Research Article - Clinical Practice (2018) Volume 15, Issue 3
One-stage endovascular management of penetrating thoracic aorta ulcer accompanied by multisupraarch branches stenosis and brainstem infarction: A case report and literature review
- Corresponding Author:
- Lefeng Qu
Department of Vascular and Endovascular Surgery
Changzheng Hospital, Second Military Medical University, Shanghai, China
E-mail: qulefeng@smmu.edu.cn
Abstract
Keywords
aortic arch, penetrating aortic ulcer, carotid artery stenosis, thoracic endovascular aortic repair, in situ fenestration
Abbreviation
CTA: Computed tomography angiography; PAU: Penetrating aortic ulcer; LSA: Left subclavian artery; VA: vertebral artery
Introduction
Classified as acute aorta syndrome, acute thoracic aortic diseases, including dissection, aneurysm, penetrating ulcer, and intramural hematoma, necessitate emergent surgery intervention, especially for patients with simultaneous supraarch pathologies [1-3]. During the past decades, open surgical therapy and hybrid procedure that combine endovascular repair with supraaortic trunk surgery may be treatments of choice in conventional algorithm for proximal thoracic aortic disease with aorta branches lesions. Examples include total arch replacement with bypass or transposition of the branches, fluoroscopy-guided aneurysm exclusion combined with retrograde fenestration for surgically dissected aorta branches [4,5]. However, due to the elevated frequency of injuries and cerebro-vascular complications of hospitalized patients and hence the elongating recovery intervals among them, the usage of open surgery and hybrid procedure for selected patients is limited.
Although thoracic endovascular aortic repair (TEVAR) has emerged as a promising, non- invasive modality for thoracic aortic disease and achieved technical advances [6], endovascular manipulation of concomitant multi-supraarch pathologies remains to be elucidated especially when the characteristic of lesions, vascular anatomy, and the state of illness differ. Thoracic aorta disease is usually accompanied by insufficient proximal endograft landing zones and progressive with lumen dilated, and thus poses a great difficulty for endovascular repair. Therefore, the adequate proximal sealing zone, reservation of perfusion into aorta branches, the prevention of endoleak and translocation become essential components of satisfying treatment results during the implementation of TEVAR.
Left subclavian artery (LSA) coverage has been recommended as an indication in order to acquire adequate proximal landing zone [7]. As the most significant ingredient of performing TEVAR, additional endograft revascularization of the supraarch vessels was thought to be a viable alternative to extend the availability of TEVAR. The present techniques evolve with the advancement of chimney techniques, branch stent or fenestrated grafts, and in situ fenestration (ISF) [8]. These procedures are generally adopted in supraarch vasculature preservation during TEVAR, indicating high rate of technical success and relatively good follow-up results. However, chimney technique has a high risk of endoleak. Branched aortic endograft and in vitro fenestration, which mandate customization before endorepair, may not be utilized in emergent situation.
ISF, as a less invasive option, can be performed in urgent cases, such as a progressive illness state predisposed to stroke, and possible lesion-related complication including bleeding and rupture [9]. As reported in previous cases, laser-generated in situ fenestration, as an off-label procedure, may obtain desirable outcome with LSA revascularized during TEVAR in patients’ cohort and animal model [8]. But regarding the cases where symptom-responsible carotid artery and vertebral artery stenosis co-occurred, there is still a lack of experience and evidence as to the management algorithm of other branches than LSA, the sequential performing order of LSA fenestration and other branches angioplasty still lack experience and evidence. Here we reported a case in which TEVAR combined with vascular angioplasty and retrograde in situ fenestrated LSA revascularization at one stage was used for the treatment of PAU with two supraarch branches stenosis complicated by onset of transient ischemic attack (TIA) and fresh cerebral stem infarction, and analyzed retrospectively similar cases in relevant literature to evaluate the effectiveness of the present management of the disease.
