Perspective - Interventional Cardiology (2014) Volume 6, Issue 1
Optimal stent design: past, present and future
- Corresponding Author:
- Pasi P Karjalainen
Heart Center, Satakunta Central Hospital,
Sairaalantie 3, FIN-28100, Pori, Finland
Tel: +358 2 627 7755
Fax: +358 2 627 7757
E-mail: pasi.karjalainen@satshp.fi
Abstract
The advancement of coronary stents has already come a long way since their first introduction in the mid-1980s. Coronary stent implantation has become the standard of care of percutaneous coronary interventions (PCIs), offering a safer strategy of coronary dilatation and securing a better outcome at short- and long-term follow-up, compared with ‘plain old’ balloon angioplasty.
Keywords
bare-metal stents, bioabsorbable vascular scaffolds, bioactive stents, biodegradable polymer, drug-eluting stents, self-expandable stents
The advancement of coronary stents has already come a long way since their first introduction in the mid-1980s. Coronary stent implantation has become the standard of care of percutaneous coronary interventions (PCIs), offering a safer strategy of coronary dilatation and securing a better outcome at short- and long-term follow-up, compared with ‘plain old’ balloon angioplasty [1,2]. Nevertheless, since the first-in-man deployment of coronary stents, in-stent restenosis (ISR) has always been the ‘Achilles heel’ of this technique, quite often leading to repeat revascularization by percutaneous or surgical means, and hence, an increased healthcare cost [3]. ISR results from neointimal hyperplasia, an exaggerated arterial healing response to vessel trauma induced by angioplasty and stent implantation. The incidence of target vessel revascularization (TVR) after first-generation bare-metal stent (BMS) implantation ranged from 20 to 30% at long-term follow-up [2,4], and was even higher in certain patient subsets; for example, diabetics [5]. This limited ‘efficacy’ of BMS was the main drive for the development of drug-eluting stents (DES). Over the past decade, the introduction of firstgeneration DES has revolutionized the practice of coronary intervention, reducing the rates of target lesion revascularization (TLR) by a half to two-thirds at long-term follow-up [6,7]. However, accumulating evidence from registries and meta-analyses has questioned the long-term safety of first-generation DES, raising concerns about a higher risk of late and very late stent thrombosis (ST) [8–10]. Further development of stent platforms with a better ‘safety’ profile has therefore become the focus of attention of the stent industry. This review discusses the progression of developments in stent platforms, and their impact on the efficacy and safety of contemporary PCI.
Bare-metal stents
The most commonly used material for BMS platforms is 316L stainless-steel; and the advantages include adequate mechanical properties and high corrosion resistance [11]. Its limitations, however, include low density, which limits its fluoroscopic visibility, and limited biocompatibility owing to the release of heavy-metal ions that may trigger a local inflammatory and immune response leading to neointimal hyperplasia, and contributes to ISR [12,13]. The cobalt–chromium alloy has an excellent radial strength, which allows the development of stents with ultrathin struts; yet, a preserved radial force. Other favorable characteristics include adequate radio-opacity and better deliverability [14,15].
DES with durable polymer
▪ First-generation durable polymer DES
The classic DES is composed of a metal stent platform coated with a polymer containing an active drug. From the polymer, the drug is slowly released over a predefined period. The first DES, Cypher® (Cordis Corporation, NJ, USA) had a stent platform made of the 316L stainlesssteel- based BX Velocity™ (Cordis Corporation) with a strut thickness of 140 μm, coated with a 10–15 μm thick layer of durable polymer matrix (1:1 mixture of the polymers polyethylenevinylacetate and polybutylmethacrylate) at a 30% drug-to-polymer weight ratio [16]. Sirolimus (rapamycin), a macrolide antibiotic initially approved for prevention of transplant organ rejection, acts by binding to an intracellular receptor (FK 506-binding protein 12) to inhibit a protein kinase (mTOR); hence it induces reversible arrest of the cell in the G1 phase, and thereby inhibits the proliferation of vascular smooth muscle cells, a key element of ISR. Early randomized controlled trials (RCTs) comparing the Cypher sirolimus-eluting stent (SES) with its BMS platform in relatively low-risk patient and lesion subsets demonstrated a significant reduction of binary restenosis at 1-year follow-up [17], as well as significantly lower rates of TLR and major adverse cardiac events (MACE) at 9-month, 2-year and 5-year follow-ups, compared with BMS controls [18–20]. Furthermore, other RCTs comparing the performance of SES versus BMS in higher-risk patient subsets, clinical settings and lesion types, consistently reported significant reductions of angiographic late loss, binary restenosis and repeat revascularization at short- and long-term follow-up [21–26]. Registries reporting the outcome of real-world patients similarly demonstrated lower rates of TLR and MACE with SES versus BMS at long-term follow-up [27,28]. Additionally, meta-analyses of former RCTs corroborated the benefit of SES over BMS in terms of decreased repeat revascularization, with similar rates of death and myocardial infarction (MI) at long-term follow-up [29,30].
In parallel, reports of the outcome of the Taxus™ Express™ (Boston Scientific, MA, USA) paclitaxel-eluting stent (PES) were published. Initially, the Taxus PES had a stent platform made of the 316L stainless-steelbased Express BMS (Boston Scientific) with a strut thickness of 132 μm; this platform has been replaced by the Liberté™ (Boston Scientific) platform with a strut thickness of 97 μm. Recently, the Taxus™ Element™ (Boston Scientific) stent was introduced, based on the platinum–chromium Element platform with a strut thickness of 81 μm, characterized by superior flexibility, deliverability and radial force, with a better radio-opacity. The Taxus PES is coated with a 16 μm layer of durable polymer matrix (polylactide-co-e-caprolactone) for controlled drug release [16]. The drug employed, paclitaxel, binds to microtubules that form the mitotic spindle during cell division, and are crucial to maintenance of cell shape, intracellular transport and motility, leading to inhibition of vascular cell proliferation, migration and signal transduction. Consequently, it induces irreversible arrest of the cell cycle during mitosis (G2/M phase). Similar to the Cypher SES, the Taxus PES was initially studied in low-risk patient and lesion categories. The first RCT comparing the Taxus PES with its control BMS, the TAXUS I trial, reported no binary restenosis at a 6-month follow-up [31]. Subsequent RCT similarly demonstrated significant reduction of angiographic late loss, binary restenosis and repeat revascularization in various patient and lesion subsets [32–34]. The T-SEARCH registry showed a similar efficacy in terms of reduction of restenosis comparable to SES [35]. Similarly, a patient-level meta-analysis confirmed the superior efficacy and comparable safety of PES versus BMS in up to 4 years of follow-up [36]. RCTs comparing SES with PES in unselected populations, as well as in various patient and lesion subsets demonstrated superior reduction of late loss and binary restenosis with SES versus PES at short-term follow-up [37–40]. However, the 5-year report of the largest RCT comparing SES versus PES (SIRTAX trial) demonstrated similar rates of MACE, cardiac death, MI and TLR in the two stent groups [41]. However, there was more delayed late loss and more TLR between 1 and 5 years associated with SES, compared with PES [41]. However, a meta-analysis of 16 RCTs comparing SES with PES reported a significant reduction of TLR and ST in the SES pooled group, with similar rates of death and MI at a median of 2-year follow-up [42].
The Endeavor® (Medtronic Vascular, CA, USA) zotarolimus-eluting stent (ZES) was studied at a later stage, relative to the Cypher and Taxus. It has the Driver BMS as its platform, made of L-605 cobalt–chromium alloy, with a strut thickness of 91 μm. The Endeavor ZES is coated with a 5-μm layer of phosphorylcholine durable polymer with a zotarolimus concentration of 1.6 μg/mm2 [16]. The ENDEAVOR III noninferiority trial (n = 436) compared ZES with SES. ZES was inferior to SES for the primary end point of in-segment late loss (and insegment binary restenosis) at 8-month angiographic follow-up [43]. In the same trial, ZES was associated with higher TLR at 9-month follow-up; however, clinically driven TLR, target vessel failure (TVF), ST and MACE were similar [43]. Alternatively, in the ENDEAVOR IV noninferiority trial (n = 1548), which compared ZES with PES, the Endeavor stent met the primary noninferiority clinical end point of TVF at 9 months [44]. At 12-month follow-up, the two stent groups were similar for the rates of cardiac death, MI, TVR, TLR and ST [44].
▪ Safety concerns with
first-generation DES
Attempts to quantify a rare event such as ST would logically necessitate the enrollment of thousands of patients and/or increasing the length of follow-up for many years. In this context, well-executed meta-analyses might possibly be of help. In a first meta-analysis by Stone et al., which pooled data from nine RCTs (including 5261 patients) comparing DES versus BMS, the incidence of ST was almost identical between the two stent types during the first year of follow-up (0.6%); however, between 1 and 4 years, that incidence was much higher with DES (0.5 vs 0.1%) [6]. In the same year, Mauri et al. published another meta-analysis of eight of these same trials [45]. This trial had access to patient-level data and the adjudication of events was now based on the newly introduced Academic Research Consortium (ARC) classification of ST. Ultimately, they concluded that the 4-year incidence of definite or probable ST was similar for SES versus BMS (1.5 vs 1.7%, respectively) as well as for PES versus BMS (1.8 vs 1.4%, respectively). Interestingly, almost a half of ST events occurred very late following DES implantation in comparison with approximately a third of such events with BMS. However, evidence of these meta-analyses clearly results from RCTs that were heavily constrained by long lists of exclusion criteria and, therefore, might not truly reflect real-world practice. In this sense, a registry provides important additional information. Daemen et al. reported a large real-life registry from two large European centers with a mean follow-up of 1.7 years [46]. The cumulative rate of definite ST was 1.1% early (within 1 month) following DES implantation; however, importantly thereafter, it occurred at a steady rate of 0.6% per year.
