Review Article - Interventional Cardiology (2012) Volume 4, Issue 6
Optical coherence tomography: research applications, potential clinical utility and future directions
- Corresponding Author:
- Ik-Kyung Jang
Cardiology Division, Massachusetts General Hospital & Harvard Medical School, GRB 800, 55 Fruit Street, Boston, MA, USA
Tel: +1 617 726 9226
Fax: +1 617 726 7419
E-mail: ijang@partners.org
Abstract
Keywords
fourier domain-optical coherence tomography,intravascular imaging,neoatherosclerosis,optical coherence tomography,plaque characterization,time domain-optical coherence tomography
Introduction
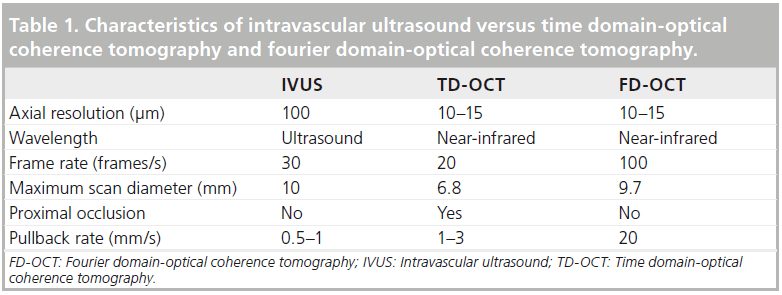
Optical coherence tomography (OCT) is an intravascular imaging modality akin to intravascular ultrasound (IVUS). It utilizes near-infrared frequency (1300 nm) light waves instead of sound waves for image acquisition and consequently provides a quantum leap in coaxial resolution. The first scientific proof of concept was described in 1991 in the retina and its application in ophthalmology is well established beyond that in cardiology [1]. OCT, unlike IVUS, requires a bloodless field as erythrocytes produce severe scatter of the light source. In its original iteration (i.e., time domain-OCT [TD-OCT]), OCT generally required proximal vessel occlusion and injection of Ringer’s lactate solution for image acquisition. Subsequently, a nonocclusive TD-OCT technique with contrast injection [2,3] was developed, which was associated with a significantly reduced incidence of transient ischemic electrocardiographic changes [3]. However, due to the slow pullback of the TD-OCT system, almost 30 ml of contrast [2] was needed per acquisition (see Table 1). The current generation of commercially available OCT, namely Fourier domain-OCT (FD-OCT), obviated the need for proximal occlusion and can be performed with contrast injection. The main advantage of the FD-OCT over TD-OCT is the faster pullback speed (20 mm/s), and hence much less contrast volume, with clearer images [4]. This significantly improved the user-friendliness of OCT and was critical in its widespread adoption. The characteristics of IVUS, TD-OCT and FD-OCT are detailed in Table 1.
Procedural detail
The currently available OCT catheter is a rapid-exchange catheter compatible with a 6 Fr guiding catheter or above. Larger guiding catheters can be used and may theoretically provide better contrast flushing, but would entail more contrast and hence, for the pure purpose of performing an OCT, are not necessary. The OCT catheter has several markers and the position of the imaging optical lens should be noted to be proximal to the radio-opaque transition. Test image acquisition and depth calibration should be performed prior to image acquisition. We advocate injecting contrast and waiting for the lumen to be completely clear of blood before the initiation of OCT pullback. As such, manual activation of pullback may be preferable. With an automated contrast injection system, a setting of 3–4ml/s should suffice. For manual injection, usually 10 ml contrast at reasonably sustained injection pressure will be sufficient to opacify the vessel. Ischemic electrocardiographic changes are not infrequent but almost always self-limiting; arrhythmia is rare and less frequent than with occlusive TD-OCT. Other complications, such as those with guiding catheters and coronary wires, are not attributable to OCT per se and are almost definitely due to operator inexperience. The safety and feasibility of FD-OCT has been widely reported [4–6].
Histopathological correlation
The first study on the use of OCT in the assessment of coronary plaques came in 1996 from the Fujimoto group [7]. An extensive body of crucial data in the early 2000s addressed the fundamental prerequisite for the use of OCT in clinical applications – that is, correlation of OCT appearances with histopathology. Multiple studies based on TD-OCT compared OCT images [8–11] with cadaveric specimens, including coronary arteries, aortae and carotid arteries, focusing on arterial wall definition. Arterial plaques, the composition of which were defined as lipid, fibrous and calcific, were identified histologically and OCT findings were then compared, and sensitivities and specificities established. The overall accuracy of TD-OCT approached 90% in this early iteration of the technology. However, these studies also highlighted the difficulty of differentiating Ca2+ from lipid cores, and this continues to be a challenging aspect in OCT interpretation [12]. Another point of contention has been the characterization of lipid plaques under a fibrous cap with variable scattering, potentially confounding the accuracy of fibrous cap depth measurement and plaque characterization [13]. In addition, recent data suggest a discrepancy between the traditional autopsy cut-off (65 μm) for a thin cap, derived from the seminal work from Burke et al. [14], and on OCT (80 μm) [15]. Most of the histological correlation with OCT had been performed with TD-OCT; however, FD-OCT has been shown to produce clearer images with fewer artifacts [4]. FD-OCT is assumed to be as accurate in its depiction of histopathology as its predecessor.
Current application: an investigational tool
The ability to visualize coronary arteries and pathologies in vivo with ten-times the resolution of IVUS had opened a floodgate of research possibilities. The current phase of OCT research is somewhat reminiscent of that initial introduction of IVUS to clinical use. Multiple studies had already demonstrated the superiority of OCT over IVUS in definition [16–22]. The availability of OCT in many catheterization laboratories means a lot of invasive cardiologists have become familiar with the OCT procedure and image interpretation. Nevertheless, it must be stressed that the clinical application of OCT at this stage remains less than evidence guided. There are currently two expert review documents mostly based on data derived from case reports and case series of observational findings [23,24].
As with any technology, the procedure/device must be safe, feasible in a wide variety of clinical situations or patients and provide reproducible results with tolerable interobserver accuracy [25–28]; OCT in its current iteration appears to have achieved these goals. However, perhaps more importantly, its use should improve patient outcomes.
Current application: understanding pathology in vivo
The main strengths of OCT relate to the nearhistological resolution and the possibility of in vivo assessment of disease. This enables a whole spectrum of opportunities in terms of longitudinal assessment of preclinical disease (i.e., vulnerable plaques), assessment of plaques and their response to treatment, and elucidation of processes previously only possible postmortem, findings beyond the resolution of IVUS. To date, many observational studies have been published pertaining to this important application of OCT.