Case report
A 71-year-old female, weakened by a sudden onset of impotence in left extremity and inarticulate speech lasting for one week, was admitted to Department of Vascular and Endovascular Surgery. She manifested no vomiting, nausea, ipsilateral extremity numbness or other posterior cerebral dysfunction-derived symptoms. Physical examination showed immobile left limb (muscle strength level 1) and an apparently decreased sensation of left extremity. Positive neurological signs of this case included right-deviated mouth, shallow nasolabial groove and positive right-side pathology. She is non-drinking, non-smoking, with severe hypertension uncontrollable by medications. A history of surgical management for intervetebral disc protrusion in 1996 and brain trauma in 2003 were also noted.
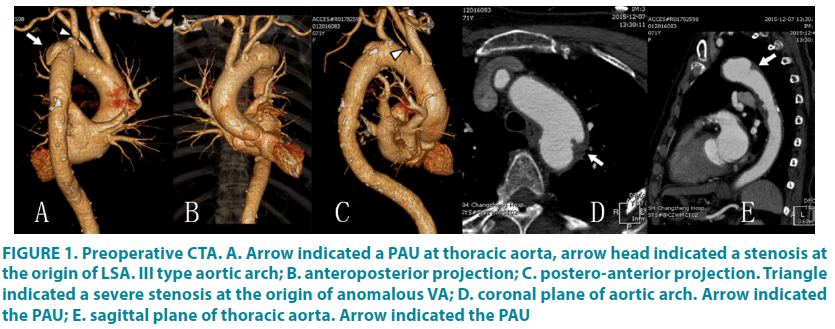
As was shown by the Computed tomography angiography (CTA) (FIGURE 1), and thoracic aneurysm (4 × 4 cm), caused by penetrating ulcer, was identified adjacent to the origin of LSA invading LSA. Besides, severe stenosis generated by non-calcified, vulnerable plaque (Echo inhomogeneity identified by duplex ultrasound) were observed at left vertebral artery, the origin of left subclavian artery, and left internal carotid artery (ICA), while moderate stenosis led by calcified plaque was noted in right common carotid artery and internal artery. In addition, segmental occlusion and multi-loci severe stenosis were observed in basilar artery and anterior cerebral artery, respectively. Notably, the left vertebral artery was anomalously originated from aortic arch. Skull computed tomography (CT) revealed a brain stem infarction. In the prevention of stroke and aneurysm-related complication, we chose endovascular reconstructions to dispose all responsible lesions at one stage, which reduced possible injuries and complication.
Figure 1: Preoperative CTA. A. Arrow indicated a PAU at thoracic aorta, arrow head indicated a stenosis at the origin of LSA. III type aortic arch; B. anteroposterior projection; C. postero-anterior projection. Triangle indicated a severe stenosis at the origin of anomalous VA; D. coronal plane of aortic arch. Arrow indicated the PAU; E. sagittal plane of thoracic aorta. Arrow indicated the PAU
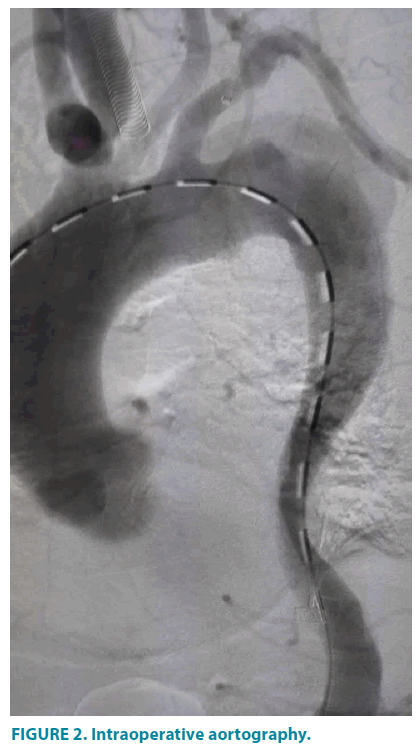
Under general anesthesia, the patient was positioned supine, with left upper limb and bilateral inguinal regions routinely sterilized. The right femoral artery was exposed through an oblique groin incision. A total of 5000 units of intravenous heparin were given to the patient. A 5F introducer sheath (Terumo, Shibuya-ku, Tokyo, Japan) was inserted into the left brachial artery. A Straight Pigtail catheter (Terumo, Shibuya-ku, Tokyo, Japan) was used to guide a 0.035-inch soft guidewire (Terumo, Shibuya- ku, Tokyo, Japan)in its path into the aortic arch under the guidance of road map, followed by a 6F renal artery sheath(Cook, Bloomington, Indiana, USA)introduced into the ostium of left subclavicular artery. An 8F introducer sheath (Terumo, Shibuya-ku, Tokyo, Japan) was implanted via the right femoral artery access leading 0.035-inch soft wire and a Straight Pigtail catheter to the ascending aorta. Then, an angiography was performed (FIGURE 2).