▪ Second-generation durable polymer DES
Second-generation DES were developed to address the safety concerns of late (beyond 30 days and up to 1 year) and very late (beyond 1 year) ST associated with first-generation DES. The XIENCE V® (Abbott Vascular, CA, USA) everolimus-eluting stent (EES) has a stent platform made of L-605 cobalt–chromium alloy, with a strut thickness of 81 μm, based on the MultiLink Vision® (Abbott Vascular) BMS. The XIENCE V EES is coated with a 6–8 μm layer of a durable polymer (consists of acrylic and fluoro polymers) for predefined controlled drug release [16]. Everolimus is a member of the limus family that is chemically modified in order to increase its solubility, binding affinity for FK 506-binding protein 12 and immunosuppressive activity. It is blended with the polymer in a concentration of 1 μg/mm2, and the stent is designed to release 80% of the drug within 30 days after its implantation with nearly all the drug released within 4 months. The SPIRIT RCT program compared the XIENCE V EES with PES (Taxus Express). In the early small (n = 300) SPIRIT II trial, in relatively low-risk patients and less complex lesions, with an angiographic primary end point, the XIENCE V EES was associated with a significant reduction of in-stent late loss compared with PES (0.11 vs 0.36 mm; p < 0.001), together with reduction of in-stent volume obstruction, and ischemia-driven MACE at 180-day follow-up [47]. At 3 years, individual clinical events were numerically lower, with a trend to lower MACE with EES [48]. Likewise, the SPIRIT III trial (n = 1002) demonstrated a significant reduction of the angiographic primary end point of in-segment late loss with EES compared with PES (0.14 vs 0.28 mm; p = 0.004) [49]. At 9-month follow-up, EES was associated with a significant reduction of TVF and MACE (a composite of cardiac death, MI and TLR; p < 0.001 and p = 0.03, respectively) [49]. In the 2-year report of the SPIRIT III trial, EES was associated with a significant reduction of TVF (10.7 vs 15.4%; p = 0.04) and MACE (7.3 vs 12.8%; p = 0.004) with a numerically lower rate of protocol-defined ST in patients who stopped thienopyridine at 6 months (0.4 vs 2.6%; p = 0.10) [50]. In the largest (n = 3687) SPIRIT IV trial, the primary clinical end point of target lesion failure was significantly lower with EES (4.2 vs 6.8%; p = 0.001) compared with PES at 12-month follow-up [51]. The 12-month rates of MI and ST were also lower with EES (1.9 vs 3.1%; p = 0.02 for MI and 0.17 vs 0.85%; p = 0.004 for ST) [51]. The results of the SPIRIT IV trial with a selected population were corroborated with those of the COMPARE trial (n = 1800) in an unrestricted allcomer population, comparing the XIENCE V EES with the Taxus Liberté PES for the primary composite end point of all-cause death, MI and TVR, at 12-month follow-up [52]. The primary end point occurred in 6% of patients in the EES group versus 9% in the PES group (p = 0.02). The difference was attributable to a lower rate of ST (0.7 vs 3%; p = 0.002), MI (3 vs 5%; p = 0.007) and TVR (2 vs 6%; p = 0.0001) [52]. The RESET noninferiority trial (n = 3197) compared the XIENCE V stent with a SES (Cypher Select™ Plus, Cordis Corporation, NJ, USA) for the primary efficacy end point of TLR at 1-year follow-up. The rates of TLR were similar (4.3% for EES and 5% for SES; p = 0.34, p noninferiority < 0.0001) [53]. Cumulative incidence of definite ST was low and similar (0.32 vs 0.38%, respectively; p = 0.77). In an angiographic substudy at 9 months, the primary angiographic end point of in-segment late loss was also similar [53].
The PROMUS Element™ (Boston Scientific) EES has a stent platform made of a platinum– chromium alloy, with a strut thickness of 81 μm, based on the Element platform design. The PROMUS Element EES is coated with a 8-μm layer of the same durable biocompatible fluoropolymer as the XIENCE V EES; however, the modified scaffold is designed to provide improved deliverability, vessel conformability, side-branch access, radio-opacity, radial strength and fracture resistance [54]. It was comparable to the XIENCE V EES regarding the everolimus release kinetics, arterial tissue levels and vascular responses in a noninjured porcine coronary artery model [55]. In the PLATINUM randomized trial (n = 1530), there were no significant differences between XIENCE V EES and PROMUS Element EES in the 12-month rates of target lesion failure (3.2 vs 3.5%; p = 0.72), cardiac death or MI (2.5 vs 2.0%; p = 0.56), TLR (1.9 vs 1.9%; p = 0.96), or ARC definite or probable ST (0.4 vs 0.4%; p = 1.00) [55]. The SORT OUT IV noninferiority trial (n = 2774) compared EES (XIENCE V and PROMUS Element) versus SES (Cypher Select Plus) for a primary composite clinical end point of safety and efficacy (cardiac death, MI, definite ST and TVR) at 9-month follow-up. The primary end point occurred in 4.9 versus 5.2%, respectively, p for noninferiority = 0.01 [56]. At 2 years, the composite primary end point was also similar; however, definite ST was lower in the EES group (0.2 vs 0.9%; p = 0.02) [57]. Moreover, in a noninferiority trial design, the EXCELLENT trial compared EES (XIENCE V and PROMUS Element) with SES (Cypher Select) for a primary angiographic end point of reducing late loss. At 9 months, in-segment late loss was 0.11 ± 0.38 mm and 0.06 ± 0.36 mm for EES and SES, respectively (p noninferiority = 0.038). In-stent late loss was also noninferior and the incidence of clinical end points was not statistically different between the two groups, including ST (0.37 vs 0.83%; p = 0.38) [58].
The Endeavor Resolute (Medtronic Vascular) ZES was modified from the Endeavor ZES to employ the BioLinx hydrophilic biocompatible polymer to release zotarolimus (at a concentration of 1.6 μg/mm2), based on the Driver BMS as its platform. BioLinx consists of three polymers, a biocompatible hydrophilic C19 polymer, water-soluble polyvinyl pyrrolidinone to allow an early burst of drug release, and a hydrophobic C10 polymer that provides a more delayed release of zotarolimus relative to the Endeavor stent. Nearly 50% of the drug is released during the first 7 days, and 85% released at 60 days after stent implantation [16]. The first-in-human study showed an in-stent late loss of 0.22 ± 0.27 mm, and in-segment binary restenosis of 2.1% at 9 months. At 12-month follow-up, the cumulative rate of MACE was 8.7% [59]. The RESOLUTE All Comers was a noninferiority RCT comparing the Endeavor Resolute ZES with the XIENCE V EES in an unrestricted population of 2292 patients. The Endeavor Resolute stent was noninferior for the primary end point of target lesion failure (a composite of cardiac death, any MI or clinically indicated TLR) to XIENCE V EES (8.2 and 8.3%, respectively; p < 0.001 noninferiority) at 12 months [60], and at 2 years (11.2 vs 10.7%; p = 0.73) [61].
The XIENCE PRIME™ (Abbott Vascular) EES was modified from the XIENCE V, with an enhanced stent delivery system that improves flexibility and deliverability, a higher rate burst pressure of the balloon and balloon tapers, to minimize edge dissection. In a small preclinical study in a rabbit iliac artery model comparing the XIENCE PRIME versus the Endeavor RESOLUTE ZES, vascular remodeling and endothelial coverage were similar in the stent groups; however, arterial drug-level concentration was lower in the EES group [62].
DES with biodegradable polymer
An important limitation to the DES technology is the permanent polymer left after the drug is completely released. Clinical and histopathological evidence identified the durable polymer as a potential trigger for vascular chronic inflammatory and hypersensitivity reaction, which might play a key role in very late ST, as well as delayed restenosis [63,64]. In order to address this concern, DES with biodegradable polymers were developed, which ultimately leave only the BMS platform behind.
▪ Biolimus-eluting biodegradable polymer DES
Biolimus-A9™ (Biosensors International PTE Ltd, Singapore) is a semisynthetic analog with a similar potency to sirolimus. It is immersed (concentration of 15.6 μg/mm2) in a biodegradable polymer made of polylactic acid applied to the abluminal surface of a stainless-steel stent; polylactic acid is degraded into carbon dioxide and water in 6 months [16]. DES utilizing Biolimus-A9 eluted from polylactic acid polymer include: BioMatrix Flex™ (Biosensors International PTE Ltd), Nobori® (Terumo Corporation, Tokyo, Japan), AXXESS™ bifurcation stent (Devax, Inc., CA, USA) and the XTENT® Custom NX™ modular system (Xtent, Inc., CA, USA). The Nobori I was compared with PES in two small randomized trials where the stent achieved a significant reduction of in-stent late loss at 9 months compared with PES [65,66]. In the multicenter LEADERS randomized noninferiority trial (n = 1707), BioMatrix Flex biolimus-eluting stent (BES) was compared with the Cypher Select SES. At 9-month follow- up, BES was noninferior to SES for the primary clinical end point (a composite of cardiac death, MI and TVR): 9.2 versus 10.5%, p for noninferiority = 0.003, p for superiority = 0.39 [67]. BES was also noninferior to SES for the in-stent percent diameter stenosis: 20.9 versus 23.3%, respectively [67]. At 4-year follow-up of the LEADERS trial, BES remained noninferior to SES for the primary end point: 18.7 versus 22.6%; p for noninferiority < 0.0001 and p for superiority = 0.05 [68]. The rate of ARC definite or probable ST at 4 years was numerically lower with BES (3 vs 5%; p = 0.2) [68].