▪ Assessment of de novo atherosclerotic coronary artery disease
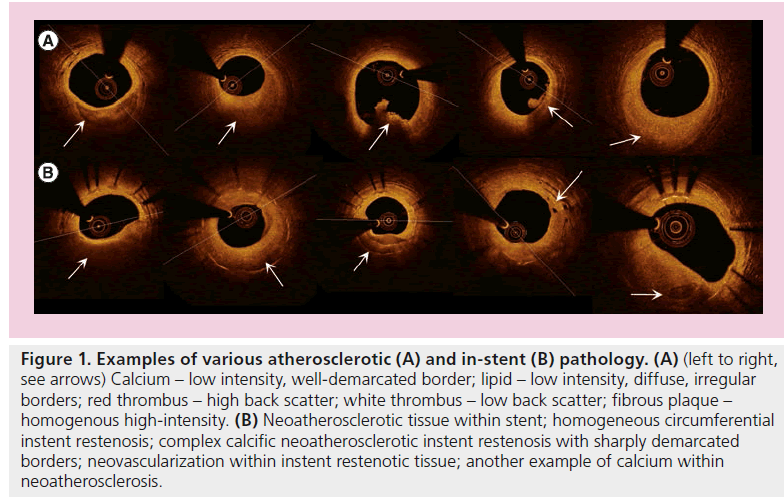
Ever since the first in vivo assessment of CAD in 2002 [29] with TD-OCT, with its illustration of the trilaminar structure of a normal coronary artery and characterization of atherosclerosis, this continues to be an evolving field with many innovative in vivo studies. Particular pathological entities (Figure 1) had been characterized on OCT, including the differentiation of fibrous, fibro-calcific and lipid-rich plaques; thrombi, differentiating white from red thrombi; extensive studies of thin-capped fibroatheroma (TCFA); characterization of plaque rupture and plaque erosion; and even surrogate identification of intralesional macrophages [30]. Whilst data exists for TD-OCT correlation with identification of macrophages, there exists only extrapolation of such for FD-OCT.
Figure 1: Examples of various atherosclerotic (A) and in-stent (B) pathology. (A) (left to right, see arrows) Calcium – low intensity, well-demarcated border; lipid – low intensity, diffuse, irregular borders; red thrombus – high back scatter; white thrombus – low back scatter; fibrous plaque – homogenous high-intensity. (B) Neoatherosclerotic tissue within stent; homogeneous circumferential instent restenosis; complex calcific neoatherosclerotic instent restenosis with sharply demarcated borders; neovascularization within instent restenotic tissue; another example of calcium within neoatherosclerosis.
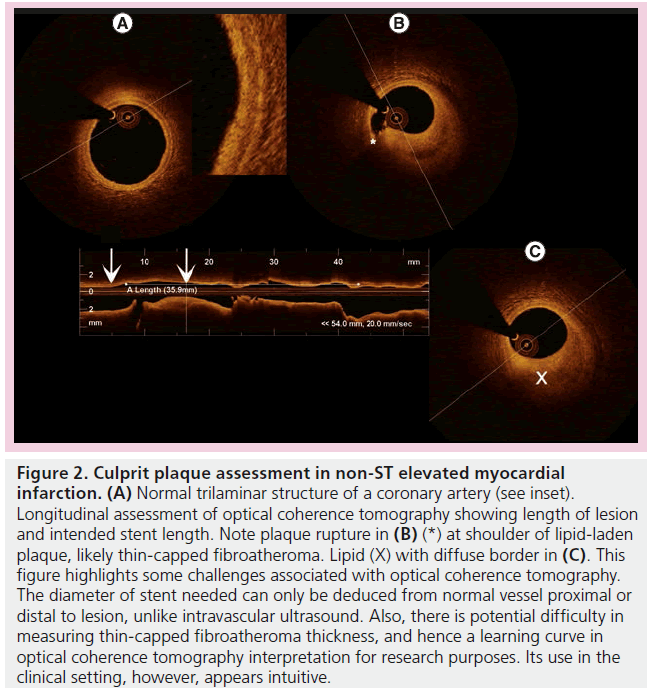
These pathological processes had been correlated with various clinical presentations and their differences were highlighted (Figure 2). Jang et al. first highlighted the characteristic plaque differences between patients with acute coronary syndromes (ACS) as compared with those with stable angina, with significant differences in fibrous cap thickness and frequency of TCFA [31]. Kubo et al. extended these findings by demonstrating, in a cohort of ACS patients, that OCT was superior to both IVUS and angioscopy in detecting TCFA, thrombus, plaque rupture, fibrous cap erosion and in thickness assessment [19]. The same group, led by Akasaka, was successful in remarkably demonstrating that plaque rupture sites differed significantly depending on the mode of ACS presentation (rest vs exertion initiated) [32], culprit lesion differences between types of ACS (non-ST elevated myocardial infarction vs ST-elevated myocardial infarction [STEMI]) [33], and even between differing degrees of unstable angina [34]. Toutouzas et al. also demonstrated morphology differences between non-STEMI and STEMI culprit plaques – the former exhibiting greater minimal luminal area, less lipid with shorter culprit rupture sites [35]. Similarly, Tian et al. found that plaques differed depending on the mode of presentation and whether they were of culprit lesions, correlating neovascularization with more vulnerable features only in ACS culprit lesions [36].OCT has also been used to assess differences in ACS plaque morphology between diabetic and nondiabetic patients [37]. OCT had significantly furthered the understanding of ACS by providing such timely data simply not attainable with postmortem time-delayed histology samples (Figure 1).
Figure 2: Culprit plaque assessment in non-ST elevated myocardial infarction. (A) Normal trilaminar structure of a coronary artery (see inset). Longitudinal assessment of optical coherence tomography showing length of lesion and intended stent length. Note plaque rupture in (B) (*) at shoulder of lipid-laden plaque, likely thin-capped fibroatheroma. Lipid (X) with diffuse border in (C). This figure highlights some challenges associated with optical coherence tomography. The diameter of stent needed can only be deduced from normal vessel proximal or distal to lesion, unlike intravascular ultrasound. Also, there is potential difficulty in measuring thin-capped fibroatheroma thickness, and hence a learning curve in optical coherence tomography interpretation for research purposes. Its use in the clinical setting, however, appears intuitive.
▪ Assessment of vulnerable plaques
OCT has also been used to assess the morphology of nonculprit lesions (NCL) in ACS. The idea of a ‘paninflammatory’ process in ACS was examined in several studies [38,39] in which nonculprit lesions in ACS patients appeared more ‘inflamed’ than lesions in non-ACS patients – with more macrophages, thinner fibrous cap and more lipid-rich plaques. The management of nonculprit lesions in STEMI remains controversial. The traditional paradigm had been generally symptom- or ischemia-driven, usually via noninvasive imaging.