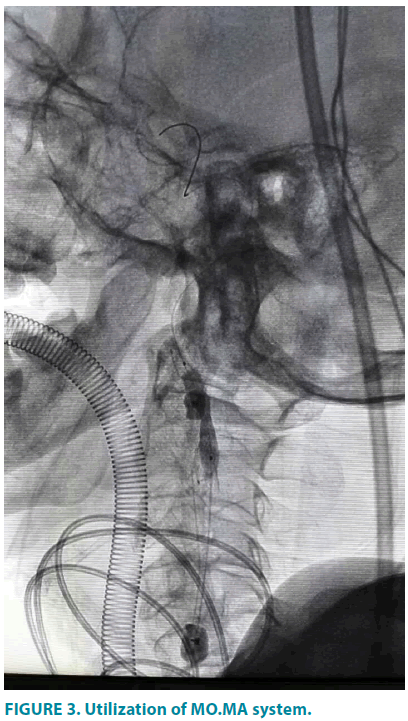
Next, in the retrograde access of femoral artery, the Straight Pigtail catheter was exchanged with vertebral catheter (Terumo, Shibuya-ku, Tokyo, Japan) and engaged into left external carotid artery. After that, the soft wire was replaced by a Supercore guidewire(Abbott, Santa Clara, California, USA), over which the MO.MA ULTRA(Invatec, Roncadelle, Brescia, Italy) was positioned in the left external carotid artery(ECA) and common carotid artery (CCA). Cautious inflation of the CCA and ECA balloons was observed under fluoroscopy (FIGURE 3) until the mark points of distal balloon was oriented at the vascular segment which was 0.5 cm far from the bifurcation of CCA and complete occlusion was indicated by the cylindrical change in balloon shape. Then a 6 mm-9 mm × 40 mm Cristallo Ideale stent graft (Invatec, Freuenfeld, Switzerland) was introduced into ICA and CCA under the guidance of 0.014-inch V-14 control wire (Boston Scientific, Marlborough, Massachusetts, USA) and deployed. Post dilatation by 5 mm × 20 mm Sterling balloon (Boston Scientific, Marlborough, Massachusetts, USA) was then performed. CCA and ECA were recanalized by deflation of the MO.MA ULTRA balloons and angiography was completed to confirm the exclusion of stenosis.
For the next step, a 5F Vertebral catheter was selectively advanced into left vertebral artery in coordination with 0.035-inch softwire, and then exchanged with a 0.018-inch V-18control wire (Boston Scientific, Marlborough, Massachusetts, USA) which was transported to the distal end of left vertebral artery. A 6F90 cm sheath (Cook, Bloomington, Indiana, USA) was inserted into aortic arch and a 5 mm × 15 mm Express SD Vascular stentgraft(Boston Scientific, Marlborough, Massachusetts, USA) was advanced across the diseased part of the artery and dilated to dissolve the stenosis.