The AXXESS stent is a self-expanding nitinolbased biodegradable polymer bifurcation BES. The AXXESS Plus registry (n = 139) reported 93.5% successful implantation of the device in the main branch; however, 80% of patients required two additional stents, 42% required three stents. At 6 months, in-stent late loss was 0.09 mm and TLR was 7.5% [69]. In the DIVERGE study (n = 302), 21.7% of patients required additional stenting of one side branch, 64.7% required stenting of both. At 9 months, TLR was 6.4% [70]. The XTENT Custom NX™ (Xtent, Inc.) stent is a customizable stent made up of multiple interdigitating 6-mm segments. This enables onsite customization of its length according to the lesion length. Its efficacy and safety were demonstrated both angiographically and clinically in the CUSTOM II trial (n = 100), with 9% MACE and in-segment late loss of 0.22 mm at 6 months [71].
▪ Sirolimus-eluting biodegradable polymer DES
The NEVO™ stent (Cordis, FL, USA) is a SES with a stent platform made of L-605 cobalt– chromium alloy, with a strut thickness of 99 μm, based on the COSTAR BMS. The NEVO SES is coated with a bioresorbable polylactic glycolic acid polymer that is absorbed within 3–4 months as assessed in porcine models. Nearly 80% of sirolimus is released during the first 30 days, and achieves a drug concentration in the arterial wall similar to that of the Cypher SES [16]. In the small randomized RES I trial (n = 394), the NEVO SES was compared with the Taxus Liberté PES for the primary angiographic end point of in-stent late loss in patients with single de novo native coronary artery lesions. At 6-month follow-up, in-stent late loss was significantly reduced with the NEVO SES (0.13 vs 0.36; p for noninferiority < 0.001, p for superiority < 0.001) with a trend to lower in-segment binary restenosis (3.9 vs 8.6%; p = 0.08) [72]. In one of the early attempts to use a biodegradable polymer SES, the TIVOLI stent (Essen Technology Beijing Co. Ltd, Beijing, China) was compared with the Endeavor ZES in a nonrandomized fashion [73]. In-stent late lumen loss and in-stent binary restenosis at 8-month follow-up were significantly lower with the TIVOLI stent, and at 2-year follow-up, TLR was significantly lower with the biodegradable polymer SES [73]. Another nonrandomized study showed similar in-stent late loss in patients who received EXCEL™ SES (JW Medical Co., Ltd, Shandong, China) and a durable polymer SES (0.14 vs 0.12 mm, respectively; p = 0.629) at 9-month follow-up [74]. A first-in-man report of the FIREHAWK® (MicroPort Medical, Shanghai, China), a novel biodegradable polymer SES with L-605 cobalt–chromium stent platform implanted in single de novo coronary lesions demonstrated an in-stent late loss of 0.13 mm at 4-month follow-up [75]. Optical coherence tomography performed at 4 months revealed a frequency of uncovered struts of 3.8%; the prevalence of malapposed struts was 0.1% [75]. In the TARGET I noninferiority RCT, the FIREHAWK SES was compared with the XIENCE V EES in patients with single de novo native coronary lesions [76]. At 9-month follow-up, the primary end point of in-stent late loss was similar in the two stent groups (0.13 vs 0.13 mm, respectively; p = 0.94, p for noninferiority < 0.0001); at 12 months, target lesion failure was similar (2.2 vs 2.2%). No definite or probable ST was observed at the 12-month follow-up in either stent group [76]. In a single-arm cohort of 50 patients with long lesions (35.2 ± 9.4 mm) who received the FIREHAWK stent with angiographic follow-up at 9 months, in-stent late loss was 0.16 ± 0.16 mm; no binary restenosis was observed [77]. The Orsiro (Biotronik AG, Bülach, Switzerland) SES has a stent platform coated with passive and active coating layers. The stent platform is the Pro-Kinetic Energy (Biotronik AG) BMS made of L-605 cobalt–chromium alloy with a helicoidal design and a strut thickness of 60 μm. The stent surface is completely coated with a layer of silicon carbide (PROBIO®, Biotronik AG) that acts as a diffusion barrier reducing ion release. The active coating layer (BIOlute®, Biotronik AG) consists of high-molecular weight poly-l-lactic acid that completely disintegrates into carbon dioxide and water. It covers the whole stent surface with an abluminal thickness of 7.5 μm, and a luminal thickness of 3.5 μm. The sirolimus concentration is 1.4 μg/mm2, with elution kinetics designed to achieve complete drug release in approximately 100 days. The BIOFLOW-I was a first-in-man study evaluating the efficacy and safety of the Orsiro SES in 30 patients with single de novo native coronary lesions [78]. Angiographic in-stent late loss was 0.05 ± 0.22 mm at the 9-month follow- up; at 12 months, the cumulative incidence of device-oriented MACE was 10%; no ST was observed. In a small subgroup of nine patients, intravascular ultrasound at 9-month follow-up revealed a very low mean net volume obstruction (0.07%) [78].
▪ Everolimus-eluting biodegradable polymer DES
The SYNERGY™ (Boston Scientific) EES consists of the platinum–chromium alloy Element stent platform delivering abluminal everolimus from a poly-lactide-co-glycolide biodegradable polymer with a polymer thickness of 3–4 μm. The abluminal drug delivery and biodegradable polymer provide more targeted drug delivery with complete absorption of drug and polymer by 4–6 months. In the EVOLVE prospective randomized noninferiority trial, comparing the SYNERGY EES in two dose formulations with the PROMUS Element durable polymer EES, the primary angiographic end point of in-stent late loss at 6 months was 0.15, 0.1 and 0.13 mm for the PROMUS Element, SYNERGY and SYNERGY half-dose, respectively, with p-value for noninferiority < 0.001 for both dose formulations [79].
DES with new antiproliferative drugs
▪ Novolimus-eluting durable polymer DES
The Elixir DESyne® (Elixir Medical, CA, USA) novolimus-eluting stent is based on a cobalt–chromium platform, with a durable polymer similar to that of the Cypher SES, with a polymer thickness <3 μm. Novolimus is a metabolite of sirolimus with a similar efficacy to currently available agents, but loaded at a lower drug dose at 5μg/mm2. The first-in-man small EXCELLA study (n = 15) demonstrated an angiographic in-stent late loss of 0.31 mm at 8 months [80]. Furthermore, the prospective randomized EXCELLA II trial (n = 210) compared the Elixir DESyne stent with a ZES. At 9 months, the primary angiographic end point in-stent late loss was 0.11 versus 0.63 mm, respectively; p < 0.0001. The device-oriented MACE was similar between the two stent groups: 2.9 versus 5.6%, respectively; p = 0.45 [81].
▪ Myolimus-eluting biodegradable polymer DES
The Elixir (Elixir Medical) myolimus-eluting stent is a thin-strut based on a cobalt–chromium platform, with a biodegradable polylactic acid polymer. Myolimus is a synthetic analog to sirolimus with a similar potency for inhibition of smooth muscle cells. The first-in-man small study (n = 15) demonstrated a 0.15 mm in-stent late loss at 6 months [82]. A multicenter registry (n = 30) showed a late loss of 0.08 mm at 6 months, and 0.13 mm at 12 months [83].
DES with no polymer
Further endeavors to eliminate the potential hazardous effect of polymers used for drug elution led to the introduction of polymer-free DES. The currently available technology is based on impregnation of the drug (in pure form) onto microporous surfaces. The YUKON® (Translumina, Hechingen, Germany) polymer-free SES is based on a stainless-steel platform with a microporous surface, on which sirolimus is applied directly without any polymer. More than two-thirds the total dose is released during the first 6 days, and sirolimus release remains measurable for over 21 days. The ISAR TEST 3 randomized 605 patients to receive one of three stent designs: a biodegradable polymer SES, the Cypher durable polymer SES or YUKON polymer-free SES [84]. At 6–8 months, the mean late lumen loss was 0.17, 0.23 and 0.47 mm, respectively. The biodegradable polymer SES met the prespecified criteria for noninferiority (p < 0.001), whereas the polymer- free SES did not (p = 0.94) [84]. At 2 years, there were no significant differences in TLR (8.4, 10.4 and 13.4%, respectively; p = 0.19), death/ MI (5.9, 6.4 and 6.5%, respectively; p = 0.97) or definite/probable ST (0.5, 1.0 and 1.0%, respectively; p = 0.82). However, paired angiographic follow-up at 6–8 months and 2 years revealed that delayed late loss was significantly different across the treatment groups: 0.17 (0.42), 0.16 (0.41) and -0.01 (0.36) mm, respectively (p < 0.001) [85].
The BIOFREEDOM™ (Biosensors SA, Morges, Switzerland) polymer-free BES is made of a stainless-steel platform (strut thickness 112 μm) with a microstructured surface alteration at the abluminal stent side onto which biolimus is applied with no polymer. More than 90% of the total dose is released within 50 h, and biolimus release remains detectable at 28 days. In a preclinical study in a rabbit model of denuded radiated iliac arteries, the BIOFREEDOM polymer- free BES significantly decreased neointimal hyperplasia at 4-week follow-up compared with BMS [86]. In a small, randomized clinical study, the BIOFREEDOM polymer-free BES in two different drug doses was compared with the Taxus Liberté durable polymer PES. At 4-month follow-up, the primary angiographic end point of late loss was significantly reduced in polymer-free BES with both doses compared with the Taxus Liberté. Intravascular ultrasound demonstrated the lowest neointimal volume obstruction in the polymer-free BES with standard drug dose [87].