The unrivalled resolution of OCT to delineate TCFA, the central pathological process for ACS elucidated by Burke et al. [14], lends itself to an explosion of studies on TCFA with OCT. Studies on vulnerable plaques potentially form a path to the holy grail of CAD management: the ability to prevent acute coronary syndrome, myonecrosis and its sequelae, by identifying and treating vulnerable plaques. An important, exhaustive study utilizing IVUS [40] revealed longitudinal data on NCL event rates and highlighted at-risk features such as TCFA. In a small study using a cap thickness cutoff of 200 μm, ‘vulnerable’ NCL were found in 26% of study subjects [41]. Distribution of TCFA was found to cluster in the proximal segments of major epicardial arteries [42,43]. Longitudinal data was assessed in a similar fashion in an OCT study [44], focusing on NCL at 7 months post-ACS, which demonstrated that the presence of TCFA and microchannels may be predictors for significant, though nonclinical, progression. It is likely that OCT will continue to play a crucial role in the management of preclinical vulnerable plaques and NCL in ACS patients. Purely speculatively speaking, OCT may shed light on an ‘inflammatory profile’ of a particular lesion, which may then be correlated with future clinical events, and possibly guide management in the future.
▪ Assessment of other pathologies
Several OCT studies assessed in vivo saphenous vein graft (SVG) pathologies [45–47]. Given the poor outcomes with SVG interventions, any effort to better understand this entity is clinically relevant; unfortunately, the studies to date have not been conclusive in addressing intravascular factors that may quantify risk for distal embolization. Nonetheless, these studies suggest that OCT in frequently large-caliber vein grafts may be more feasible than previously perceived.
Another interesting application had been towards cardiac allograft vasculopathy (CAV). Previous IVUS studies had demonstrated increased accuracy of CAV diagnosis when compared with angiography. The first study on the use of OCT in CAV [48,49] demonstrated lower interobserver variability in the diagnosis and better plaque characterization, compared with IVUS. This application of OCT is currently the subject of two ongoing trials (NCT01527344; NCT01403142). These will be important in characterizing the as-yet-undetermined OCT appearance of CAV, and this will hopefully enable earlier, more accurate diagnosis and possibly earlier institution of antiproliferative medications.
Given its ability to delineate the trilaminar arterial structure, OCT had also been studied in the relatively rare condition of spontaneous coronary artery dissection (SCAD) [50]. Two large case series [51,52] had been reported concerning the use of OCT in SCAD. OCT appears to improve the diagnosis of SCAD, although the clinical ramification for this remains to be clarified.
Assessment of stent pathology
Along with its arguably gold-standard status for strut-level analysis in stent trial follow-up (see later), the enhanced resolution from OCT had been applied to stent pathology characterization such as stent thrombosis and in-stent restenotic tissue characterization (Figure 1). Despite widespread clinical use and publication, there had been little data correlating FD-OCT findings with actual histopathology until a recent important study by Nakano et al. [53], which correlated FD-OCT and IVUS findings with postmortem histological examination of stents. Previous histopathological studies on stents had only been performed on animals [54]. Nakano et al. demonstrated that FD-OCT was significantly superior to IVUS for characterization of neointimal thickness assessment and strut coverage, and also for specific histological findings such as fibrin, hypersensitivity and macrophages. This atlas of identified histological pathologies will provide an important benchmark for interpreting FD-OCT findings for all future research.
▪ Stent thrombosis
OCT provides incremental and complementary data to that from IVUS when used in stent thrombosis mechanistic analysis [55–57]. These studies compared IVUS with OCT in patients with stent thrombosis. OCT provides unique insights into strut-level coverage, and both modalities provided information on malapposition. However, IVUS provided unique information on positive remodeling not visible on OCT. Rupture of neointima, particularly when associated with lipid-laden neointima, appears to be a frequent finding with late or very late stent thrombosis.
▪ In-stent restenosis
Perhaps the most interesting finding thus far in the OCT assessment of stent pathology had been the presence of neoatherosclerosis in instent restenotic tissue, which is beyond the resolution of IVUS, seen previously only on postmortem [58–61]. A decade ago it was noted that in-stent restenosis may be less benign than previously thought and could present with ACS [62]. The presence of neoatherosclerosis and neointimal disruption on OCT provides a unifying theory. Another interesting observation is that neoatherosclerosis is found at different time-points between bare-metal stents (BMS) and drug-eluting stents (DES); with the former more likely to develop neoatherosclerosis later (after 5 years of implantation [63], 3 years in another study [64]), While evidence so far suggest the earlier development of neoatherosclerosis in DES, with more lipid-rich plaques in DES in the early phase than BMS [65], and factors such as smoking, kidney disease and stent age identified as predictors of neoatheroscloerosis [66]. This difference in time-course of neoatherosclerosis had been supported in a postmortem analysis – DES 420 days versus BMS 2160 days [60]. Neoatherosclerosis is likely to be an important factor in late stent thrombosis and OCT has been critical in this research area. To speculate, this may have implications for cessation of antiplatelet therapy, for example, for perioperative management.
▪ Assessment of response to pharmacotherapy: statins
The resolution of OCT had also been capitalized in assessing histological responses to pharmacotherapy, such as statin therapy. Patients who had been on prior statin therapy were found to have reduced plaque ruptures [67], and in longitudinal studies such ‘pacifying’ effects of statins were further demonstrated, with a reduction in the inflammatory nature of plaques, such as production of thicker TCFA and reduced total atheroma volume [68,69]. Whilst such measures are surrogate end points, these studies are important in furthering understanding of pharmacotherapy, and OCT may be used in this manner for other pharmacotherapy.
Gold standard in stent follow-up
Given the strut-level resolution available with OCT (10–20 μm), it has quickly established itself as the gold standard in stent trial followup, particularly with reference to stent healing and neointimal coverage. Multiple published and ongoing studies have adopted the use of OCT (e.g., ABSORB [70], LEADERS [71], OCTAMI [72], ENDEAVOUR OCT [73]) to assess for stent coverage and vascular response to stent placement. OCT will allow comparative data between different stent designs, polymers and drug delivery systems. Its use was demonstrated well in bioabsorbable stent trials [70,74], as well as drug eluting balloon trials [75]. The presence of endothelization (<15 μm), however, cannot be determined by OCT [53,54]. Further technological advances may address this, although the translation to clinical utility will be some time yet.