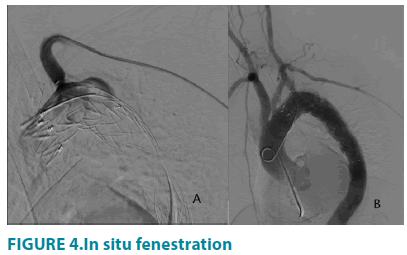
Upon the exchange of Lunderquist guidewire(Cook, Bloomington, Indiana, USA) into aorta arch, a 34 mm × 150 mm Capitivia dacron stentgraft(Medtronic, Minneapolis, Minnesota, USA) was deployed just distal to the proximal end of left vertebral endograft, covering LSA. Through the previously settled renal artery sheath, a 5F CXI supporting catheter (Cook, Bloomington, Indiana, USA) combined with laser fiber were placed at the ostium of the LSA and made perpendicular contact with the proximal endograft. After that an application of lasermar1500 circular laser energy (Eufoton, Trieste, Italy) with the parameters of 10 Watt, 1470nm wavelength, was made for 3 seconds to create the fenestration. The stiff 0.035-inch soft guidewire was advanced through the CXI laser catheter and crossed the fenestration into the endograft lumen. The stent fenestration was predilated by a 6 mm × 40 mm Mustang balloon(Boston Scientific, Marlborough, Massachusetts, USA), followed by precise deployment of an 8 mm × 37 mm Express LD Vascular balloon expandable stent graft(Boston Scientific, Marlborough, Massachusetts, USA). Finally, the last aortography was performed to substantiate the patency of aortic stent, left vertebral artery stent and LSA fenestration and to distinguish whether endoleak existed (FIGURE 4).
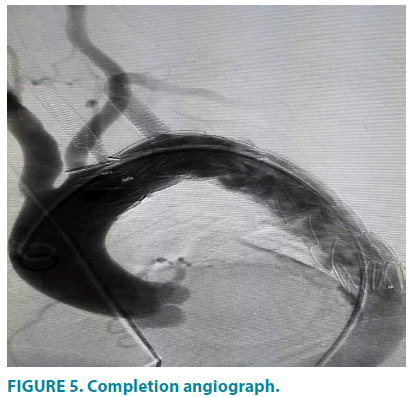
Through final DSA examination (FIGURE 5) the penetrating ulcer was successfully excluded and the stenosis of LSA, left ICA and VA were completely handled with no tearing of the fabric and no endoleak observed. Then guide wire and catheter were retracted while incision and high- pressure dressing were made on right femoral artery and left brachial artery, respectively. During procedure and post-precedure, no TIA, perioperative strokes, myocardial and cerebral infarction, or other neurologic complications occurred. The patient was discharged after 5 days, with symptoms resolved, the aortic aneurysm well repaired and a normal position and morphology of the stent confirmed.
Discussion
TEVAR has become a valid treatment option for thoracic aorta disease with less injury, shortening hospitalization, and promising long- term results, compared to total arch replacement by sternotomy or hybrid techniques [10,11]. However, to achieve an adequate proximal landing zone during TEVAR, aortic branches including innominate arery, LCA or LSA may need to be covered to stabilize endograft, of which LSA coverage is required in approximately 26-40% of TEVAR cases [8]. But such procedure may sacrifice the perfusion into the distal target organ, which can lead to ischemic symptom. The alternative procedure for supra- aortic arteries revascularization during TEVAR indicated debranching technique, parallel stent technique, branched stent, fenestration, etc.
