case training to be used as a substitution for proctored cases. For the physician learning the procedure, every step can be practiced, from inserting the delivery system to deploying the device to verifying device positioning. This allows for not only practicing and gaining comfort with the proper techniques for device usage, but also for dealing with errors in device deployment, all within a ‘safe’ educational environment. Edwards Lifesciences has established a similar simulation-based training program for use with the percutaneously implanted aortic stent valves.
As technology continues to advance and new devices are approved for broader uses, continuing education on proper device use including techniques in deployment is imperative. Carroll and Messenger stated ‘It is likely that the major forces for implementing simulation will come from the medical device industry, which will need acceleration in training on new products’ [28]. Yet when devices are used off-label, this educational safety net is not available. Simulation experiences or educational protocols are not designed for devices used outside their labeled indications. According to FDA policy, manufacturers cannot promote or advocate their devices for off-label use. Thus, industry cannot design effective educational protocols for devices that are being used beyond their established indications.
Future perspective
In 2004, the US Health and Human Services and the FDA recommended performance of a comprehensive needs assessment to determine the best mechanism to promote the development of pediatric-specific devices. The Health and Human Services and FDA reported to Congress, ‘it is clear that further study is warranted to evaluate the scope of unmet needs’ of pediatric medical devices [27]. The response from Congress in 2007 was the passage of the Pediatric Medical Device Improvement and Safety Act (PL-110–85), which sought to stimulate industry by removing profit prohibitions on devices approved through the humanitarian device exemption pathway. The act also gave the FDA authority to improve postmarket safety monitoring of devices, and encouraged the creation of nonprofit consortia to stimulate innovation in the development of medical devices for children. The intention of this legislation was to encourage and foster the development of cardiac devices specifically for children with congenital heart disorders, and begin to move away from relying so heavily on the off-label use of devices. In the fall of 2010, with the sponsorship of the FDA, the NIH, the American College of Cardiology, and the Society for Cardiovascular Angiography and Interventions, the American Academy of Pediatrics sponsored a workshop titled ‘Optimizing Clinical Trial Design for the Development of Pediatric Cardiovascular Devices.’ This workshop was attended by cardiologists, scientists, representatives of industry and government regulators and proved to be a valuable forum for focusing on children’s cardiac device needs.
Beyond legislation, enhanced postmarket surveillance and national workshops, two additional solutions to the problem of the frequency of off-label use include the use of objective performance criteria (OPC) or performance goals (PG), and the use of registry data. An OPC or PG is an estimate of an acceptable performance for a device created to determine whether a new application can be marketed for that given device. It is based on historical data, and replaces the gold standard randomized, controlled clinical trial (RCCT) as an alternative pathway to evaluate whether a device meets criteria for approval. In small, diverse pediatric populations, conducting an adequately powered RCCT may be statistically impossible. OPC’s and PG can augment the data where a RCCT is lacking in achieving safe, efficacious use of approved devices for new applications. This has been demonstrated by the use of an OPC in the approval process for the patent ductus arteriosus occluder device [29].
A second possible solution is the creation and use of registry data. A prospective registry dataset can be created to provide key clinical information and long-term longitudinal followup data on devices that are being used for offlabel indications. These datasets can then be evaluated by the FDA to support review and approval processes for new applications. An example of a congenital heart disease-specific registry is the Improving Pediatric and Adult Congenital Treatment (IMPACT) registry [30]. IMPACT was created by the American College of Cardiology in 2011 as a national registry of pediatric cardiac and congenital catheter-based procedures, whose role is to serve as a resource for pediatric and congenital interventional cardiologists to guide and improve treatment on a national level.
For those involved in the care of children, it is very encouraging and exciting to see the attention their specific needs are receiving on a national level, and it is hoped that these developments will usher in the dawn of a new age in pediatric interventional cardiology. Future devices used by pediatric practitioners ideally will be engineered and evaluated specifically for the pediatric patient and their specific conditions. The innovations imparted by using approved cardiac devices for off-label indications will never be entirely replaced. However, children deserve more than simply extrapolating data from adult medicine.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
The scope of off-label use
▪▪ There is an extensive literature defining the off-label use of medications in pediatric patients.