One of the most pressing issues has been the need for standardized definitions [76]. Definitions for malapposition and strut coverage are important. Two recent expert consensus documents [23,24] provided some recommendations on such definitions as protruding and embedded apposition, and OCT strut coverage. It is foreseeable that core lab image analysis will eventually develop, as had been the case for IVUS to ensure impartial, standardized, expert analysis.
Potential application: defining a clinical role
The application of OCT in the clinical arena is yet to be defined. There is emerging, but far from conclusive, data on its clinical use. Purely seen as a tool to overcome limitations in 2D luminogram, to define angiographic uncertainties, the use of OCT appears to be intuitive. Indeed, such use for IVUS has been advocated in the American College of Cardiology/American Heart Association guidelines, and it is foreseeable that the same may happen in due course for OCT.
A question often arises as to whether OCT is ready to replace IVUS in routine clinical applications. In most situations, OCT is applicable and possesses advantages over IVUS: speed, resolution, overall user-friendliness and less interobserver variability. Some have even managed to overcome theoretical limitations, such as depth of penetration, particularly in vein grafts and left main lesions [77]. One area of limitation that will be difficult, if not impossible, to overcome will be true ostial lesions. This is particularly pertinent in left main interventions where the use of intravascular imaging to optimize acute outcomes may be best justified [78]. If a laboratory is limited to acquire only one intravascular modality, the director and interventional cardiologist must be aware of this.
The overriding question is whether OCT, with all the exquisite beauty of its images, can improve clinical outcomes. One observational study remarkably suggested improved clinical outcomes [79]. If OCT can indeed achieve such lofty goals then its enthusiastic uptake will be well justified and indeed the idea of ‘OCT-guided percutaneous coronary intervention (PCI)’ may be a new paradigm. As yet however, OCT should be viewed as purely a research tool.
Several theoretical applications are being actively investigated and observational data has already surfaced. From a theoretical perspective, in current practice, OCT information should be used: to guide reference diameter and lesion length [17]; for plaque characterization proximal and distal to the lesion, which may be used to predict risk of stent edge dissection; and for lesion characterization to guide strategy (e.g., calcified plaque – predilatation with a noncompliant balloon or rotational atherectomy lesion preparation; versus soft plaque – direct stenting strategy). All of these remain speculative and hypothesis generating – the next phase of OCT clinical research will be exciting. For now, the currently available published data will be analyzed.
Assessment of lesions prior to interventions
▪ Diagnostic uncertainty – overcoming limitations with 2D luminology
Some case report experience had been published using OCT to better define haziness, tortuosity and calcification in coronary vessels [20]. One such use is exemplified in the diagnosis of SCAD.
▪ Aiding decision-making
IVUS had previously been proposed as an anatomical guide for coronary interventions, based on minimal luminal area. This had been controversial area, and recent data suggest a revamping of the IVUS threshold for interventions [80]. Several studies had applied this idea of OCT to define correlation between IVUS and fractional flow reserve (FFR) [81–83].
The idea of functional angioplasty may challenge the use of minimal luminal area (MLA) data to guide PCI. Data from physiology or FFR is particularly robust in prognostic implications [84–86]. The decision that a lesion should be revascularized should, one would hope, come down to clinical acumen and adequate historytaking, possibly with noninvasive functional data. In the current era of appropriateness for interventions, this may be particularly pertinent. FFR is an excellent on-table tool in the absence of such data. The use of MLA, however, benchmarked to FFR, would hence appear to be a second best option. One suspects OCT may be equally appropriate to IVUS as a surrogate marker for FFR, if FFR was not available.
▪ Predicting adverse periprocedural outcomes
In a similar fashion to using IVUS to gauge degree of calcification to guide the need for rotablation, a novel concept had been examined with OCT with regards to lipid content. The first study to use OCT to predict potential adverse outcomes demonstrated a correlation between lipid arc in the culprit lesions and noreflow in patients presenting with non-STEMI [87]. The degree of preintervention lipid content and plaque characteristics, such as TCFA [88], had been correlated with procedural outcomes, such as coronary flow and biomarkers [89,90].
Application during PCI
▪ Assessment of immediate PCI outcomes
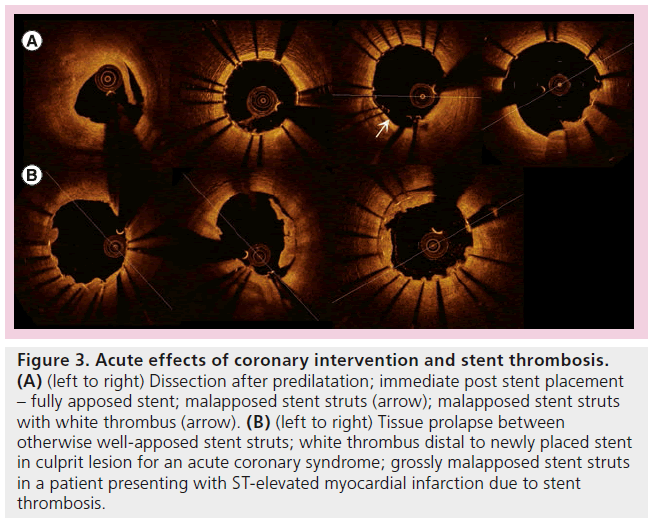
Acute effects of stent placement were characterized well [91]. Not surprisingly, tissue prolapse and dissection were better-appreciated than IVUS [91,92]. This had been known since 2001 with TD-OCT [93]. The relevance of such detailed findings (Figure 3), whilst gratifying to identify, remains to be defined. One study suggests these findings almost resolved completely at 6-month follow-up [94]. In addition, the management of such subtle and ubiquitous findings [91] (97.5% of stents with tissue prolapse, 87.5% with intrastent dissection) is unclear and it is inconceivable that additional treatment would be needed in this situation. The clinical implications of all of these findings require significant further research.
Figure 3: Acute effects of coronary intervention and stent thrombosis. (A) (left to right) Dissection after predilatation; immediate post stent placement – fully apposed stent; malapposed stent struts (arrow); malapposed stent struts with white thrombus (arrow). (B) (left to right) Tissue prolapse between otherwise well-apposed stent struts; white thrombus distal to newly placed stent in culprit lesion for an acute coronary syndrome; grossly malapposed stent struts in a patient presenting with ST-elevated myocardial infarction due to stent thrombosis.
