Research Article - Interventional Cardiology (2020) Volume 12, Issue 2
One Year Outcomes of Orbital versus Rotational Atherectomy for the Treatment of Heavily Calcified Coronary Disease
- Corresponding Author:
- Anbukarasi Maran
Department of Medicine,
Cardiology,
Medical University of South Carolina,
Charleston, SC, USA
E-mail: maran@musc.edu
Received date: March 09, 2020; Accepted date: March 17, 2019; Published date: March 25, 2019
Abstract
Objectives: Compare long term outcomes between orbital versus rotational atherectomy for the treatment of heavily calcified coronary disease at a single institution.
Background: Plaque modification with atherectomy facilitates stent delivery and optimization in severely calcified coronaries. Rotational Atherectomy (RA) (Boston Scientific) has been in use for several decades while orbital atherectomy (OA) (CSI Diamondback 360®) is a newer atherectomy device that is rapidly gaining momentum. Small trials and meta-analyses have compared these 2 devices; however, longer term outcomes have not been evaluated.
Methods: We retrospectively identified 75 patients who underwent RA or OA, and had at least 1-year follow up, at a single veteran center from March 2016 to October 2017. The primary endpoint was 1-year major adverse cardiac and cerebrovascular events (MACCE) (composite of all-cause mortality, myocardial infarction (MI), target vessel revascularization (TVR), and stroke). The secondary endpoint was cardiovascular death at 1 year.
Results: Out of 75 patients, 46 underwent 54 unique RA procedures and 28 underwent 28 unique OA procedures. More patients in the RA group had a prior MI (45.8% vs. 20.7%, p=0.03). Otherwise, baseline demographics and comorbidities were similar in both groups. Procedural success was achieved in all patients. Angiographic complications were uncommon. There was no statistically significant difference in the primary endpoint at one year between RA and OA groups (26% vs. 11%, p=0.14), respectively, as well as the individual components of all-cause-mortality (13% vs. 7%, p=0.70), MI (11% vs. 0%, p=0.15), TVR (13% vs. 7%, p=0.71), and stroke (0% vs. 4%, p=0.38). No significant difference in cardiovascular death was observed at 1 year (9% vs. 4%, p=0.64).
Conclusion: Both RA and OA are safe and effective in treating severe coronary artery calcification as they provide similar outcomes at 1 year
Keywords
Percutaneous coronary intervention • Coronary artery disease • Plaque modification • Coronary atherectomy
Introduction
Coronary artery calcification (CAC) is angiographically present in up to one third of all atherosclerotic lesions, and this prevalence increases to 74% when intravascular ultrasound (IVUS) is used [1]. Percutaneous Coronary Intervention (PCI) of severely calcified vessels is technically challenging and is associated with worse clinical outcomes [2-4]. Furthermore, the higher inflation pressures necessary to achieve appropriate vessel preparation and overcome severe calcific lesions can lead to increased dissections, perforations, and ischemia [5]. CAC impedes optimal stent delivery and expansion and may also damage the drug polymer and stent platform. This all leads to a higher incidence of stent thrombosis and in stent restenosis [6]. No mortality benefit has been proven with atherectomy however, atherectomy remains an important tool in the armamentarium of the interventional cardiologist as it allows for plaque modification and optimization of stent delivery and expansion. The two most commonly used atherectomy modalities are rotational atherectomy (RA) and orbital atherectomy (OA).
Coronary atherectomy with the Rotablator (Boston Scientific, Marlborough, Massachusetts) has been in use since the 1990’s. It employs a diamond-coated elliptical burr with sizes ranging from 1.25 mm to 2.5 mm on its distal tip and is advanced linearly along a 0.009” RotaWire. Thus, the Rotablator system burr only has one axis of rotation on the RotaWire and ablates a fixed diameter. The older RA system required the operator to simultaneously activate the pneumatic gas with a foot pedal, activate fluoroscopy with the other foot and advance the burr manually. These issues with operator balance and synchronization have been reduced with the Rota-Pro Atherectomy system available now. Alternatively, the Diamondback 360º® Coronary Orbital Atherectomy (OA) System (Cardiovascular Systems, Inc., St. Paul, Minnesota) employs a 1.25 mm eccentrically mounted crown advanced over a 0.014” ViperWire. The crown expands its luminal orbit through increased rotational speed. The Orbital system does not require compressed gas or a separate operator, and the crown allows for bidirectional sanding.