Surgical debranching technique, via extra- anatomic vascular transposition into covered aorta arch branches after interposition of aorta endograft, is effectively used in patients’ cohort with proximal thoracic aorta disease [12]. This modality, combining endovascular procedure and open surgery, can be performed to prevent the incidence of endoleak of type III [13], but meanwhile it changes hemodynamic state and is usually accompanied by a high mortality, increased nerve injuries and elevated stroke frequency. Parallel stent technique, including periscope and chimney, has gradually been used for complete reconstruction of supra- aortic branches with different types of lesions in patients who are not suitable for open or hybrid repair [14]. However, this technique can add up to the incidence of endoleak and its long-term effect remained to be observed. In the indicated case, this technique may not be adaptable because the patient has undergone a brainstem infarction and may not be tolerable to multi-stage treatment if endoleak or dislocation of graft happens. Branched stent deployment can be effective and safe, accompanied by desirable technical and clinical outcomes at early and mid-term follow-up during TEVAR. However, in this emergent case with short proximal anchoring zone and Type III aortic arch, branched techniques may not be suitable because it necessitates preoperative detailed modification of aortic endograft on the basis of patients’ vasculature anatomy, which needs meticulous planning. Although some originally devised multibranched endograft has gradually emerged [15], the long-term effect of the graft and the detailed experience still remain to be reported.
Fenestration, created in vitro or in vivo, has been gradually utilized as an attractive approach to treat proximal thoracic aorta disease, especially for old patients with concurrent diseases. In vitro designed fenestrated graft, similar to branched graft, requires detailed devise before surgery and the endograft has to be rotated repeatedly to confirm whether the fenestration is positioned towards the orifice of branches. Such procedure may not be applicable to the patients with critical condition, or special anatomical vasculature, because it may prolong the procedure time, and thereby elevate the risk of stroke. As to in vivo fenestration, total endovascular aortic arch reconstruction via fenestration in situ, though not indicated as routine procedure, has been proved in animal experiments and patients with promising results but without destruction of fabric or structure of endograft. In addition, the intraoperative fenestration of aortic stent can be instantly carried out on patients in emergent conditions as the patient reported here manifested TIA and brain stem infarction [16].
Since the first case was reported by McWilliams et al., various techniques has been created for in situ fenestrations: penetration by a needle or the sharp end of a guide wire; use of a radiofrequency probe and laser generated fenestrations [8,17]. But these techniques all have their own disadvantages: A needle or guide wire-generated fenestration, in the principle of mechanistic pressure, is usually time-consuming and prone to being affected by particular vessel anatomy, which may not be appropriate for this patient with specific anatomy and emergent condition. In principle of energy burning, laser-created fenestration may produce graft fabric particles or embolism into circulation or encroach on the surrounding blood and vessel wall during the melt or vaporization of the stent. However, as Sonesson et al. has demonstrated that no macroscopically visible embolus could be observed during the procedure of laser-generated in situ fenestrations for the reservation of left iliac artery while implanting a Dacron aortic endograft from the infrarenal aorta to the right iliac artery in pig models, which at experimental level implies the appropriate applicability of laser-guided in situ fenestrations for LSA revascularization during TEVAR [8]. Furthermore, in clinical practice, in situ retrograde laser fenestration has been successively performed (technical success rate was 95.8%) on 24 patients with aortic disease during TEVAR with no fenestration-related complications or neurological adversity occurred [18]. Weighing the pros and cons of Laser in situ fenestration while confronting patients with progressive deteriorating illness and anomalous vessel anatomy, such procedure may be more adaptable.
As to the vascular access, surgically exposed LCA, RCA and LSA have long been used as a puncture site for retrograde ISF during the coverage of aorta branches. However, as the less- invasive brachial artery or radial artery access has been introduced to revascularize LSA, the puncture of LSA should be gradually abandoned. Multi-stage disposition of LCA atherosclerotic lesion and TEVAR with LSA revascularization may present a more conventional option for patients; however, old patient with comorbidities may not endure such by-stage procedure which can bring more stimuli to hospitalized patients. Thus, we decided to perform one- stage endovascular reconstruction for two aorta branches with thoracic aortic disease without retrograde ISF for plaque-loaded LCA.