Stents with bioactive coating
▪ Titanium–nitride–oxide-coated bioactive stents
The Titan2® (Hexacath, Paris, France) stent is a laser-cut slotted tube made of 316L stainless-steel coated with a thin atomic layer of titanium–nitride–oxide, and has the helistent BMS as its platform. Titanium exhibits a better biocompatibility as compared with other surface coating materials, since it minimizes toxic ion release, which would reduce tissue reaction and inflammation. In a preclinical study, coating with titanium oxide reduced platelet aggregation and fibrin deposition over the titaniumcoated carbon cylinders compared with uncoated ones, after implantation in a canine ventral aorta for 14 days [88]. A preclinical study in a porcine restenosis model investigated the outcome of a titanium–nitride–oxide-coated stent, and showed almost a 50% reduction of neointimal hyperplasia at 6-week follow-up (p < 0.05), compared with an uncoated BMS [89]. In an early prospective randomized comparison in an unselected population, titanium–nitride–oxide-coated stents significantly reduced late lumen loss by 40% (p = 0.03) versus BMS, at 6-month follow-up [90]. Thereafter, the safety of titanium–nitride–oxide-coated bioactive stents was established in several reports from real-world unselected populations [91,92]. In the TITAX AMI randomized multicenter trial (n = 425) in patients with acute MI, the Titan2 stent was associated with a comparable rate of MACE as PES at a 12-month follow-up (10.3 vs 12.8%; p = 0.5), with a lower rate of ARC-defined ST (0.9 vs 4.3%; p = 0.03) [93]. At 5‑year followup, the incidence of MACE was significantly lower in patients assigned to a Titan2 stent versus PES (16.4 vs 25.1%, respectively; p = 0.03). The rates of cardiac death, MI and definite ST were also significantly reduced (p < 0.05 for all) with a similar rate of ischemia-driven TLR (p = 0.92) [94]. The BASE ACS prospective multicenter randomized noninferiority trial (n = 827) compared the Titan2 stent with EES in patients with acute coronary syndrome. At 12-month follow-up, Titan2 proved noninferior to EES for the composite clinical end point of MACE (9.6 vs 9%, respectively; p = 0.81 and p for noninferiority = 0.001), with lower nonfatal MI (p = 0.007) and similar ischemia-driven TLR (p = 0.37) [95]. At 2 years, the rate of MACE was similar, with lower MI (p = 0.005) and similar TLR (p = 0.84) [96].
▪ Polyzene F-coated stents
The CATANIA™ (CeloNova BioSciences, GA, USA) stent based on a cobalt–chromium platform has a unique ultrathin (40 nm) surface coating of Polyzene F polymer (CeloNova BioSciences), a biocompatible, biostatic polymer with anti-inflammatory and prohealing properties. In the first-in-man prospective singlecenter ATLANTA study (n = 55), late loss was 0.60 ± 0.48 mm, the binary angiographic restenosis rate was 6.8% at 6 months and clinically driven TLR was 3.6% at 12 months [97]. In the single-arm ATLANTA 2 registry (n = 300), the incidence of MACE was 8.8%, cardiac death was 2.5%, MI was 0.7% and TLR was 6.5%, at 12-month follow-up [98].
▪ Endothelial progenitor cell-capturing stent
The Genous™ (OrbusNeich, FL, USA) stent, based on the R stent stainless-steel platform, is uniquely coated with immobile anti-CD34 antibodies on its luminal surface, capable of binding circulating endothelial progenitor cells bearing CD34+ cell surface antigens. In a small singlearm first-in-man study (n = 16), angiographic late loss was 0.63 ± 0.52 mm, with a MACE rate of 6.3% at 9 months [99]. In the HEALING II prospective registry (n = 63), the primary safety end point of MACE occurred in 7.9%, whereas clinically justified TLR occurred in 6.3%, at 9 months. At 6 months, in-stent late loss was 0.78 mm and in-stent volume obstruction was 22.9% [100]. However, from 6 to 18 months, a significant late regression (24.4% reduction) of neointimal hyperplasia was observed: late loss 0.59 mm at 18 months [101]. At 12 months, MACE and TLR increased to 15.6 and 11.5%, respectively [102].
The COMBO™ (OrbusNeich) stent was further modified from the Genous stent with sirolimus eluting from an abluminal biodegradable polymer (SynBiosys, Surmodics Inc., MN, USA). In a porcine model preclinical study, both optical coherence tomography and histology demonstrated that the COMBO stent technology promotes endothelialization while reducing neointimal formation and inflammation [103]. The REMEDEE trial was a first-inman prospective randomized multicenter trial comparing the COMBO stent versus a PES, with the primary end point of noninferiority for angiographic in-stent late loss. At 9 months, in-stent late loss was 0.39 versus 0.44 mm, respectively (p for noninferiority = 0.0012). At 12 months, the rate of MACE was 8.9 versus 10.2%, respectively (p = 0.80) [104].
Other stent designs
▪ Self-expandable stents
The Stentys stent (Stentys® SA, Paris, France) is a self-expandable stent made of nitinol, an alloy of nickel and titanium characterized by shape memory, biocompatibility, fatigue resistence and highly elastic qualities. It is available into two forms: a BMS and a PES (80 μm/cm2) with a durable polymer. In the OPEN I study – a small (n = 60) prospective, single-arm study – the Stentys stent (33 BMS and 27 PES) was implanted in bifurcation lesions. At 6 months, clinically driven TLR was 3.7 and 24.2%, and angiographic late loss in the proximal main branch was 0.39 and 0.86 mm, with PES and BMS, respectively [105]. In the APPOSITION I, another small (n = 25) prospective study, implantation of Stentys BMS in patients with ST-elevation MI undergoing primary PCI was associated with in-stent late loss of 0.71 mm, binary restenosis of 25% and clinically driven TLR of 12% at 6-month follow-up [106]. In the APPOSITION II (n = 80), implantation of Stentys BMS in patients with ST-elevation MI undergoing primary PCI was associated with a lower rate of malapposed struts at 3 days compared with a balloon-expandable BMS (0.58 vs 5.46%, respectively; p < 0.001) [107].
The CardioMind Sparrow™ stent (Cardio- Mind, Inc., CA, USA) is a guidewire-based, selfexpandable, ultrathin nitinol stent with smaller profile, improved flexibility and deliverability. The vProtect™ Luminal Shield is a self-expandable vascular shield with a rapid-exchange delivery system, made of a nickel–titanium alloy with strut thickness less than 70 μm. It was developed to scaffold soft nonobstructive coronary lesions, such as thin-cap fibroatheroma.
▪ Mesh-covered stents
The MGuard™ (InspireMD, Tel Aviv, Israel) is a balloon-expandable thin-strut (100 μm) stainless-steel BMS covered with a polymer mesh sleeve of polyethylene terephthalate (thickness 20 μm) on its outer surface. The fibers act like a net (aperture size 150 × 180 μm) preventing distal embolization of atherothrombotic debris. In the INSPIRE single-arm study (n = 80), implantation of MGuard in patients with lesions at high risk of distal embolization (55% acute coronary syndrome and 57% saphenous vein grafts) was associated with final thrombolysis in MI three flow in all cases; however, at 6-month follow-up, in-stent late loss was 1.0 ± 0.4 mm, and at 12-month follow-up, ischemia-driven TLR was 20% [108]. The MASTER prospective multicenter trial randomized 433 patients with ST-elevation MI undergoing primary PCI for either the MGuard stent or a control BMS or DES. The primary end point of complete ST segment resolution was better with the MGuard stent (57.8 vs 44.7%; p = 0.008); final thrombolysis in MI flow was superior (91.7 vs 82.9%; p = 0.006), with comparable rates of MACE at 30 days (p > 0.05) [109]. In another small (n = 40) randomized trial in patients with ST-elevation MI undergoing primary PCI, the MGuard stent was associated with better myocardial blush grade and corrected thrombolysis in MI frame count, compared with a BMS [110].
▪ Dedicated bifurcation stents
The Tryton Side Branch Stent™ (Tryton Medical, Inc., NC, USA) is a balloon-expandable cobalt–chromium thin-strut (76 μm) BMS. Its design uses the Tri-Zone™ technology: a distal zone scaffolding the side branch, a transition zone at the carina and a main branch zone with a minimal amount of metal allowing easy delivery of a standard stent in the main branch. The stent delivery system has four markers to delineate the proximal and distal end of the stent, as well as the proximal and distal extent of the transition zone [111]. In the first-in-man Tryton I trial (n = 30), implantation of the Tryton stent with a standard DES in the main branch was associated with a MACE rate of 9.9%, and a late loss of 0.17 ± 0.35 mm in the side branch and 0.24 ± 0.43 mm in the main branch, at 6-month follow-up [112]. In a real-world registry (n = 96), TLR was 4%, MI was 3% and cardiac death was 1% at the 6-month follow-up [113]. In another prospective registry (n = 302), procedural success was 94.4%, and the cumulative 6-month MACE was 6.4%, including 4.7% was MI (3.7% was periprocedural) and 3.4% was TLR [114]. In the PYTON study, 20 patients with bifurcation lesions were treated with the Tryton stent with XIENCE V EES implantation in the main branch. At the 9-month follow-up (n = 16), angiographic late loss was 0.34, 0.29 and 0.57 mm in the proximal main branch, distal main branch and side branch, respectively [115]. Binary ISR occurred in four (25%) patients, and the percentage of uncovered struts by optical coherence tomography was 4, 0.7 and 2.5% in the proximal main branch, distal main branch and side branch, respectively [115].
The Sideguard® (Cappella Medical Devices Ltd, Galway, Ireland) side branch stent is a balloon- deployed self-expandable thin-strut (76 μm) nitinol stent with anatomic funnel-shaped flaring at the side branch ostium. It secures the side branch for easy access after main branch stenting, preventing side branch closure from carina or plaque shift [116]. In the Sideguard 1 first-in-man study (n = 20), 6-month follow-up with intravascular ultrasound (available for 11 patients) demonstrated preserved side branch ostial lumen area due to an additional increase of stent area that compensated for the occurrence of neointimal hyperplasia [117]. Another study with intravascular ultrasound immediately following implantation demonstrated that acute lumen gain in the side branch stented by the Sideguard stent is less than that in the main branch stented by a balloon-expandable stent, and suggested plaque shift from the proximal main vessel to the side branch [118].