Rotational atherectomy is associated with a higher procedural success rate with similar outcomes when compared to Plain Old Balloon Angioplasty (POBA) prior to stent deployment [7]. The PCI guidelines of the American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI) recommend rotational atherectomy in fibrotic or heavily calcified plaques that cannot be crossed by balloon or adequately predilated before stent delivery (Level of Evidence: C) [8]. The OA system, while novel compared to the established RA system, has been shown to have similar outcomes when compared to a historical cohort of rotational atherectomy, including a low rate of target lesion revascularization [9]. Furthermore, several studies have reported similar outcomes between the two modalities at short term follow-up [10-13]. Here, we report outcomes at one year in patients undergoing OA versus RA for the treatment of heavily calcified coronary disease.
Materials and Methods
Consecutive patients who underwent rotational or orbital atherectomy followed by deployment of one or more drug eluting stents at the Ralph H. Johnson VA Medical Center between March 2016 and October 2017, with at least one-year follow-up, were included. Pre-specified clinical, procedural, and laboratory data were retrospectively collected for all patients as approved by the Institutional Review Board at the Ralph H. Johnson VA Medical Center. Data included demographic information, medical history, clinical data, and laboratory indices. Procedural data collected in the database included the amount of contrast injected, total time under fluoroscopy, procedural length, and total number of passes with the atherectomy device. Immediate procedural complications were also noted. All angiograms were reviewed by two interventional cardiologists and syntax scores were calculated. The choice of atherectomy procedure primarily dependent on preference and comfort of the operator. The RA flush solution consisted of 10,000 units of heparin diluted in 1 L of normal saline. The OA flush solution consisted of 20 mL Viper Slide (soybean oil 10%, egg yolk phospholipids 1.2%, glycerin 25% and water), 5 mL nitroglycerin and 2.5 mg verapamil in 1 L of normal saline.
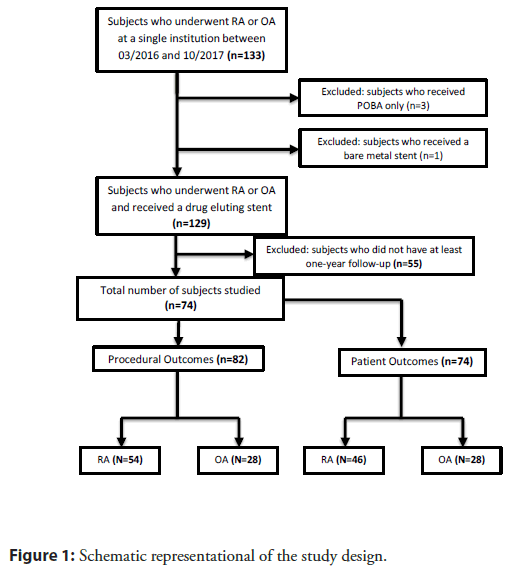
The primary endpoint was 1-year major adverse cardiac and cerebrovascular events (MACCE) (composite of all-cause mortality, myocardial infarction (MI), target vessel revascularization (TVR), and stroke). The secondary endpoint was cardiovascular death at 1 year, which was defined as death resulting from an acute MI, death due to heart failure, death due to stroke, death due to cardiovascular procedures, death due to cardiovascular hemorrhage and death due to other cardiovascular causes. Intra-procedural hypotension was defined as hypotension requiring neosynephrine. Intra-procedural bradycardia was defined as bradycardia requiring atropine, theophylline or a temporary pacer wire. 82 unique atherectomy procedures were identified on 76 patients in this study (Figure 1).