Carotid angioplasty and stenting, as an acceptable alternative to CEA, can be prior to CEA because of shortened hospitalization and convalescence in reducing carotid stenosis, while CAS brings about no increased risks for complications of death, myocardial infarction, and ipsilateral stroke during the procedure or longer duration of follow-up compared to CEA, which were indicated by single or multi- centre randomized trials [19,20]. During the procedure of CAS, brain protection device (EPD) is required to potentially reduce the risk of embolization [21]. The proximal Mo.Ma protection system, in the principle of back blood pressure produced by blockage of CCA and ECA, has been applied to the area of performing endovascular therapy for high- risk carotid stenosis with no requirement of advancement through lesion area and relevant multi-center trials had demonstrated that CAS combined with proximal flow blockage was safe and feasible with a high procedural success rate [22-24]. As to the indicated case here with vulnerable plaque, MO.MA proximal protection system may adapt more to the case compared to distal protection system [25-27].
In respective of acute thoracic disease near LSA with at least one supraaotic branches pathology, the selection of one-stage or multi- stage treatment for thoracic lesion and LCA, innominate artery and the sequential disposing order of them still lacks clinical experience. In search of relevant literature, we have found only other 5cases of patients with thoracic disease combined with aortic branches construction (TABLE 1). A total of 5 patients suffering from this situation have been described.
| Author | Year | Age/sex | Indication | Treatment | Outcome |
|---|---|---|---|---|---|
| Harada et al. [25] | 2001 | 55/M | Aneurysm of thoracic and RSA | RCCA–RSA bypass, and open arch graft replacement | recovery |
| Takagi et al. [26] | 2003 | 68/M | occlusion of the left ICA and thoracic aneurysm | Two-stage superficial temporal artery–middle cerebral artery anastomosis and total aortic arch replacement under Cardiopulmonary bypass | recovery |
| Giulio Illuminati et al. [27] | 2004 | 76/F | stenosis of the left ICA and thoracic aneurysm | LSA to LCCA transposition, left CEA, and TEVAR | recovery |
| Rajagopal, et al. [28] | 2007 | 73/F | Stenosis of left CCA and thoracic aortic aneurysm | ascending aorta-to-LCCA bypass grafting and TEVAR |
transient ischemic attack on postoperative day 1 and recover |
| Satoshi Yamashiro et al. [29] | 2014 | 68/M | Dissecting intima of the right CCA and stanford type A aortic dissection | Graft replacement of ascending aorta under cardiopulmonary bypass | recovery |
Table 1: Summary of all reported cases of thoracic aorta disease with supraarch pathologies
One stage treatment first with carotid and vertebral angioplasty and then TEVAR accompanied by LSA retrograde in situ fenestration may provide an optimal treatment plan for the selected patients. The principle of the modality is demonstrated as two steps: First, endograft interposition to the exclusion of lesioned thoracic aorta with adjacent branches covered is performed. Second, perforate the stent fabrics covering supra-aortic branches for sufficient perfusion in strictly controlled time window. It can widen the use of TEVAR while multiple supraaortic comorbidities exists and simultaneously disposes aortic branch lesion through which patients get a fast convalescence period and avoid injuries and stimuli caused by multi-stage surgery routinely with vascular transposition or bypass graft [28,29].
The technique skills of fenestration in our case is that the selection of fenestration site on endograft need to be guided by fluoroscopy in multiple angle to confirm that the site is in the central part of the ostium of covered LSA. Furthermore, prompt performance of fenestration followed by deployment of balloon and stent in fenestration to recover perfusion of LSA is considered as a requirement to largely shorten ischemic time. In addition, the laser head should be supplemented with fluorescent, in order to manipulate the laser head through vision in case that the laser advanced too deep into the branches, causing injuries of LSA. The angle between LSA and adjacent aortic arch should be emphasized because too large (>150°) or small (<30°) angle may add up to the difficulties of management and is therefore not recommended for retrograde ISF.