▪ Using OCT to optimize immediate results
One of the most difficult lessons to reconcile from IVUS had been the overall lack of clinical benefit in using IVUS to guide PCI. A recent meta-analysis of an IVUS-guided strategy of PCI with BMS [95] failed to demonstrate reduction in death (2.4 vs 1.6%, p = 0.18) or even myocardial infarction (3.6 vs 4.4%, p = 0.51). Target vessel revascularization, however, was significantly reduced with IVUS (13 vs 18%, p < 0.001). With regards to DES, several underpowered studies [96,97] failed to provide conclusive evidence of improved restenosis rates despite higher postdilatation rates and presumably better stent expansion. Like IVUS, OCT can be applied to assess PCI results (Figure 3), and some data had been published regarding this. No impact on outcomes has yet been published. One paper evaluated the utility of OCT in optimizing acute procedure appearance in unprotected left main stenting [77]. Only 69% ± 20% of the stent inner area was suitable for analysis. The limitations of the current iteration of OCT technology were cited as a reason, particularly given that the study was based on TD-OCT. The clinical implications of using OCT to optimize left main stenting remain to be seen.
▪ Bifurcation lesion
OCT has been used to analyze bifurcation lesions. One group demonstrated, by using OCT to isolate a distal cell recrossing, that the number of malapposed stent struts was significantly lower than with angiography alone [98]. Such result was replicated with a 3D model [99]. These applications harvest the in vivo strength of OCT, mimicking what had been learnt from micro-CT, but the clinical implications of such strategies remain to be defined.
Several observational studies assessed the fate of side branch ostia, particularly the outcomes of malapposed stent struts; one suggested different neointimal hyperplasia between different types of DES [100]; another suggested impaired strut coverage on malapposed struts compared with apposed struts on the same side branch stent [101]. This appears to be more of an issue with DES than with BMS [102].
Future development
The current iteration of FD-OCT has taken several years to translate into widespread clinical use since commercialization. Various refinements had already been developed and applied to the current FD-OCT system, including 3D reconstruction and automated interpretation. Technically, the next iteration will allow up to 100,000–200,000 axial scans, essentially leading to quicker pullback speed and a longer segment to be imaged. Even more interestingly, micro-OCT [103] will provide an even higher resolution, although there is currently little clinical data. A cellular level of detail appears interesting, but one suspects this may remain in the research arena and be unlikely to have a clinical impact in the foreseeable future. Polarization-sensitive OCT [104] focuses on the identification of collagen and is actively investigated in other areas of medicine, such as orthopedics.
Aside from technical advancements, several pertinent issues will need to be addressed going forward. Definitions for malapposition and strut coverage are important. Two recent expert consensus documents provided some recommendations on such definitions as protruding and embedded apposition, and OCT strut coverage. A consensus on OCT measurable parameters and accuracy of measurements is critical for the validity of multiple observational in vivo studies in the pipeline. As many studies are forthcoming, fundamental questions on histological correlation have surfaced. It is foreseeable that core lab image analysis will eventually transpire as had been the case for IVUS, to ensure impartial, standardized, expert analysis.
Perspectives on clinical use
The exquisite imagery available from OCT has arguably opened a floodgate of findings previously not available. It is often the case that the cardiologist wonders, “what does that mean?”. This perhaps summarizes the current state of OCT for clinical use: in the research setting, OCT has become the gold standard for understanding in vivo pathology and has advanced our understanding significantly on many disease processes. The incorporation of OCT in the clinical arena is encouraging, but caution should be exercised. Data on how the newly gained information from OCT may translate to improved diagnosis and outcome is eagerly awaited. Maybe a sobering lesson can be taken from the development of IVUS – an important tool used for almost two decades. Its evidencebased use has only been better formulated in the recent past. Still, its use has yet to be associated with a statistically robust reduction in hard end points such as mortality, apart from a small subset of lesions.
Indeed, it is difficult to quantify how the use of OCT has already enhanced interventional practice. An angiographically ‘perfect’ PCI result often appears less than so on OCT, and interventional cardiologists experienced in OCT would have learnt this from previous experience and would perhaps frequently be more aggressive in balloon and stent sizing. Robust double-blinded randomized controlled studies on the clinical benefits of OCT will be difficult to undertake. Large-scale international registries, such as the Massachusetts General Hospital, OCT registry, coupled with rigorous follow-up to correlate with clinical end points, may be the most important mode of research going forward.
An expensive procedure with additional risks but no impact on clinical outcomes will forever be relegated as a research tool and, particularly in the current economic environment, unacceptable from a cost-effective perspective. The next phase of OCT research in cardiology – finding an indication, formulating guidelines, using OCT to improve outcomes – is perhaps the most exciting in intravascular imaging research in the foreseeable future. Investigators and clinicians in this area are strongly encouraged to continue to collaborate, share findings and participate in multicenter registries, such that the answer to the question, “what does that mean?”, may be resoundingly answered in the future with evidence-based outcome data.
Conclusion & future perspective
A decade has passed since the publication of the in vivo characterization of atherosclerosis with OCT, providing a ‘live microscope’ in human coronary arteries. Significant research findings have been published in areas such as plaque characterization in a whole variety of clinical scenarios and anecdoctal findings on OCT. This light-based intravascular imaging modality is now the gold standard for research applications. Its use in the clinical arena had been enthusiastically adopted by OCT protagonists worldwide and anecdotally appears very useful based on observational data. Over the next few years, its role in clinical applications will be defined, standardized definitions for lesion characterization and stent analysis will be developed and widely applied in the research arena. More information will become available from observational data, generated from its widespread use, and this will enhance our understanding of atherosclerotic disease, particularly that of vulnerable plaques. It is hoped that OCT can avoid the pitfalls of IVUS and data will demonstrate that its use improves clinical outcomes.
Financial & competing interests disclosure
I-K Jang holds a research grant and is a consultant for St Jude Medical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪▪ Commercially available Fourier domain-optical coherence tomography has been widely adopted as a clinical tool in many laboratories around the world after a decade of basic research development.
▪▪ Optical coherence tomography is now arguably a gold standard for intravascular imaging, having provided revolutionary insights in atherosclerotic plaque characterization, stent assessment and follow-up, in the research arena.
▪▪ Clinical scientists, however, should be aware of some of its limitations in plaque characterization.
▪▪ Its value as a clinical tool remains to be defined. Preliminary data suggest usefulness as a diagnostic tool, and possibly the potential to improve clinical outcomes.
▪▪ The next decade will likely see further pathophysiology research, its dominance in stent follow-up in research, establishment of guidelines and standardized definitions, and, hopefully, data on improved clinical outcomes in percutaneous coronary interventions.