Bioabsorbable stents
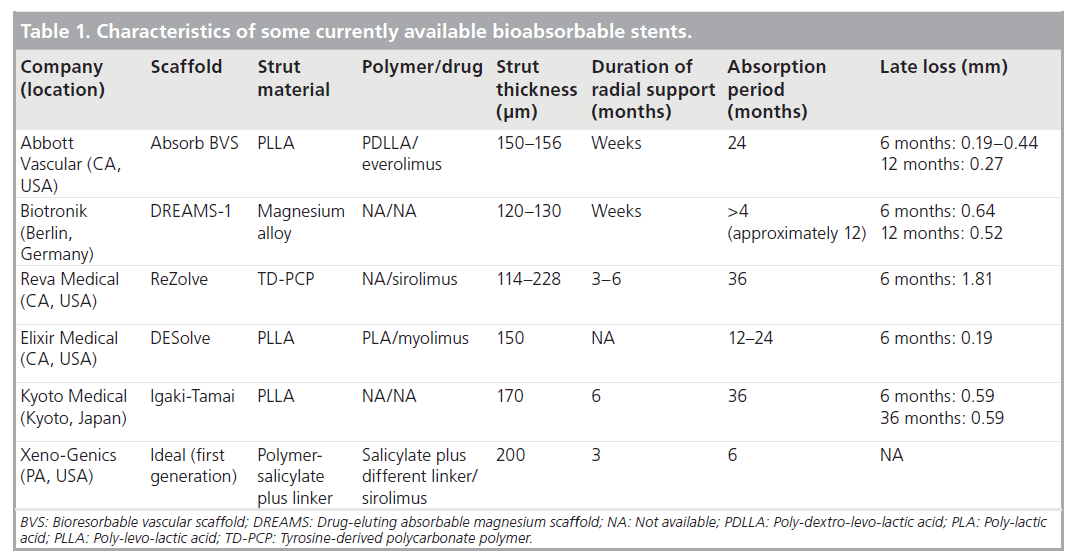
The advantages of completely absorbable stents include the potential reduction of chronic inflammation and hypersensitivity reaction in the vessel wall, improved vasomotion and adaptive shear stress, the feasibility of subsequent percutaneous and surgical revascularization, preservation of side branches, and not interfering with noninvasive imaging techniques (Table 1) [119].
▪ Everolimus-eluting polymer-based bioresorbable vascular scaffolds
The bioresorbable vascular scaffold (BVS; Abbott Vascular) EES is based on a backbone of poly-llactic acid, coated with a thin layer of amorphous poly-d,l-lactic acid that contains everolimus at a dose of 8.2 μg/mm2 and enables elution of 80% of everolimus content by 30 days (similar to XIENCE V). The natural loss of polymer mass is nearly 30% at 12 months, 60% at 18 months and the polymer is fully absorbed over 2 years. The stent is radiolucent with two platinum markers at each end. The initial radial strength following implantation is similar to the XIENCE V stent, as indicated by comparable acute vessel recoil [119]. The first BVS (revision 1.0) has a strut thickness of 150 μm, consisting of out-of-phase zigzag hoops, with struts connected either directly or by thin straight bridges, furthermore it has to be stored below -20°C. The second generation device (revision 1.1) has improved stent design with in-phase zigzag hoops, struts connected by bridges, providing a more consistent drug release, and a more uniform strut distribution and vessel wall support. Although it has a similar absorption time to the first BVS (2 years), the modified manufacturing process of the polymer provides longer radial support compared with revision 1.0. It also provides a greater stent security with less risk of stent dislodgement. ABSORB was a prospective open-label study that explored revision 1.0 BVS in 30 patients (cohort A) with single de novo lesions. The device success rate was 94%. At 12 months, only one case of non-Q-wave MI occurred (3.3%). At 6 months, angiographic instent late loss was 0.44 mm [120]. The 6-month late loss was a combination of neointimal hyperplasia and a reduction of scaffold area, the latter resulting from acute and chronic scaffold recoil. Chronic recoil occurs as a result of loss of radial strength with bioresorption. At the 2-year follow-up, no further MACE (cardiac death, MI and ischemia-driven TLR) were observed, angiographic in-stent late loss (0.48 mm) was comparable to that at 6 months, and 34.5% of strut locations presented no discernible features by optical coherence tomography. Vasomotion was preserved at the stented site and adjacent coronary artery in response to vasoactive agents [121]. At 3 years, with 29 patients available for follow-up, MACE remained at 3.4%, two patients underwent nonischemia-driven TVR and no cases of ST were reported [122]. Revision 1.1 BVS was then studied in 56 patients (cohort B) with multiple imaging modalities at baseline and 12 months. Overall scaffold area remained unchanged with both intravascular ultrasound and optical coherence tomography, with an angiographic late loss of 0.27 mm. MACE rate was 7.1%: two MI and two repeat interventions [123]. However, in the ABSORB EXTEND study (n = 435), BVS was associated with a higher incidence of postprocedural side-branch occlusion (6%), compared with the corresponding incidence reported with the XIENCE V stent (n = 237) in the SPIRIT I and II trials (4.1%). The effect was more pronounced in side branches with small reference vessel diameter (0.5 mm) [124]. The ABSORB II trial is underway with the prospect to randomize 501 patients to either the new generation Absorb BVS or the XIENCE EES, with the primary end point of superiority in vasomotor reactivity of the treated segment at 2-year follow-up [125].
▪ Magnesium-based bioabsorbable scaffolds
The AMS-1 (Biotronik, Berlin, Germany) absorbable metal scaffold (93% magnesium, strut thickness 165 μm) has mechanical properties comparable to a stainless-steel stent: low elastic recoil, a high collapse pressure and minimal shortening after inflation. Preclinical assessment demonstrated rapid endothelialization and magnesium degradation within 60 days into inorganic salts with little inflammatory response [126]. In the PROGRESS AMS, a small prospective (n = 63) nonrandomized study, AMS-1 stent was associated with 23.8% ischemia-driven TLR at 4 months, and 45% overall TLR at 1 year; in-stent late loss was 1.08 mm at 4 months [127]. Substantial late loss was due to a lower initial radial force, compared with a BMS, and progressive loss of such radial force by early rapid degradation. The AMS-2 (strut thickness 120 μm) was then designed to address these downsides with a different magnesium alloy, higher collapse pressure and slower degradation time.
The DREAMS (Biotronik) is an absorbable metal scaffold (based on the AMS-2 stent) that elutes paclitaxel (80 μm/cm2) from a 1-μm thick layer of biodegradable polymer (polylactic-co-glycolic acid). Controlled paclitaxel release and polymer degradation occurs in 3 months, and scaffold absorption is complete after 9–12 months. In the BIOSOLVE-1 firstin- man, multicenter, single-arm trial (n = 46), clinically driven TLR at 12 months was 5%, and in-scaffold late loss was 0.65 and 0.52 mm at 6 and 12 months, respectively [128].
Future perspective
Coronary stents are a pivotal component of current PCI. Over the past decade, the introduction of DES has revolutionized the practice of coronary intervention, reducing the rates of TLR by a half to two-thirds at long-term follow-up The improved outcomes with DES have led to expanding the indications for PCI, which is now an accepted treatment for diabetic patients and patients with complex coronary artery disease such as acute coronary syndrome. However, accumulating evidence from registries and metaanalyses has questioned the long-term safety of first-generation DES, raising concerns about a higher risk of late and very late ST. The persisting concerns over ST have led to improvements in stent design, and the later generation DES have demonstrated early improvements in safety compared with previous generation DES. Further development of stent platforms with a better ‘safety’ profile has therefore become the focus of attention of the stent industry. At the present time, the selection of BMS or DES for various lesions and patients, and the duration of antiplatelet therapy remain debated areas; therefore, long-term RCTs are urgently required.
BVS combined with optimal medical therapy could provide an alternative treatment modality to metallic stents. Currently, at least 16 different scaffolds are being developed. These devices provide structural support function for the first year after deployment, and are then completely absorbed into the vascular wall. These novel devices could have a number of potential advantages including normalization of vascular function, restoration of physiological vascular responses to shear stress, and completion of the vascular response to stenting. This could be the next revolution in interventional cardiology, and many of these scaffolds are currently undergoing preclinical and clinical trials; nevertheless, early results seem promising.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Bare-metal stents
• Cobalt–chromium alloys offered greater radial strength and better radio-opacity compared with stainless-steel-based bare-metal stents.
Drug-eluting stents with durable polymer
• First-generation drug-eluting stents (DES) demonstrated superior efficacy in terms of reduction of angiographic late loss as well as target lesion revascularization, compared with bare-metal stents, in unselected patients, as well as in high-risk patient and lesion subsets.
• Registries and meta-analyses of randomized controlled trials raised concerns about higher rates of late (and very late) stent thrombosis associated with first-generation DES.
• Second-generation DES with a durable polymer demonstrated an efficacy outcome similar to first-generation DES in terms of reduction of late loss and target lesion revascularization, with a better safety outcome in terms of stent thrombosis reduction.
DES with biodegradable polymer
• The biolimus-eluting stent proved noninferior to a durable polymer sirolimus-eluting stent (SES) for the composite end point of safety and efficacy outcome with a numerically lower rate of stent thrombosis at long-term follow-up.
• Various biodegradable polymer SES proved superior to a durable polymer palcitaxel-eluting stent and Endeavor zotarolimus-eluting stents, and comparable to durable polymer SES and everolimus-eluting stents (EES) for angiographic in-stent and in-segment late loss.
• Novel bioabsorbable polymer-coated EES proved noninferior to a durable polymer EES for the primary angiographic end point of in-stent late loss at the 6-month follow-up.
DES with no polymer
• The polymer-free SES failed to show noninferiority for the reduction of late lumen loss compared with a durable polymer SES, with a similar clinical outcome in the long term.
• The polymer-free biolimus-eluting stent proved superior to a durable polymer palcitaxel-eluting stent for angiographic late loss.