To the best of our knowledge, this is the first reported case of aortic arch lesions with ectopic origin of the left vertebral artery complicated by multi-supraarch branches stenosis, one- stage endovascular managed by simultaneously performing MO.MA-protected CAS and laser-generated in situ LSA fenestration during TEVAR. But our study has limitations. The referred methods have only been utilized in one case and the confirmation of its effectiveness and safety remains to be demonstrated by retrospective or prospective studies with larger population compared with conventional procedures.
Conclusion
We reported a one-stage endovascular management procedure with laser-guided in situ fenestration, distal cerebral protection led carotid angioplasty and vertebral artery revascularization combined for multiple supra arch lesions. We demonstrate that the technique of in situ laser-guided fenestration angioplasty in retrograde access is safe and effective. Considering the overall condition of patient, one-stage endovascular treatment may provide patient with optimal results. However, studies of the endovascular therapy of proximal thoracic aortic aneurysm with multi-supra arch pathologies in larger populations with longerterm follow-up now should be conducted.
Conflict of interest
The authors declare no conflicts of interest.
Consent
We appropriately obtained written consent from the patient before the procedure.
Funding
This work was supported by the Specifically Invited Professor of Oriental Scholar of Shanghai Colleges and Universities Tracking Program (GZ2016008), the Guiding Project of Western medicine from the Science and Technology Commission of Shanghai Municipality (16411966500), and grants from the National Natural Science Foundation of China (8157020854).
References
- Dake MD, Kato N, Mitchell RS, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N. Engl. J. Med. 340(20), 1546-1552 (1999).
- Kouchoukos NT, Dougenis D. Surgery of the thoracic aorta. N. Engl. J. Med. 336(26), 1876-1888 (1997).
- Ganaha F, Miller DC, Sugimoto K, et al. Prognosis of aortic intramural hematoma with and without penetrating atherosclerotic ulcer: A clinical and radiological analysis. Circulation. 106(3), 342-348 (2002).
- Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J. Vasc. Surg. 17, 357-368 discussion 368-370 (1993).
- Schumacher H, Von Tengg-Kobligk H, Ostovic M, et al. Hybrid aortic procedures for endoluminal arch replacement in thoracic aneurysms and type b dissections. J. Cardiovasc. Surg. 47(5), 509-517 (2006).
- Murphy EH, Dimaio JM, Dean W, Jessen ME, Arko FR. Endovascular repair of acute traumatic thoracic aortic transection with laser-assisted in-situ fenestration of a stent-graft covering the left subclavian artery. J. Endovasc. Ther. 16(4), 457-463 (2009).
- Alsac JM, Boura B, Desgranges P, et al. Immediate endovascular repair for acute traumatic injuries of the thoracic aorta: A multicenter analysis of 28 cases. J. Vasc. Surg. 48(6), 1369-1374 (2008).
- Sonesson B, Dias N, Resch T, et al. Laser generated in situ fenestrations in dacron stent grafts. Eur. J. Vasc. Endovasc. Surg. 51(4), 499-503 (2016).
- Ahanchi SS, Almaroof B, Stout CL, et al. In situ laser fenestration for revascularization of the left subclavian artery during emergent thoracic endovascular aortic repair. J. Endovasc. Ther. 19(2), 226-230 (2012).
- Zamor KC, Eskandari MK, Rodriguez HE, et al. Outcomes of thoracic endovascular aortic repair and subclavian revascularization techniques. J. Am. Coll. Surg. 221(1), 93-100 (2015)
- Lee TC, Andersen ND, Williams JB, et al. Results with a selective revascularization strategy for left subclavian artery coverage during thoracic endovascular aortic repair. Ann. Thorac. Surg. 92(1), 97-102; discussion 102-103 (2011).
- Andrasi TB, Grossmann M, Zenker D, et al. Supra-aortic interventions for endovascular exclusion of the entire aortic arch. J. Vasc. Surg. 66(1), 281-297 e282 (2017).