References
Papers of special note have been highlighted as:
▪▪ of considerable interest
- Huang D, Swanson EA, Lin CP et al. Optical coherence tomography. Science 254, 1178–1181 (1991).
- Prati F, Cera M, Ramazzotti V et al. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention 3, 365–370 (2007).
- Barlis P, Gonzalo N, Di Mario C et al. A multicenter evaluation of the safety of intracoronary optical coherence tomography.EuroIntervention 1, 90–95 (2009).
- Takarada S, Imanishi T, Liu Y et al. Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc. Interv. 75, 202–206(2010).
- Imola F, Mallus M, Ramazzotti V et al. Safety and feasibility of frequency domain optical coherence tomography to guide decision making in percutaneous coronary intervention. EuroIntervention 6, 575–581 (2010).
- Yoon JH, Di Vito L, Moses JW et al. Feasibility and safety of the second-generation, frequency domain optical coherence tomography (FD-OCT): a multicenter study. J. Invasive Cardiol. 24, 206–209 (2012).
- Brezinski ME, Tearney GJ, Bouma BE et al. Optical coherence tomography for optical biopsy. Properties and demonstration of vascular pathology. Circulation 93, 1206–1213 (1996).
- Yabushita H, Bouma BE, Houser SL et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 106, 1640–1645 (2002).
- Kume T, Akasaka T, Kawamoto T et al. Assessment of coronary arterial plaque by optical coherence tomography. Am. J. Cardiol. 97, 156–159 (2006).
- Kawasaki M, Bouma BE, Bressner J et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J. Am. Coll. Cardiol. 48, 81–88 (2006).
- Rieber J, Meissner O, Babaryka G et al. Diagnostic accuracy of optical coherence tomography and intravascular ultrasound for the detection and characterization of atherosclerotic plaque composition in ex-vivo coronary specimens: a comparison with histology. Coron. Artery Dis. 17, 425–430 (2006).
- Manfrini O, Mont E, Leone O et al. Sources of error and interpretation of plaque morphology by optical coherence tomography. Am. J. Cardiol. 98, 156–159 (2006).
- Kume T, Akasaka T, Kawamoto T et al. Measurement of the thickness of the fibrous cap by optical coherence tomography. Am. Heart J. 152, 755e1–e4 (2006).
- Burke AP, Farb A, Malcom GT et al. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N. Engl. J. Med. 18, 1276–1282 (1997).
- Yonetsu T, Kakuta T, Lee T et al. In vivo critical fibrous cap thickness for rupture-prone coronary plaques assessed by optical coherence tomography. Eur. Heart J. 32, 1251–1259 (2011).
- Ramesh S, Papayannis A, Abdel-karim AR et al. In vivo comparison of Fourier-domain optical coherence tomography and intravascular ultrasonography. J. Invasive Cardiol. 24, 111–115 (2012).
- Tahara S, Bezerra HG, Baibars M et al. In vitro validation of new Fourier-domainoptical coherence tomography. EuroIntervention 6, 875–882 (2011).
- Tu S, Xu L, Ligthart J et al. In vivo comparison of arterial lumen dimensions assessed by co-registered three-dimensional (3D) quantitative coronary angiography, intravascular ultrasound and optical coherence tomography. Int. J. Cardiovasc. Imaging 28, 1315–1327 (2012).
- Kubo T, Imanishi T, Takarada S et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 50, 933–939 (2007).
- Kume T, Okura H, Kawamoto T et al. Assessment of the coronary calcification by optical coherence tomography. EuroIntervention 6, 768–772 (2011).
- Low AF, Kawase Y, Chan YH et al. In vivo characterization of coronary plaques with conventional gray-scale intravascular ultrasound: correlation with optical coherence tomography. EuroIntervention 4, 626–632 (2009).
- Capodanno D, Prati F, Pawlowsky T et al. Comparison of optical coherence tomography and intravascular ultrasound for the assessment of in-stent tissue coverage after stent implantation. EuroIntervention 5, 538–543 (2009).
- Prati F, Guagliumi G, Mintz GS et al.Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur. Heart J. 33(20), 2513–2520 (2012).
- Tearney GJ, Regar E, Akasaka T et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 59, 1058–1072 (2012).
- Jamil Z, Tearney G, Bruining N et al. Interstudy reproducibility of the second generation, Fourier domain optical coherence tomography in patients with coronary artery disease and comparison with intravascular ultrasound: a study applying automated contour detection. Int. J. Cardiovasc. Imaging doi:10.1007/s10554-012-0067-8 (2012) (Epub ahead of print).
- Okamura T, Gonzalo N, Gutiérrez-Chico JL et al. Reproducibility of coronary Fourier domain optical coherence tomography: quantitative analysis of in vivo stented coronary arteries using three different software packages. EuroIntervention 3, 371–379 (2010).
- Gonzalo N, Tearney GJ, Serruys PW, Regar E. Second-generation optical coherence tomography in clinical practice. High-speed data acquisition is highly reproducible in patients undergoing percutaneous coronary intervention. Rev. Esp. Cardiol. 63, 893–903 (2010).
- Gonzalo N, Garcia-Garcia HM, Serruys PW et al. Reproducibility of quantitative optical coherence tomography for stent analysis. EuroIntervention 5, 224–232 (2009).
- Jang IK, Bouma BE, Kang DH et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J. Am. Coll. Cardiol. 39, 604–609 (2002).
- Tearney GJ, Yabushita H, Houser SL et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation 107, 113–119 (2003).
- Jang IK, Tearney GJ, MacNeill BD et al. In vivo characterization of coronaryatherosclerotic plaque by use of optical coherence tomography. Circulation 111, 1551–1555 (2005).
- Tanaka A, Imanishi T, Kitabata H et al. Distribution and frequency of thin-capped fibroatheromas and ruptured plaques in the entire culprit coronary artery in patients with acute coronary syndrome as determined by optical coherence tomography. Am. J. Cardiol. 102, 975–979 (2008).
- Ino Y, Kubo T, Tanaka A et al. Difference of culprit lesion morphologies between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. J. Am. Coll. Cardiol. Interv. 1, 76–82 (2011).
- Mizukoshi M, Imanishi T, Tanaka A. Clinical classification and plaque morphology determined by optical coherence tomography in unstable angina pectoris. Am. J. Cardiol. 3, 323–328 (2010).
- Toutouzas K, Karanasos A, Tsiamis E et al. New insights by optical coherence tomography into the differences and similarities of culprit ruptured plaque morphology in non-ST-elevation myocardial infarction and ST-elevation myocardial infarction. Am. Heart J. 161, 1192–1199 (2011).