Procedural complications and characteristics were recorded for each unique encounter. Patient outcomes were recorded for each unique patient, and in the cases that required staged PCIs or multiple atherectomies, outcomes were recorded based on the index procedure i.e. first encounter for atherectomy. Continuous variables are presented as mean ± SD or median and interquartile range (IQR) as appropriate and compared using Student’s t-test or Mann-Whitney U test, respectively. Categorical variables are presented as numbers and percentage and compared using a chisquare test or Fisher’s exact test as appropriate. Statistical analysis was performed using SigmaPlot (Systat Software, San Jose, CA).
Results
Our registry included 133 patients who underwent either rotational or orbital atherectomy from March 2016 to October 2017. We identified 129 patients who received drug eluting stents following atherectomy and 75 patients with at least 1-year followup. Of these, 46 patients underwent 54 unique RA procedures and 28 underwent 28 unique OA procedures. Patients in the RA group had a higher percentage of prior myocardial infarction history (p=0.03) otherwise baseline characteristics were similar (Table 1).
| Variable | OA (n=28) | RA (n=46) | p-value |
|---|---|---|---|
| Age (years, mean, SD) | 70.1 (6.8) | 70.7 (7.1) | 0.706 |
| Male | 100 | 100 | |
| White (%) | 19 (68%) | 38 (83%) | 0.164† |
| BMI (mean, SD) | 30.5 (5.1) | 30.3 (5.0) | 0.869 |
| Comorbidities (%) | |||
| COPD | 10 (36%) | 10 (22%) | 0.280† |
| MI History | 6 (21%) | 22 (48%) | 0.028† |
| Type 2 Diabetes Mellitus | 24 (86%) | 30 (65%) | 0.075† |
| Heart Failure | 10 (36%) | 26 (57%) | 0.098† |
| Hyperlipidemia | 26 (93%) | 43 (93%) | - |
| Hypertension | 24 (86%) | 45 (98%) | 0.067† |
| Major Depression | 8 (29%) | 9 (20%) | 0.404† |
| Obstructive Sleep Apnea | 7 (25%) | 13 (28%) | - |
| Peripheral Artery Disease | 6 (21%) | 12 (26%) | 0.783† |
| Hypothyroidism | 3 (11%) | 5 (11%) | 0.766‡ |
| Smoking Status (%) | |||
| Smoker | 10 (38%) | 9 (20%) | 0.171† |
| Former Smoker | 5 (18%) | 8 (17%) | - |
| Never Smoker | 13 (46%) | 29 (63%) | 0.23† |
| Medications (%) | |||
| Aspirin | 17 (61%) | 37 (80%) | 0.104† |
| ACEi/ARB | 19 (68%) | 33 (72%) | 0.796† |
| Beta-Blocker | 24 (86%) | 35 (76%) | 0.383† |
| Statin | 23 (83%) | 42 (91%) | - |
| Clinical Presentation (%) | |||
| Arrhythmia | 3 (11%) | 2 (4%) | 0.360‡ |
| NSTEMI | 3 (11%) | 7 (15%) | 0.733‡ |
| Worsening HF | 6 (21%) | 13 (29%) | 0.591‡ |
| Stable Angina | 2 (7%) | 6 (13%) | 0.702‡ |
| Unstable Angina | 16 (57%) | 20 (44%) | 0.338† |
| STEMI | 0 | 2 (4%) | 0.523‡ |
*Continuous variables show as mean ± SD Student t-test or Mann-Whitney Rank Sum Test as appropriate unless otherwise indicated †: Chi-Square Test; ‡: Fisher Exact Test BMI: Body Mass Index; COPD: Chronic Obstructive Pulmonary Disease; ACEI: Angiotensin Converting Enzyme Inhibitor; NSTEMI: Non ST-elevation Myocardial Infarction; HF: Heart Failure; STEMI: ST-elevation Myocardial Infarction
Table 1: Baseline characteristics.