▪▪ Recent documentation demonstrated that off-label device use in pediatric interventional cardiology is very common, particularly for stent procedures and dilation balloons.
▪▪ Approved indications for medical devices in the USA specify procedures, not patient ages.
Drawbacks of off-label use of medical devices include patient safety, entrepreneurial development & educational support
▪▪ Safety and performance: the use of an approved medical device for an off-label indication falls within the bounds of acceptable practice; however, medical devices may not have the same excellent performance characteristics when used for an off-label indication.
▪▪ Development: due to multiple factors, there is limited motivation for industry entrepreneurial development of pediatric-specific devices, especially when the ‘standard of care’ in many circumstances has become the off-label use of approved devices.
▪▪ Education and training: industry is limited in the design of effective educational protocols for devices that are being used beyond their approved indications.
The future: legislation & organizational programs
▪▪ The Pediatric Medical Device Improvement and Safety Act (PL-110–85, 2007) was created to encourage the development of cardiac devices specifically for children with congenital heart disease and improved US FDA postmarket safety monitoring pathways.
▪▪ National workshops have proven to be valuable forums for focusing on children’s cardiac device needs.
▪▪ Objective performance criteria or performance goals can augment the data available for FDA review in achieving safe, efficacious use of approved devices for new applications.
▪▪ Registry data such as the IMPACT registry can serve as a resource for pediatric and congenital interventional cardiologists to guide and improve treatment on a national level.
References
Papers of special note have been highlighted as:
▪ of interest
- Grines CL. Off-label use of drug-eluting stents putting it in perspective. J. Am. Coll. Cardiol. 251, 607–614 (2008).
- Russel ME, Friedman MI, Mascioli SR et al. Off-label use: an industry perspective on expanding use beyond approved indications. J. Interv. Cardiol. 19, 432–438 (2006).
- Ansel GM, Jaff MR. The ‘art’ of medicine and the ‘smokescreen’ of the randomized trial off-label use of vascular devices. Catheter Cardiovasc. Interv. 72, 998–1002(2008).
- Price MJ, Teirstein PS. The off- versus on-label use of medical devices in interventional cardiovascular medicine: clarifying the ambiguity between regulatory labeling and clinical decision-making, Part I: PCI. Catheter Cardiovasc. Interv. 72, 500–504 (2008).
- Hsien L, Breddemann A, Frobel AK et al. Off-label drug use among hospitalised children: identifying areas with the highest need for research. Pharm. World Sci. 30, 497–502 (2008).
- Jain SB, Bavdekar SB, Gogtay NJ et al. Off-label drug use in children. Indian J. Pediatr. 75, 1133–1136 (2008).
- Kumar P, Walker JK, Hurt KM et al. Medication use in the neonatal intensive care unit: current patterns and off-label use of parenteral medications. J. Pediatr. 152, 412–415 (2008).
- Shah SS, Hall MH, Goodman DM. Off-label drug use in hospitalized children. Arch. Pediatr. Adolesc. Med. 161, 282–290(2007).
- Sturkenboom MCJM, Verhamme KMC, Nicolosi A et al. Drug use in children: cohort study in three European countries. Brit. Med. J. 337, a2245 (2008).
- Tafuri G, Trotta F, Leufkens HGM et al. Off-label use of medicines in children: can available evidence avoid useless pediatric trials? Eur. J. Clin. Pharmacol. 65, 209–216 (2009).
- Eiland LS, Knight P. Evaluating the off-label use of medications in children. Am.J. Health-Syst. Pharm. 63, 1062–1065(2006).
- Schirm E, Tobi H, de Jong-van den Berg LT. Risk factors for unlicensed and off-label drug use in children outside the hospital. Pediatrics 111, 291–295 (2003).