- Tian J, Hou J, Xing L et al. Significance of intraplaque neovascularisation for vulnerability: optical coherence tomography study. Heart 98, 1504–1509 (2012).
- Feng T, Yundai C, Lian C et al. Assessment of coronary plaque characteristics by optical coherence tomography in patients with diabetes mellitus complicated with unstable angina pectoris. Atherosclerosis 2, 462–465 (2010).
- Kubo T, Imanishi T, Kashiwagi M. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. Am. J. Cardiol. 3, 318–322 (2010).
- Kato K, Yonetsu T, Kim SJ et al. Non-culprit plaques in patients with acute coronary syndromes (ACS) have more vulnerable features compared with those with non-ACS: A 3-vessel optical coherence tomography study. Circ. Imaging 5, 433–440 (2012).
- Stone GW, Maehara A, Lansky AJ et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 3, 226–235 (2011).
- Barlis P, Serruys PW, Gonzalo N et al. Assessment of culprit and remote coronary narrowings using optical coherence tomography with long-term outcomes. Am. J. Cardiol. 4, 391–395 (2008).
- Fujii K, Kawasaki D, Masutani M et al. OCT assessment of thin-cap fibroatheroma distribution in native coronary arteries. JACC Cardiol. Imaging 2, 168–175 (2010).
- Toutouzas K, Karanasos A, Riga M et al. Optical coherence tomography assessment of the spatial distribution of culprit ruptured plaques and thin-cap fibroatheromas in acute coronary syndrome. EuroIntervention 4, 477–485 (2012).
- Uemura S, Ishgami K, Soeda T. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur. Heart J. 1, 78–85 (2012).
- Fusuzaki T, Itoh T, Koeda T. Angioscopy and OCT in repeated in-stent restenosis in saphenous vein graft. J. Am. Coll. Cardiol. Imaging 3(7), 785–786 (2010).
- Davlouros P, Damelou A, Karantalis V et al. Evaluation of culprit saphenous vein graft lesions with optical coherence tomography in patients with acute coronary syndromes. Am. Coll. Cardiol. Interv. 6, 683–693(2011).
- Adlam D, Antoniades C, Lee R et al. OCT characteristics of saphenous vein graft atherosclerosis. J. Am. Coll. Cardiol. Imaging7, 807–809 (2011).
- Garrido IP, García-Lara J, Pinar E et al. Optical coherence tomography and highly sensitivity troponin T for evaluating cardiac allograft vasculopathy. Am. J. Cardiol. 110(5), 655–661 (2012).
- Hou J, Lv H, Jia H et al. OCT assessment of allograft vasculopathy in heart transplant recipients. J. Am. Coll. Cardiol. Imagimg 6, 662–663 (2012).
- Poon K, Bell B, Raffel OC et al. Spontaneous coronary artery dissection: utility of intravascular ultrasound and optical coherence tomography during percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2, e5–e7 (2011).
- Alfonso F, Paulo M, Gonzalo N et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. Am. Coll. Cardiol. 12, 1073–1079 (2012).
- Saw J, Poulter R, Fung A et al. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ. Cardiovasc. Interv. 1, 134–137 (2012).
- Nakano M, Vorpahi M, Otsuka F et al. Ex vivo assessment of vascular response tocoronary stents by optical frequency domain imaging. J. Am. Coll. Cardiol. Imaging 5, 71–82 (2012).
- Templin C, Meyer M, Muller MF et al. Coronary optical frequency domain imaging (OFDI) for in vivo evaluation of stent healing: comparison with light and electron microscopy. Eur. Heart J. 14, 1792–1801 (2010).
- Guagliumi G, Sirbu V, Musumeci G et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. J. Am. Coll. Cardiol. Interv. 5(1), 12–20 (2012).
- Alfonso F, Dutary J, Paulo M et al. Combined use of optical coherence tomography and intravascular ultrasound imaging in patients undergoing coronary interventions for stent thrombosis. Heart 16, 1213–1220 (2010).
- Miyazaki S, Hiasa Y, Takahashi T et al.In vivo optical coherence tomography of verylate drug-eluting stent thrombosis compared with late in-stent restenosis. Circ. J. 76, 390–398 (2012).
- Takano M, Yamamoto M, Inami S et al. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J. Am. Coll. Cardiol. 55, 26–32 (2009).
- Hou J, Qi H, Zhang M et al. Development of lipid-rich plaque inside bare metal stent: possible mechanism of late stent thrombosis? An optical coherence tomography study. Heart 15, 1187–1190 (2010).
- Nakazawa G, Otsuka F, Nakano M et al. The pathology of neoatherosclerosis in human coronary implants bare metal and drug eluting stents. J. Am. Coll. Cardiol. 11, 1314–1322 (2011).
- Park SJ, Kang SJ, Virmani R et al. In-stent neoatherosclerosis: a final common pathway of late stent failure. J. Am. Coll. Cardiol. 23, 2051–2057 (2012).
- Walters DL, Harding SA, Walsh CR et al. Acute coronary syndrome is a common clinical presentation of in-stent restenosis. Am. J. Cardiol. 5, 491–494 (2002).
- Yamaji K, Inoue K, Nakahashi T et al. Bare metal stent thrombosis and in-stent neoatherosclerosis. Circ. Cardiovasc. Interv. 5, 47–54 (2012).
- Kang SJ, Mintz, Akasaka T et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation 25, 2954–2963 (2011).
- Yonetsu T, Kim SJ, Kato K et al. Comparison of incidence and time course of neoatherosclerosis between bare metal stents and drug-eluting stents using optical coherence tomography. Am. J. Cardiol. 110, 933–939 (2012).
- Yonetsu T, Kato K, Kim SJ et al. Predictors for neoatherosclerosis: a retrospective observational study from the optical coherence tomography registry. Circ. Cardiovasc. Imaging 5, 660–666 (2012).
- Chia S, Raffel OC, Takano M et al. Association of statin therapy with reduced coronary plaque rupture: an optical coherence tomography study. Coron. Artery Dis. 4, 237–242 (2008).
- Takarada S, Imanishi T, Kubo T et al. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis 2, 491–497 (2009).
- Hattori K, Ozaki Y, Ismall TF et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. J. Am. Coll. Cardiol. Imaging 2, 169–177 (2012).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2year outcomes and results from multiple imaging methods. Lancet 9667, 897–910 (2009).
- Barlis P, Regar E, Serruys P et al. An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: aLEADERS trial sub-study. Eur. Heart J. 31, 165–176 (2010).