Procedural characteristics were also similar between groups (Table 2), however patients undergoing RA were more likely to have undergone a prior PCI on the target vessel (11% vs. 41%, p=0.01) with 33% of them undergoing staged PCIs (p=0.001) versus 0% in the OA group. Furthermore, patients undergoing RA were more likely to undergo multivessel atherectomy (31% vs. 0%, p=0.002).
| Variable | OA (n=28) | RA (n=54) | p-value |
|---|---|---|---|
| Radial Access | 19 (69%) | 44 (81%) | 0.267† |
| Circulation (%) | |||
| Left-Dominant | 7 (28%) | 8 (18%) | 0.487† |
| Right-Dominant | 17 (68%) | 34 (76%) | 0.689† |
| Co-Dominant | 1 (4%) | 5 (11%) | 0.410‡ |
| Target Lesion (%) | |||
| LAD | 15 (54%) | 33 (61%) | 0.674† |
| LCX | 6 (21%) | 21 (39%) | 0.178† |
| RCA | 4 (14%) | 14 (26%) | 0.354† |
| Left-Main Intervention | 3 (11%) | 7 (13%) | - |
| Multi-vessel Atherectomy | 17 (31%) | 0 | 0.002† |
| Cardiac Intervention History (%) | |||
| Prior CABG | 4 (14%) | 14 (26%) | 0.354† |
| Prior PCI | 7 (25%) | 29 (54%) | 0.025† |
| Prior PCI on Target Vessel | 3 (11%) | 22 (41%) | 0.011† |
| Staged PCI | 0 | 18 (33%) | 0.001† |
| Atherectomy on Dominant Vessel (%) | 10 (40%) | 21 (48%) | 0.712† |
| Surgical Turndowns | 12 (43%) | 28 (52%) | 0.589† |
| Syntax 1 Score (median, IQR) | 28 [25-40] | 29 [20-38] | 0.734† |
| Syntax 2 PCI Score (mean, SD) | 44 (11) | 47 (13) | 0.375† |
| Procedural Characteristics | |||
| Total Op Time (min, median, IQR) | 101 [85-138] | 94 [75-111] | 0.04† |
| Atherectomy time (sec, median, IQR) | 10 [8-15] | 10 [6-13] | 0.5783† |
| Total Contrast used (mL, median, IQR) | 150 [110-179] | 125 [95-154] | 0.056† |
| Total Fluoro time (sec, median, IQR) | 27 [23-40] | 26 [19-40] | 0.380† |
| Total # of passes (median, IQR) | 5 [3-7] | 3 [2-5] | 0.004† |
| Mechanical Support (%) | |||
| Pre-Atherectomy | 7 (25%) | 2 (4%) | 0.005† |
| Intra-Aortic Balloon Pump | 4 (14%) | 2 (4%) | 0.174† |
| Impella | 3 (11%) | 0 | 0.037‡ |
| Post-Atherectomy | 4 (14%) | 2 (4%) | 0.174† |
| ECMO | 0 | 0 | - |
| Temporary Pacemaker (%) | 0 | 0 | - |
| Heparin Anticoagulation (%) | 28 (100%) | 54 (100%) | - |
*Continuous variables show as mean ± SD Student t-test or Mann-Whitney Rank Sum Test as appropriate unless otherwise indicated †: Chi-Square Test; ‡: Fisher Exact Test; LAD: Left Anterior Descending Artery; LCX: Left Circumflex Artery; RCA: Right Coronary Artery; CABG: Coronary Artery Bypass Grafting; PCI: Percutaneous Coronary Intervention; Op: Operative; Fluoro: Fluoroscopic; ECMO: Extracorporeal Membrane Oxygenation
Table 2: Procedural characteristics.