Stents with bioactive coating
• The titanium–nitride–oxide-coated stent reduced late loss versus bare-metal stents. In two randomized controlled trials, the Titan2 stent was comparable to a palcitaxel- and an EES for the clinical efficacy end point, with superiority for the safety outcome, at long-term follow-up.
Bioabsorbable stents
• In the ABSORB cohort A, the revision 1.0 bioabsorbable vascular scaffold fully bioresorbable EES was associated with a modest reduction of late loss at 6 months, and 2 years: a combination of neointimal hyperplasia and chronic scaffold recoil. In the ABSORB cohort B, revision 1.1 bioabsorbable vascular scaffold, with improved stent design and longer radial support compared with Revision 1.0, demonstrated a better reduction of late loss at 12 months.
• Although the absorbable magnesium scaffold stent was associated with substantial late loss at 4 months, and a high rate of target lesion revascularization at 12 months, the DREAMS biodegradable polymer paclitaxel-eluting magnesium-based absorbable stent was associated with modest reduction of late loss at 6 and 12 months, and 5% target lesion revascularization at 12 months.
References
Papers of special note have been highlighted as: • of interest
- Serruys PW, de Jaegere P, Kiemeneij F et al. A comparison of balloon-expandable stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 331, 489–495 (1994).
- Fischman DL, Leon MB, Baim DS et al. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 331, 496–501 (1994).
- Peterson ED, Cowper PA, DeLong ER, Zidar JP, Stack RS, Mark DB. Acute and long-term cost implications of coronary stenting. J. Am. Coll. Cardiol. 33, 1610–1618 (1999).
- Kastrati A, Hall D, Schömig A. Long-term outcome after coronary stenting. Curr. Control. Trials Cardiovasc. Med. 1, 48–54 (2000).
- Abizaid A, Kornowski R, Mintz GS et al. The influence of diabetes mellitus on acute and late clinical outcomes following coronary stent implantation. J. Am. Coll. Cardiol. 32, 584–589 (1998).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356, 998–1008 (2007).
- Morice MC, Serruys PW, Barragan P et al. Long-term clinical outcomes with sirolimus-eluting coronary stents: five-year results of the R AVEL trial. J. Am. Coll. Cardiol. 50, 1299–1304 (2007).
- Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur. Heart J. 27, 2784–2814 (2006).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293, 2126–2130 (2005).
- Lagerqvist B, James SK, Stenestrand U et al.Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N. Engl. J. Med. 356, 1009–1019 (2007).
- Taylor A. Metals. In: Endoluminal Stenting. Sigwart U (Ed.), WB Saunders Company Ltd, London, UK, 28–33 (1996).
- Köster R, Vieluf D, Kiehn M et al. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet 356, 1895–1897 (2000).
- Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials 28, 1689–1710 (2007).
- Kereiakes D, Cox D, Hermiller J et al. Usefulness of a cobalt chromium coronary stent alloy. Am. J. Cardiol. 92, 463–466 (2003).
- Klocke A, Kemper J, Schulze D, Adam G, Kahl-Nieke B. Magnetic field interactions of orthodontic wires during magnetic resonance imaging (MRI) at 1.5 Tesla. J. Orofac. Orthop. 66, 279–287 (2005).
- Räber L, Windecker S. Current status of drug-eluting stents. Cardiovasc. Ther. 29, 176–189 (2011).
- Morice MC, Serruys PW, Sousa JE et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl.J. Med. 346, 1773–1780 (2002).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349, 1315–1323 (2003).
- Weisz G, Leon MB, Holmes DR Jr et al. Two-year outcomes after sirolimus-eluting stent implantation: results from the Sirolimus-Eluting Stent in de novo Native
Coronary Lesions (SIRIUS) trial. J. Am. Coll. Cardiol. 47, 1350–1355 (2006). - Weisz G, Leon MB, Holmes DR Jr et al. Five-year follow-up after sirolimus-eluting stent implantation: results of the SIRIUS (Sirolimus-Eluting Stent in de-novo Native Coronary Lesions) trial. J. Am. Coll. Cardiol. 53, 1488–1497 (2009).
- Schofer J, Schluter M, Gershlick AH et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (ESIRIUS). Lancet 362, 1093–1099 (2003).
- Jimenez-Quevedo P, Sabate M, Angiolillo DJ et al. Long-term clinical benefit of sirolimus-eluting stent implantation in diabetic patients with de novo coronary
stenoses: long-term results of the DIABETES trial. Eur. Heart J. 28, 1946–1952 (2007). - Atary JZ, van der Hoeven BL, Liem SS et al. Three-year outcome of sirolimus-eluting versus bare-metal stents for the treatment of ST-segment elevation myocardial infarction (from the MISSION Intervention study). Am. J. Cardiol. 106, 4–12 (2010).
- Violini R, Musto C, De Felice F et al. Maintenance of long-term clinical benefit with sirolimus-eluting stents in patients with ST segment elevation myocardial infarction 3-year results of the SESAMI
(Sirolimus-Eluting Stent Versus Bare-Metal Stent in Acute Myocardial Infarction) trial. J. Am. Coll. Cardiol. 55, 810–814 (2010). - Rahel BM, Laarman GJ, Kelder JC, Ten Berg JM, Suttorp MJ. Three-year clinical outcome after primary stenting of totally occluded native coronary arteries: a randomized comparison of bare-metal stent implantation with sirolimus-eluting stent implantation for the treatment of total coronary occlusions (Primary Stenting of Totally Occluded Native Coronary Arteries [PRISON] II study). Am. Heart J. 157, 149–155 (2009).
- Vermeersch P, Agostoni P, Verheye S et al. Increased late mortality after sirolimus-eluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC trial. J. Am. Coll. Cardiol. 50, 261–267 (2007).
- Daemen J, Kukreja N, van Twisk PH et al. Four-year clinical follow-up of the rapamycin-eluting stent evaluated at Rotterdam Cardiology Hospital registry. Am. J. Cardiol. 101, 1105–1111 (2008).
- Serruys PW, Onuma Y, Garg S et al. 5-Year clinical outcomes of the ARTS II (Arterial Revascularization Therapies Study II) of the sirolimus-eluting stent in the treatment of patients with multivessel de novo coronary artery lesions. J. Am. Coll. Cardiol. 55, 1093–1101 (2010).
- Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356, 989–997 (2007).
- Kastrati A, Mehilli J, Pache J et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356, 1030–1039 (2007).
- Grube E, Silber S, Hauptmann KE et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 107, 38–42 (2003).
- Stone GW, Ellis SG, Cannon L et al. Comparison of a polymer based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA 294, 1215–1223 (2005).
- Stone GW, Lansky AJ, Pocock SJ et al. Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N. Engl. J. Med. 360, 1946–1959 (2009).
- Erglis A, Narbute I, Kumsars I et al. A randomized comparison of paclitaxel-eluting stents versus bare-metal stents for treatment of unprotected left main coronary artery stenosis. J. Am. Coll. Cardiol. 50, 491–497 (2007).
- Daemen J, Keiichi T, Kristensen SD et al. Two-year clinical follow-up of the unrestricted use of the paclitaxel-eluting stent compared with the sirolimus-eluting stent as part of the Taxus-Stent Evaluated at Rotterdam Cardiology Hospital (T-SEARCH) registry. EuroIntervention 2, 330–337 (2006).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356, 998–1008 (2007).
- Morice MC, Colombo A, Meier B et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions. The REALITY trial: a randomized controlled trial. JAMA 295, 895–904 (2006).
- Galloe AM, Thuesen L, Kelbaek H et al. Comparison of paclitaxel and sirolimus-eluting stents in everyday clinical practice: the SORT OUT II randomized trial. JAMA 299, 409–416 (2008).
- Kim HS, Lee JH, Lee SW et al. Long-term safety and efficacy of sirolimus- vs. paclitaxel-eluting stent implantation for acute ST-elevation myocardial infarction: 3-year follow-up of the PROSIT trial. Int. J. Cardiol. 147, 253–257 (2011).
- Lee SW, Park SW, Kim YH et al. A randomized comparison of sirolimus- versus paclitaxel-eluting stent implantation in patients with diabetes mellitus: 2-year clinical outcomes of the DESDIABETES trial. J. Am. Coll. Cardiol. 53, 812–813 (2009).
- Räber L, Wohlwend L, Wigger M et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the sirolimus-eluting versus paclitaxel-eluting stents for coronary revascularization late trial. Circulation 123, 2819–2828 (2011).
- Schomig A, Dibra A, Windecker S et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J. Am. Coll. Cardiol. 50, 1373–1380 (2007).
- Kandzari DE, Leon MB, Popma JJ et al. Comparison of zotarolimus-eluting and sirolimus-eluting stents in patients with native coronary artery disease: a randomized controlled trial. J. Am. Coll. Cardiol. 48, 2440–2447 (2006).
- Leon MB, Mauri L, Popma JJ et al. A randomized comparison of the Endeavor zotarolimus-eluting stent versus the Taxus paclitaxel-eluting stent in de novo native coronary lesions 12-month outcomes from the ENDEAVOR IV trial. J. Am. Coll. Cardiol. 55, 543–554 (2010).
- Mauri L, Hsieh WH, Massaro JM et al. Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356, 1020–1029 (2007).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369, 667–678 (2007).
- Serruys PW, Ruygrok P, Neuzner J et al. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent: the SPIRIT II trial. EuroIntervention 2, 286–294 (2006).
- Garg S, Serruys P, Onuma Y et al. Three-year clinical follow-up of the XIENCE V everolimus-eluting coronary stent system in the treatment of patients with de novo coronary artery lesions. The SPIRIT II trial. JACC Cardiovasc. Interv. 2, 1190–1198 (2009).
- Stone GW, Midei M, Newman W et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA 299, 1903–1913 (2008).