- Guagliumi G, Sirbu V, Bezerra H et al. Strut coverage and vessel wall response to zotarolimus-eluting and bare-metal stents implanted in patients with ST-segment elevation myocardial infarction: the OCTAMI (Optical Coherence Tomography in Acute Myocardial Infarction) Study. JACC Cardiovasc. Interv. 3, 680–687(2010).
- Kim JS, Jang IK, Fan C et al. Evaluation in 3 months duration of neointimal coverage after zotarolimus-eluting stent implantation by opticalcoherence tomography: the ENDEAVOR OCT trial. JACC Cardiovasc. Interv. 2, 1240–1247 (2009).
- Onuma Y, Serruys PW, Perkins LE et al. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation 22, 2288–2300 (2010).
- Gutiérrez-Chico JL, van Geuns RJ, Koch KT et al. Paclitaxel-coated balloon in combination with bare metal stent for treatment of de novo coronary lesions: an optical coherence tomography first-in-human randomised trial, balloon first vs. stent first. EuroIntervention 6, 711–722 (2011).
- Lowe HC, Narula J, Fujimoto JG et al. Intracoronary optical diagnostics current status, limitations, and potential. J. Am. Coll. Cardiol. Interv. 12, 1257–1270 (2011).
- Parodi G, Maehara A, Giullani G et al. Optical coherence tomography in unprotected left main coronary artery stenting. EuroIntervention 6, 94–99 (2010).
- Park SJ, Kim YH, Park DW et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ. Cardiovasc. Interv. 3, 166–177 (2009).
- Prati F, Di Vito L, Biondi-Zoccai G et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention (2012) (Epub ahead of print).
- Koo BK, Yang HM, Doh JH et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. J. Am. Coll. Cardiol. Interv. 7, 803–811 (2011).
- Shiono Y, Kitabata H, Kubo T et al. Optical coherence tomography-derived anatomical criteria for functionally significant coronary stenosis assessed by fractional flow reserve. Circ. J. 76, 2218–2225 (2012).
- Stefano GT, Bezerra HG, Attizzani G et al. Utilization of frequency domain optical coherence tomography and fractional flow reserve to assess intermediate coronary artery stenoses: conciliating anatomic and physiologic information. Int. J. Cardiovasc. Imaging 2, 299–308 (2011).
- Gonzalo N, Escaned J, Alfonso F et al. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. J. Am. Coll. Cardiol. 12, 1080–1089 (2012).
- Torino PA, De Bruyne B, Pijls NH et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. NEJM 3, 213–224 (2009).
- De Bruyne B, Pijls NH, Kalesan B et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. NEJM 11, 991–1001 (2012).
- Pijls NH, van Schaardenburgh P, Manoharan G et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5year follow-up of the DEFER Study. J. Am. Coll. Cardiol. 21, 2105–2111 (2007).
- Tanaka A, Imanishi T, Kitabata H et al. Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. Eur. Heart J. 11, 1348–1355 (2009).
- Ozaki Y, Tanaka A, Tanimoto T et al. Thin-cap fibroatheroma as high-risk plaque for microvascular obstruction in patients with acute coronary syndrome. Circ. Cardiovasc. Imaging 6, 620–627 (2011).
- Porto I, Di Vito L, Burzotta F. Predictors of periprocedural (type IVa) myocardial infarction, as assessed by frequency-domain optical coherence tomography. Circ. Cardiovasc. Interv. 1, 89–96 (2012).
- Yonetsu T, Kakuta T, Lee T et al. Impact of plaque morphology on creatine kinase-MB elevation in patients with elective stent implantation. Int. J. Cardiol. 1, 80–85 (2011).
- Gonzalo N, Serruys PW, Okamura T et al. Optical coherence tomography assessment of the acute effects of stent implantation on the vessel wall: a systematic quantitative approach. Heart 23, 1913–1919 (2009).
- Radu M, Jørgensen E, Kelbæk H et al. Optical coherence tomography at follow-up after percutaneous coronary intervention: relationship between procedural dissections, stent strut malapposition and stent healing. EuroIntervention 3, 353–361 (2011).
- Jang IK, Tearney G, Bouma B. Visualization of tissue prolapse between coronary stent struts by optical coherence tomography: comparison with intravascular ultrasound. Circulation 22, 2754 (2001).
- Kume T, Okura H, Miyamoto Y et al. Natural history of stent edge dissection, tissue protrusion and incomplete stent apposition detectable only on optical coherence tomography after stent implantation– preliminary observation. Circ. J. 76, 698–703 (2012).
- Parise H, Maehara A, Stone GW et al. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am. J. Cardiol. 107, 374–382 (2011).
- Jakabcin J, Spacek R, Bustron M et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidnance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc. Interv. 75, 578–583 (2010).
- Colombo A, Caussin C, Presbitero P et al. AVIO: a prospective, randomized trial of intravascular-ultrasound guided compared with angiography guided stent implantationin complex coronary lesions. J. Am. Coll. Cardiol. 56(Suppl. B), xvii (Abstract) (2010).
- Alegria-Barrero E, Foin N, Chan PH et al. Optical coherence tomography for guidance of distal cell recrossing in bifurcation stenting: choosing the right cell matters. EuroIntervention 2, 205–213 (2012).
- Okamura T, Yamada J, Nao T et al. Three-dimensional optical coherence tomography assessment of coronary wire re-crossing position during bifurcation stenting. EuroIntervention 7, 886–887 (2011).
- Kyono H, Guagliumi G, Sirbu V et al. Optical coherence tomography (OCT) strut-level analysis of drug eluting stents (DES) in human coronary artery bifurcations. EuroIntervention 1, 69–77 (2010).
- Gutierrez-Chico JL, Regar E, Nüesch E et al. Delayed coverage in malapposed and side-branch struts with respect to well-apposed struts in drug-eluting stents: in vivo assessment with optical coherence tomography. Circulation 5, 612–623 (2011).
- Liu Y, Imanishi T, Kubo T et al. Assessment by optical coherence tomography of stent struts across side branch. Comparison of bare-metal stents and drug-elution stents. Circ. J. 75, 106–112 (2011).
- Liu L, Gardecki JA, Nadkarni SK et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat. Med. 8,1010–1014 (2011).
- Giattina SD, Courtney BK, Herz PR et al. Assessment of coronary plaque collagen with polarization sensitive optical coherence tomography (PS-OCT). Int. J. Cardiol. 107, 400–409 (2006).
▪▪ Important expert review document on standardized definitions.
▪▪ Important expert review document on standardized definitions.
▪▪ One of the original papers on plaque characterization.
▪▪ Important paper on neovascularization.
▪▪ First paper assessing the clinical impact of optical coherence tomography-guided percutaneous coronary intervention.