During the procedure, patients undergoing OA were more likely to receive pre-emptive mechanical support (25%, vs. 4%, p=0.02). Angiographic complications were similarly low in both groups (Table 3), however patients in the OA group had more intraprocedural hypotension (43% vs. 15%, p=0.01).
| Variable | OA(n=28) | RA (n=54) | p-value |
|---|---|---|---|
| Procedural Success (TIMI III Flow) | 28 (100%) | 53 (98%) | - |
| Intraprocedural Events (%) | |||
| Bradycardia | 8 (29%) | 11 (20%) | 0.576† |
| Hypotension | 12 (43%) | 8 (15%) | 0.013† |
| Complications (%) | 3 (11%) | 3 (6%) | 0.406 |
| Dissection | 1 (4%) | 2 (4%) | -‡ |
| Perforation | 0 | 0 | - |
| Slow Flow/No Reflow | 0 | 1 (2%) | -‡ |
*†: Chi-Square Test; ‡: Fisher Exact Test
Table 3: Angiographic complications.
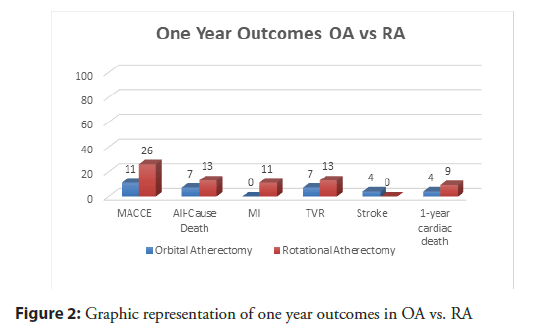
There was no statistically significant difference in the primary endpoint of MACCE at 1 year between RA and OA groups, respectively (26% vs. 11%, p=0.14, Figure 2), neither was there a difference in individual endpoints of all-cause-mortality (13% vs. 7%, p=0.70), MI (11% vs. 0%, p=0.15), TVR (13% vs. 7%, p=0.71), and stroke (0% vs. 4%, p=0.38) (Table 4).
| Variable | OA (n=28) | RA (n=46) | p-value |
|---|---|---|---|
| 1-Year Outcomes (%) | |||
| MACCE | 3 (11%) | 12 (26%) | 0.1425‡ |
| All-Cause Death | 2 (7%) | 6 (13%) | 0.702‡ |
| Myocardial Infarction | 0 | 5 (11%) | 0.1497‡ |
| Target Vessel Revascularization | 2 (7%) | 6 (13%) | 0.7‡ |
| Stroke | 1 (4%) | 0 | 0.3784‡ |
| 1-year cardiac death | 1 (4%) | 4 (9%) | 0.6438‡ |
*†: Chi-Square Test; ‡: Fisher Exact Test; MACCE: Major Adverse Cardiac and Cerebrovascular Events
Table 4: One-year patient outcomes.
There was no statistically significant difference in the secondary endpoint of cardiovascular death (9% vs. 4%, p=0.64) (Table 4). Intraprocedural hypotension did not predict MACCE (HR 0.76 95% CI [0.217-2.72], p=0.674) or cardiac death (HR 1.994 95% CI [0.33-11.94], p=0.464) at 1 year (data not presented).
Discussion
Atheroablative modalities such as Orbital and Rotational atherectomy are often needed in the catheterization laboratory to facilitate stent delivery in target vessels with severe coronary artery calcification. There is limited evidence to suggest the use of one modality over another. Here, for the first time, we compare oneyear outcomes of RA versus OA.
Similar procedural efficacy and safety was observed between OA and RA modalities in our study, in agreement with other studies [10-13]. Although a higher rate of coronary dissections [12,14,15] and perforations [15] was observed with OA in other studies, no significant difference in dissections (4% vs. 4%) and no perforations were observed in our cohort. Total operative time was longer in the OA group by a median of 10 minutes; however there was no significant difference between groups in atherectomy time and total time under fluoroscopy. A greater incidence of transient hypotension requiring neosynephrine intra-procedurally was seen in the OA group (43% vs. 15%, p=0.013). This difference can likely be accounted for by the added nitroglycerin in the OA flush solution and did not appear to affect long term outcomes.