- Tan TW, Coulter AH, Zhang WW. Percutaneous in situ left subclavian artery fenestration using reentry catheter during endovascular thoracic aortic aneurysm repair. Int. J. Angiol. 25(5), e77-e80 (2016).
- Zhao Y, Shi Y, Wang M, et al. Chimney technique in supra-aortic branch reconstruction in china: A systematic and critical review of chinese published experience. Vasc. Endovascular. Surg. 51(6), 429-435 (2017).
- Gallitto E, Gargiulo M, Freyrie A, et al. Off-the-shelf multibranched endograft for urgent endovascular repair of thoracoabdominal aortic aneurysms. J. Vasc. Surg. 66, 696-704 e695 (2017).
- Numan F, Arbatli H, Bruszewski W, et al. Total endovascular aortic arch reconstruction via fenestration in situ with cerebral circulatory support: An acute experimental study. Interact. Cardiovasc. Thorac. Surg. 7(4), 535-538 (2008).
- McWilliams RG, Murphy M, Hartley D, et al. In situ stent-graft fenestration to preserve the left subclavian artery. J. Endovasc. Ther. 11(2), 170-174 (2004).
- Qin J, Zhao Z, Wang R, et al. In situ laser fenestration is a feasible method for revascularization of aortic arch during thoracic endovascular aortic repair. J. Am. Heart Assoc. 6(4), e004542 (2017).
- Brooks WH, McClure RR, Jones MR, Coleman TC, Breathitt L. Carotid angioplasty and stenting versus carotid endarterectomy: Randomized trial in a community hospital. J. Am. Coll. Cardiol. 38(6), 1589-1595 (2001).
- Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N. Engl. J. Med. 374(11), 1021-1031 (2016).
- Reimers B, Corvaja N, Moshiri S, et al. Cerebral protection with filter devices during carotid artery stenting. Circulation. 104(1), 12-15 (2001).
- Reimers B, Sievert H, Schuler GC, et al. Proximal endovascular flow blockage for cerebral protection during carotid artery stenting: Results from a prospective multicenter registry. J. Endovasc. Ther. 12(2), 156-165 (2005).
- Ansel GM, Hopkins LN, Jaff MR, et al. Safety and effectiveness of the invatec mo.Ma proximal cerebral protection device during carotid artery stenting: Results from the armour pivotal trial. Catheter Cardiovasc. Interv. 76(1), 1-8 (2010).
- Micari A, Stabile E, Cremonesi A, et al. Carotid artery stenting in octogenarians using a proximal endovascular occlusion cerebral protection device: A multicenter registry. Catheter Cardiovasc. Interv. 76(1), 9-15 (2010).
- Harada H, Ito T, Yamamoto N, Abe T. Surgical treatment of an aneurysm of the aberrant right subclavian artery involving an aortic arch aneurysm and coronary artery disease. Ann. Thorac. Cardiovasc. Surg. 7(2), 109-112 (2001).
- Takagi H, Mori Y, Umeda Y, et al. Preoperative construction of an extracranial arterial shunt for resection of an aortic arch aneurysm with occluded left carotid artery. Ann. Thorac. Surg. 76(4), 1298-1301 (2003).
- Illuminati G, Bresadola L, D'Urso A, et al. Artery transposition and carotid endarterectomy done simultaneously with stent-graft repair of an aneurysm of the aortic isthmus. Can. J. Surg. 47(3), 215-216 (2004).
- Rajagopal K, Lima B, Harrison JK, et al. Endovascular thoracic aortic aneurysm repair with concomitant myocardial and carotid revascularization. Ann. Thorac. Surg. 84(1), e1-3 (2007).
- Yamashiro S, Arakaki R, Kise Y, et al. Emergency operation for aortic dissection with ischemic stroke. Asian Cardiovasc. Thorac. Ann. 22(2), 208-211 (2014).