- Yang CP, Veltri MA, Anton B et al. US FDA approval for medications used in the pediatric intensive care unit: a continuing conundrum. Pediatr. Crit. Care Med. 12(5), e195–e199 (2010).
- Pasquali SK, Hall M, Slonim AD et al. Off-label use of cardiovascular medications in children hospitalized with congenital and acquired heart disease. Cir. Cardiovasc. Qual. Outcomes 2, 69–71 (2008).
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch. Intern. Med. 166, 1021–1026 (2006).
- Tabbarock A. From off-label prescribing towards a new FDA. Med. Hypotheses 72, 11–13 (2009).
- Sutherell JS, Hirsch R, Beekman RH. Pediatric interventional cardiology in the USA is dependent on the off-label use of medical devices. Congenit. Heart Dis. 5, 2–7 (2010).
- Stafford RS. Regulating off-label drug use: rethinking the role of the FDA. NEJM 358, 1427–1429 (2008).
- Beekman RH, Duncan BW, Hagler DJ et al. Pathways to approval of pediatric cardiac devices in the USA: challenges and solutions. Pediatrics 124, e155–e162 (2009).
- McElhinney DB, Bergersen L, Marshall AC. In situ fracture of stents implanted for relief ofpulmonary arterial stenosis in patients with congenitally malformed hearts. Cardiol. Young 18, 405–414 (2008).
- Breinholt JP, Nugent AW, Law MA et al. Stent fractures in congenital heart disease. Catheter Cardiovasc. Interv. 72, 977–982 (2008).
- Shim D, Fedderly RT, Beekman RH et al. Follow-up of coil occlusion of patent ductus arteriosus. J. Am. Coll. Cardiol. 28, 207–211 (1996).
- Magee AG, Huggon IC, Seed PT et al. Transcatheter coil occlusion of the arterial duct; results of the European Registry. Eur.Heart J. 22, 1817–1821 (2001).
- Wang JK, Liau CS, Huang JJ et al. Transcatheter closure of patent ductus arteriosus using Gianturco coils in adolescents and adults. Catheter Cardiovasc. Interv. 55, 513–518 (2002).
- Gudausky TM, Hirsch R, Khoury PR et al. Comparison of two transcatheter device strategies for occlusion of the patent ductus arteriosus. Catheter Cardiovasc. Interv. 72, 675–680 (2008).
- Milne CP, Bruss JB. The economics of pediatric formulation development for off-patent drugs. Clin. Ther. 11, 2133–2145 (2008).
- US Department of health and human services and food and drug administration. Barriers to the availability of medical devices intended for the treatment or diagnosis of diseases and conditions that affect children. Report to Congress (2004).
- Carroll JD, Messenger JC. Medical simulation. Perspect. Biol. Med. 51, 47–60 (2008).
- Proposed standards for clinical evaluation of patent ductus arteriosus occlusion devices. Multiorganization advisory panel to FDA for pediatric cardiovascular devices. Catheter Cardiovasc. Interv. 51, 293–296 (2000).
- Martin GR, Beekman RH, Ing FF et al. The IMPACT registry: improving pediatric and adult congenital treatments. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Ann.13, 20–25 (2010).
- US FDA. New Humanitarian Device Approval. www.accessdata.fda.gov/cdrh_docs/pdf8/ H080002b.pdf
▪ Presents arguments against the restriction of off-label use of vascular devices based, in part, on the difficulty of randomized trials stemming from interventional peripheral vascular disease. Counter argument to limiting off-label use.
▪ Large multicenter retrospective cohort study performed in the USA to evaluate the extent of off-label drug use in pediatric inpatients and to identify drugs most commonly used off-label.
▪ First study to document the prevalence and nature of off-label device use in pediatric interventional cardiology.
▪ Provides an overview of the US FDA approval process, including recent legislation and possible solutions to provide novel approval pathways for off-label products in pediatrics.
▪ Report of US Department of health and human services and FDA to Congress addressing the approval process for medical devices in pediatrics, including a discussion on the major issues surrounding pediatric device development and approval.