There was no difference in intraprocedural bradycardia in OA (29%) and RA (20%) groups and no temporary pacemakers were required or used prophylactically in our study. Although there was a higher trend towards the primary endpoint at one-year in the RA group, this difference was not statistically significant (26% vs. 11%, p=0.14). No statistical significance was observed in respective individual end points of death (13% vs. 7%), TVR (13% vs. 7%) and MI (11% vs. 0%) in the RA and OA groups. Approximately 49% of patients in this cohort presented after they were deemed at prohibitive surgical risk for coronary artery bypass grafting (CABG). Although not statistically significant, the higher incidence of surgical turndowns in the RA group (70% vs. 50%) and higher prevalence of patients with prior CABG (26% vs. 14%), may in part explain the observed increased trend in MACCE in the RA group. Furthermore, patients that underwent RA were more likely to have had a prior myocardial infarction (48% vs. 21%, p=0.0279) in our cohort.
No statistical difference in syntax score was observed between groups. Although more patients in the OA group received prophylactic mechanical support (25% vs. 4%, p=0.005), there was no statistical difference in mechanical support requirements after atherectomy, suggesting this may have been operator dependent. Overall, we did not find any statistically significant differences at one year in MACCE or its individual components of all cause death, myocardial infarction, target vessel revascularization, or stroke between the OA and RA groups. RA and OA have several operating differences that may contribute to clinically observed complications. For example, due to the mechanics of linear advancement and constant contact with luminal plaque, RA lends itself to such risks as burr entrapment, thermal injury, and increased platelet aggregation [16-19]. Slower burr speeds to the recommended 140,000 to 150,000 rpm and gradual, intermittent advancement with a pecking motion are recommended to counteract these risks [17,19,20].
Furthermore, OA has a smaller average particle size of 2 um than the larger 5 um particle size of RA. This is thought to be a contributor to the higher reported slow-flow/no reflows in RA of 2-20% versus the lower 0.9% rate observed in the ORBIT II trial [21-23]. The use of RA burrs to match the diameter of target vessels may have historically led to impeded distal flow, a potential complication that has been successfully negated with implementation of short runs and using a single burr sized to 0.5- 0.6 of the reference vessel diameter [19]. In our small, retrospective analysis one case (2%) of slow flow/no-reflow was reported in the RA group and none in the OA group (p=not significant). All in all, OA and RA demonstrated similar safety and efficacy with similar peri-procedural complications and similar clinical outcomes at one year follow up.
Limitations
This was a single-center retrospective study with a small sample size. The data was non-randomized and angiographic follow up was not performed on all patients. Furthermore, many patients from the original cohort were lost to follow up, which may have biased the results.
Conclusion
While orbital atherectomy presents some theoretical as well as procedural advantages specifically pertaining to bidirectional single burr use that is 6 French compatible, higher dissection and perforation risk has been described in the literature. In our small retrospective study, no significant difference in complications was noted between the RA and OA groups and one-year outcomes were similar. Atherectomy continues to be a useful and necessary tool for plaque modification of severely calcified vessels prior to stenting. There are no data to suggest superiority of either atherectomy modality or atherectomy choice remains operator dependent. More large scale and prospective studies are needed to guide atherectomy choice.
Conflicts of interest
There are no conflicts of interest.
References
- Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 91(7): 1959-1965 (1995).
- Goel R, Garg P, Achenbach S, et al. Coronary artery calcification and coronary atherosclerotic disease. Cardiol Clin. 30(1): 19-47 (2012).
- Lee MS, Shah N. The Impact and Pathophysiologic Consequences of Coronary Artery Calcium Deposition in Percutaneous Coronary Interventions. J Invasive Cardiol. 28(4): 160-167 (2016).