- Stone GW, Midei M, Newman W et al. Randomized comparison of everolimus-eluting and paclitaxel-eluting stents: two-year clinical follow-up from the Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) III trial. Circulation 119, 680–686 (2009).
- Stone GW, Rizvi A, Newman W et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N. Engl. J. Med. 362, 1663–1674 (2010).
- Kedhi E, Joesoef KS, McFadden E et al. (COMPARE): a randomised trial. Lancet 375, 201–209 (2010).
- Kimura T, Morimoto T, Natsuaki M et al. Comparison of everolimus-eluting and sirolimus-eluting coronary stents: 1-year outcomes from the Randomized Evaluation of Sirolimus-eluting versus Everolimus-eluting stent trial (RESET). Circulation 126, 1225–1236 (2012).
- Stone GW, Teirstein PS, Meredith IT et al. A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a prospective, randomized, multicenter trial to assess an
everolimus-eluting coronary stent system [PROMUS Element] for the treatment of up to two de novo coronary artery lesions) trial. J. Am. Coll. Cardiol. 57, 1700–1708 (2011). - Wilson GJ, Huibregtse BA, Stejskal EA et al. Vascular response to a third generation everolimus-eluting stent. EuroIntervention 6, 512–519 (2010).
- Jensen LO, Thayssen P, Hansen HS et al. Randomized comparison of everolimus-eluting and sirolimus-eluting stents in patients treated with percutaneous coronary intervention: the Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). Circulation 125, 1246–1255 (2012).
- Jensen LO, Thayssen P, Christiansen EH et al. 2-year patient-related versus stent-related outcomes: the SORT OUT IV (Scandinavian Organization for Randomized Trials with Clinical Outcome IV) trial. J. Am. Coll. Cardiol. 60, 1140–1147 (2012).
- Park KW, Chae IH, Lim DS et al. Everolimus- eluting versus sirolimus-eluting stents in patients undergoing percutaneous coronary intervention: the EXCELLENT (Efficacy of XIENCE/PROMUS versus Cypher to Reduce Late Loss After Stenting) randomized trial. J. Am. Coll. Cardiol. 58, 1844–1854 (2011).
- Meredith IT, Worthley S, Whitbourn R et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc. Interv. 2, 977–985 (2009).
- Serruys PW, Silber S, Garg S et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N. Engl. J. Med. 363, 136–146 (2010).
- Silber S, Windecker S, Vranckx P et al. Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet 377, 1241–1247 (2011).
- Yazdani SK, Sheehy A, Nakano M et al. Preclinical evaluation of second-generation everolimus- and zotarolimus-eluting coronary stents. J. Invasive Cardiol. 25, 383–390 (2013).
- Cook S, Ladich E, Nakazawa G et al. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 120, 391–399 (2009).
- Byrne RA, Iijima R, Mehilli J et al. Durability of antirestenotic efficacy in drug-eluting stents with and without permanent polymer. JACC Cardiovasc. Interv. 2, 291–299 (2009).
- Chevalier B, Serruys PW, Silber S et al. Randomised comparison of Nobori, Biolimus A9-eluting coronary stent with a Taxus®, paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the Nobori 1 trial. EuroIntervention 2, 426–434 (2007).
- Chevalier B, Silber S, Park SJ et al. Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: the NOBORI 1 trial – Phase 2. Circ. Cardiovasc. Interv. 2, 188–195 (2009).
- Windecker S, Serruys PW, Wandel S et al. Biolimus eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomized noninferiority trial. Lancet 372, 1163–1173 (2008).
- Stefanini GG, Kalesan B, Serruys PW et al. Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non- inferiority trial. Lancet 378, 1940–1948 (2011).
- Grube E, Buellesfeld L, Neumann FJ et al. Six-month clinical and angiographic results of a dedicated drug-eluting stent for the treatment of coronary bifurcation narrowings. Am. J. Cardiol. 99, 1691–1697 (2007).
- Verheye S, Agostoni P, Dubois CL et al. 9-month clinical, angiographic, and intravascular ultrasound results of a prospective evaluation of the AXXESS self-expanding Biolimus A9-eluting stent in coronary bifurcation lesions: the DIVERGE (Drug-Eluting Stent Intervention for Treating Side Branches Effectively) study. J. Am. Coll. Cardiol. 53, 1031–1039 (2009).
- Stella PR, Mueller R, Pavlakis G et al. One year results of a new in situ length-adjustable stent platform with a biodegradable Biolimus A9 eluting polymer: results of the CUSTOM-II trial. EuroIntervention 4, 200–207 (2008).
- Ormiston JA, Abizaid A, Spertus J et al. Six-month results of the NEVO Res-Elution I (NEVO RES-I) trial: a randomized, multicenter comparison of the NEVO sirolimus-eluting coronary stent with the Taxus Liberté paclitaxel-eluting stent in de novo native coronary artery lesions. Circ. Cardiovasc. Interv. 3, 556–564 (2010).
- Xu B, Dou KF, Han YL et al. A prospective multicenter parallel-controlled trial of TIVOLI biodegradable-polymer-based sirolimus-eluting stent compared to Endeavor zotarolimus-eluting stent for the treatment of coronary artery disease: 8-month angiographic and 2-year clinical follow-up results. Chin. Med. J. 124, 811–816 (2011).
- Kan J, Chen F, Liu LY et al. Quantitative assessment of late lumen loss after biodegradable polymer and permanent polymer sirolimus-eluting stents implantation. Chin. Med. J. 126, 1081–1085 (2013).
- Qian J, Xu B, Lansky AJ et al. First report of a novel abluminal groove filled biodegradable polymer rapamycin-eluting stent in de novo coronary artery disease: results of the first in man FIREHAWK trial. Chin. Med. J. 125, 970–976 (2012).
- Gao RL, Xu B, Lansky AJ et al. A randomised comparison of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: clinical and angiographic follow-up of the TARGET I trial. EuroIntervention 9, 75–83 (2013).
- Xu B, Gao RL, Zhang RY et al. Efficacy and safety of FIREHAWK abluminal groove filled biodegradable polymer sirolimus-eluting stents for the treatment of long coronary lesions: nine-month angiographic and one-year clinical results from TARGET I trial long cohort. Chin. Med. J. 126, 1026–1032 (2013).
- Hamon M, Niculescu R, Deleanu D, Dorobantu M, Weissman NJ, Waksman R. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): a prospective, first-in- man study. EuroIntervention 8, 1006–1011 (2013).
- Meredith IT, Verheye S, Dubois CL et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J. Am. Coll. Cardiol. 59, 1362–1370 (2012).
- Costa JR Jr, Abizaid A, Feres F et al. EXCELLA first-in-man (FIM) study: safety and efficacy of novolimus-eluting stent in de novo coronary lesions. EuroIntervention 4, 53–58 (2008).
- Serruys PW, Garg S, Abizaid A et al. A randomised comparison of novolimus-eluting and zotarolimus-eluting stents: 9-month results of the EXCELLA II study. EuroIntervention 6, 195–205 (2010).
- Rutsch W. Multi-center first-in-man study with the lowest known limus dose on the Elixir Medical myolimus eluting coronary stent system with a durable polymer: nine month clinical and six month angiographic and IVUS follow-up. Presented at: EuroPCR. Barcelona, Spain, 19–22 May 2009.
- Schofer J. Multicentre, first-in-man study on the Elixir myolimus eluting coronary stent system with bioabsorbable polymer: 12-month clinical and angiographic/IVUS results. Presented at: EuroPCR. Paris, France, 25–28 May 2010.
- Mehilli J, Byrne RA, Wieczorek A et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur. Heart J. 29, 1975–1982 (2008).
- Byrne RA, Kufner S, Tiroch K et al. Randomised trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis: 2-year follow-up results. Heart 95, 1489–1494
(2009). - Waksman R, Pakala R, Baffour R et al. In vivo comparison of a polymer-free Biolimus A9-eluting stent with a biodegradable polymer-based Biolimus A9 eluting stent and a bare metal stent in balloon denuded and radiated hypercholesterolemic rabbit iliac arteries. Catheter Cardiovasc. Interv. 80, 429–436 (2012).
- Grube E. A prospective, randomized, noninferiority trial comparing biolimus drug coated-polymer free stent BIOFREEDOM versus paclitaxel-eluting stent with durable polymer Taxus Liberte. Presented at: Trancatheter Cardiovascular Therapeutics. San Francisco, CA, USA, 24 September 2009.
- Zhang F, Zheng Z, Chen Y, Liu X, Chen A, Jiang Z. In vivo investigation of blood compatibility of titanium oxide films. J. Biomed. Mater. Res. 42, 128–133 (1998).
- Windecker S, Mayer I, De Pasquale G et al. Stent coating with titanium–nitride–oxide for reduction of neointimal hyperplasia. Circulation 104, 928–933 (2001).
- Windecker S, Simon R, Lins M et al. Randomized comparison of a titanium–nitride–oxide-coated stent with a stainless steel stent for coronary revascularization. The TINOX trial. Circulation 111, 2617–2622 (2005).
- Mosseri M, Miller H, Tamari I et al. The titanium-NO stent: results of a multicenter registry. EuroIntervention 2, 192–196 (2006).
- Karjalainen PP, Ylitalo A, Airaksinen KE, Nammas W. Five-year clinical outcome of titanium–nitride–oxide-coated bioactive stent implantation in a real world population: a comparison with paclitaxel-eluting stents: The PORI registry. J. Interv. Cardiol. 24, 1–8 (2011).
- Karjalainen PP, Ylitalo A, Niemela M et al. Titanium–nitride–oxide coated stents versus paclitaxel-eluting stents in acute myocardial infarction: a 12-months follow-up report from the TITAX AMI trial. EuroIntervention 4, 234–241 (2008).