- Lee MS, Yang T, Lasala J, et al. Impact of coronary artery calcification in percutaneous coronary intervention with paclitaxel-eluting stents: Two-year clinical outcomes of paclitaxel-eluting stents in patients from the ARRIVE program. Catheter Cardiovasc Interv. 88(6): 891-897 (2016).
- Fitzgerald PJ, Ports TA, Yock PG. Contribution of localized calcium deposits to dissection after angioplasty. An observational study using intravascular ultrasound. Circulation. 86(1): 64-70 (1992).
- Vavuranakis M, Toutouzas K, Stefanadis C, et al. Stent deployment in calcified lesions: can we overcome calcific restraint with high-pressure balloon inflations? Catheter Cardiovasc Interv. 52(2): 164-172 (2001).
- Abdel-Wahab M, Richardt G, Büttner JH, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 6(1): 10-19 (2013).
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 58(24): e44-122 (2011).
- Lee M, Généreux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 18(4): 261-264 (2017).
- Meraj PM, Shlofmitz E, Kaplan B, et al. Clinical outcomes of atherectomy prior to percutaneous coronary intervention: A comparison of outcomes following rotational versus orbital atherectomy (COAP-PCI study). J Interv Cardiol. 31(4): 478-485 (2018).
- Lee MS, Park WK, Shlofmitz E, et al. Comparison of Rotational Atherectomy Versus Orbital Atherectomy for the Treatment of Heavily Calcified Coronary Plaques. Am J Cardiol. 119(8): 1320-1323 (2017).
- Koifman E, Garcia HM, Kuku KO, et al. Comparison of the Efficacy and Safety of Orbital and Rotational Atherectomy in Calcified Narrowings in Patients Who Underwent Percutaneous Coronary Intervention. Am J Cardiol. 121:934-939 (2018).
- Goel S, Ravi Teja P, Srilekha C, et al. Orbital atherectomy versus rotational atherectomy: A systematic review and meta-analysis. Int J Cardiol. 303:16-21 (2020).
- Kini AS, Vengrenyuk Y, Pena J, et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. 86(6): 1024-1032 (2015).
- Okamoto N, Ueda H, Bhatheja S, et al. Procedural and one-year outcomes in patients treated with rotational versus orbital atherectomy with mechanistic insights using optical coherence tomography. J Am Coll Cardiol. 71(11): Supplement (2018).
- Sulimov DS, Abdel-Wahab M, Toelg R, et al. Stuck rotablator: the nightmare of rotational atherectomy. Euro Intervention. 9(2): 251-258 (2013).
- Whitlow PL, Bass TA, Kipperman RM, et al. Results of the study to determine rotablator and transluminal angioplasty strategy (STRATAS). Am J Cardiol. 87(6): 699-705 (2001).
- Reisman M, Shuman BJ, Dillard D, et al. Analysis of low-speed rotational atherectomy for the reduction of platelet aggregation. Cathet Cardiovasc Diagn. 45(2): 208-214 (1998).
- Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy. JACC Cardiovasc Interv. 7(4): 345-53 (2014).
- Safian RD, Feldman T, David WM, et al. Coronary angioplasty and Rotablator atherectomy trial (CARAT): immediate and late results of a prospective multicenter randomized trial. Catheter Cardiovasc Interv. 53(2): 213-20 (2001).
- Sakakura K, Funayama H, Taniguchi Y, et al. The incidence of slow flow after rotational atherectomy of calcified coronary arteries: A randomized study of low speed versus high speed. Catheter Cardiovasc Interv. 89(5): 832-840 (2017).
- Kini A, Jonathan D, Srinivas D, et al. Rotational atherectomy: improved procedural outcome with evolution of technique and equipment. Single-center results of first 1,000 patients. Catheter Cardiovasc Interv. 46(3): 305-311 (1999).
- Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 7(5): 510-518 (2014).