- Tuomainen PO, Ylitalo A, Niemelä M et al. Five-year clinical outcome of titanium–nitride–oxide-coated bioactive stents versus paclitaxel-eluting stents in patients with acute myocardial infarction: long-term follow-up from the TITAX AMI trial. Int. J. Cardiol. 168(2), 1214–1219 (2013).
- Karjalainen PP, Niemelä M, Airaksinen JK et al. A prospective randomised comparison of titanium–nitride–oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: the BASE-ACS trial. EuroIntervention 8, 306–315 (2012).
- Romppanen H, Nammas W, Kervinen K et al. Stent-oriented versus patient-oriented outcome in patients undergoing early percutaneous coronary intervention for acute coronary syndrome: 2-year report from the BASE-ACS trial. Ann. Med. 45(7), 488–493 (2013).
- Tamburino C, La Manna A, Di Salvo ME et al. First-in-man 1-year clinical outcomes of the CATANIA coronary stent system with nanothin Polyzene-F in de novo native coronary artery lesions: the ATLANTA (Assessment of The LAtest Non-Thrombogenic Angioplasty stent) trial. JACC Cardiovasc. Interv. 2, 197–204 (2009).
- Tamburino C, Capodanno D, Di Salvo ME et al. Safety and effectiveness of the CATANIA Polyzene-F coated stent in real world clinical practice: 12-month results from the ATLANTA 2 registry. EuroIntervention 7, 1062–1068 (2012).
- Aoki J, Serruys PW, van Beusekom H et al. Endothelial progenitor cell capture by stents coated with antibody against CD34: the Healing-FIM (Healthy Endothelial Accelerated Lining Inhibits Neointimal Growth-First In Man) registry. J. Am. Coll. Cardiol. 45, 1574–1579 (2005).
- Duckers HJ, Silber S, de Winter R et al. Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the Healing-II trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention 3, 67–75 (2007).
- Duckers HJ, Soullié T, den Heijer P et al. Accelerated vascular repair following percutaneous coronary intervention by capture of endothelial progenitor cells promotes regression of neointimal growth at long term follow-up: final results of the Healing II trial using an endothelial progenitor cell capturing stent (Genous R stent). EuroIntervention 3, 350–358 (2007).
- den Dekker WK, Houtgraaf JH, Onuma Y et al. Final results of the Healing IIB trial to evaluate a bio-engineered CD34 antibody coated stent (Genous™ stent) designed to promote vascular healing by capture of circulating endothelial progenitor cells in CAD patients. Atherosclerosis 219, 245–252 (2011).
- Granada JF, Inami S, Aboodi MS et al. Development of a novel prohealing stent designed to deliver sirolimus from a biodegradable abluminal matrix. Circ. Cardiovasc. Interv. 3, 257–266 (2010).
- Haude M, Lee SW, Worthley SG et al. The REMEDEE trial: a randomized comparison of a combination sirolimus-eluting endothelial progenitor cell capture stent with a paclitaxel-eluting stent. JACC Cardiovasc. Interv. 6, 334–343 (2013).
- Verheye S, Ramcharitar S, Grube E et al. Six-month clinical and angiographic results of the STENTYS® self-apposing stent in bifurcation lesions. EuroIntervention 7, 580–587 (2011).
- Amoroso G, van Geuns RJ, Spaulding C et al. Assessment of the safety and performance of the Stentys self-expanding coronary stent in acute myocardial infarction: results from the APPOSITION I study. EuroIntervention 7, 428–436 (2011).
- van Geuns RJ, Tamburino C, Fajadet J et al. Self-expanding versus balloon-expandable stents in acute myocardial infarction: results from the APPOSITION II study: self-expanding stents in ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 5, 1209–1219 (2012).
- Costa JR Jr, Abizaid A, Feres F et al. One-year results of the INSPIRE trial with the novel MGuard stent: serial analysis with QCA and IVUS. Catheter Cardiovasc. Interv. 78, 1095–1100 (2011).
- Stone GW, Abizaid A, Silber S et al. Prospective, randomized, multicenter evaluation of a polyethylene terephthalate micronet mesh-covered stent (MGuard) in ST-segment elevation myocardial infarction: the MASTER trial. J. Am. Coll. Cardiol. 60, 1975–1984 (2012).
- Lindefjeld DS, Guarda E, Méndez M et al. Microvascular coronary flow comparison in acute myocardial infarction angioplasty treated with a mesh covered stent (MGuard stent) versus bare metal stent: Micami-MGuard. Cardiovasc. Revasc. Med. 14, 4–8 (2013).
- Magro M, van Geuns RJ. The Tryton side branch stent. EuroIntervention 6(Suppl.), J147–J150 (2010).
- Onuma Y, Müller R, Ramcharitar S et al. Tryton I, first-in-man (FIM) study: six month clinical and angiographic outcome, analysis with new quantitative coronary angiography dedicated for bifurcation lesions. EuroIntervention 3, 546–552 (2008).
- Magro M, Wykrzykowska J, Serruys PW et al. Six-month clinical follow-up of the Tryton side branch stent for the treatment of bifurcation lesions: a two center registry analysis. Catheter Cardiovasc. Interv. 77, 798–806 (2011).
- Agostoni P, Foley D, Lesiak M et al. A prospective multicentre registry, evaluating real-world usage of the Tryton side branch stent: results of the E-Tryton 150/Benelux registry. EuroIntervention 7, 1293–1300 (2012).
- Dubois C, Adriaenssens T, Ughi G et al. Healing responses after bifurcation stenting with the dedicated Tryton Side-Branch Stent™ in combination with XIENCE-V™ stents: a clinical, angiography, fractional flow reserve, and optical coherence tomography study: the PYTON (Prospective evaluation of the Tryton Side-Branch Stent™ with an additional XIENCE-V™ everolimus-eluting stent in coronary bifurcation lesions) study. Catheter Cardiovasc. Interv. 81, E155–E164 (2013).
- Latib A, Chieffo A. The Cappella Sideguard™ stent. EuroIntervention 6(Suppl.), J143–J146 (2010).
- Doi H, Maehara A, Mintz GS, Dani L, Leon MB, Grube E. Serial intravascular ultrasound analysis of bifurcation lesions treated using the novel self-expanding sideguard side branch stent. Am. J. Cardiol. 104, 1216–1221 (2009).
- Ma S, Maehara A, Hauptmann KE et al. Intravascular ultrasound comparison of the self-expanding sideguard stent in the side branch versus a balloon-expandable stent in the main vessel to assess mechanisms of acute lumen gain in bifurcation lesions. Catheter Cardiovasc. Interv. 82, 748–754 (2013).
- Tanimoto S, Serruys PW, Thuesen L et al. Comparison of in vivo acute stent recoil between the bioabsorbable everolimus-eluting coronary stent and the everolimus-eluting cobalt chromium coronary stent: insights from the ABSORB and SPIRIT trials. Catheter Cardiovasc. Interv. 70, 515–523 (2007).
- Ormiston JA, Serruys PW, Regar E et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet 371, 899–907 (2008).
- Serruys PW, Ormiston JA, Onuma Y et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet 373, 897–910 (2009).
- Onuma Y, Serruys PW, Ormiston JA et al. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention 6, 47–53 (2010).
- Serruys PW, Onuma Y, Dudek D et al. Evaluation of the second generation of a bioresorbable everolimus-eluting vascular scaffold for the treatment of de novo coronary artery stenosis: 12-month clinical and imaging outcomes. J. Am. Coll. Cardiol. 58, 1578–1588 (2011).
- Muramatsu T, Onuma Y, García-García HM et al. Incidence and short-term clinical outcomes of small side branch occlusion after implantation of an everolimus-eluting bioresorbable vascular scaffold: an interim report of 435 patients in the ABSORB-EXTEND single-arm trial in comparison with an everolimus-eluting metallic stent in the SPIRIT first and II trials. JACC Cardiovasc. Interv. 6, 247–257 (2013).
- Diletti R, Serruys PW, Farooq V et al. ABSORB II randomized controlled trial: a clinical evaluation to compare the safety, efficacy, and performance of the Absorb everolimus-eluting bioresorbable vascular scaffold system against the XIENCE everolimus-eluting coronary stent system in the treatment of subjects with ischemic heart disease caused by de novo native coronary artery lesions: rationale and study design. Am. Heart J. 164, 654–663 (2012).
- Waksman R, Pakala R, Kuchulakanti PK et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter Cardiovasc. Interv. 68, 607–617; discussion 618–619 (2006).
- Erbel R, Di Mario C, Bartunek J et al.; PROGRESS-AMS (Clinical Performance and Angiographic Results of Coronary Stenting with Absorbable Metal Stents) Investigators. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet 369(9576), 1869–1875 (2007).
- Haude M, Erbel R, Erne P et al. Safety and performance of the drug-eluting absorbable metal scaffold (DREAMS) in patients with de-novo coronary lesions: 12 month results of the prospective, multicentre, first-in-man BIOSOLVE-I trial. Lancet 381(9869), 836–844 (2013).
• Landmark registry of the incidence and predictors of stent thrombosis after first-generation drug-eluting stents (DES).
• Landmark randomized controlled trial (RCT) comparing the clinical outcome of a sirolimus-eluting stent with a bare-metal stent.
• The largest RCT, to date, comparing DES with bare-metal stents for clinical outcome in patients with acute myocardial infarction.
• Important meta-analysis of 16 RCTs comparing sirolimus-eluting stents with paclitaxel-eluting stents.
• Landmark registry reporting the incidence of stent thrombosis following first-generation DES in real-world practice.
• Landmark RCT comparing second- with first-generation DES.
• Landmark RCT comparing two durable-polymer second-generation DES.
• Landmark RCT comparing a biodegradablepolymer second-generation DES with a durable polymer first-generation DES.
• Landmark RCT comparing a novel bioactivecoated stent with a second-generation DES in patients with acute coronary syndrome.
• First-in-man report of the outcome of a fully bioabsorbable vascular scaffold.