Research Article - Interventional Cardiology (2024) Volume 16, Issue 5
Optimal weight based unfractionated heparin dosing during percutaneous coronary intervention
- Corresponding Author:
- Ray Sriratana Tabucanon
Department of Cardiology, Thammasat University Hospital, Pathum Thani, Thailand,
E-mail: raybm019@gmail.com
Received date: 22-Oct-2024, Manuscript No. FMIC-24-150599; Editor assigned: 24-Oct-2024, PreQC No. FMIC-24-150599 (PQ); Reviewed date: 07-Nov-2024, QC No. FMIC-24-150599; Revised date: 14-Nov-2024, Manuscript No. FMIC-24-150599 (R); Published date: 21-Nov-2024, DOI: Crossref
Abstract
Introduction: Unfractionated Heparin (UFH) is a potent and preferable anticoagulant agent during Percutaneous Coronary Intervention (PCI). Activated clotting time (ACT) is a good assay for accurate titration of UFH during PCI; however, the optimal range of ACT during PCI is narrow (250-300 sec by Hemotech system). The guideline recommendation of the loading dose of UFH is 70 U/Kg-100 U/Kg but the optimal loading dose is unclear. The aim of this study was to evaluate the number of optimal ACT levels 30 min after administration of 70 U/kg, 80 U/kg, 90 U/kg and 100 U/ kg IV UFH.
Method: This study was performed in Thammasat University Hospital from February 15, 2019 to March 15, 2019. Forty patient candidates for PCI were enrolled in the study. Patients were randomized into 4 groups (1:1:1:1), 70 U/kg, 80 U/kg, 90 U/ kg and 100 U/kg IV UFH (10 patients per group). Data including demographic and risk factors were collected. Baseline ACT and ACT 30 min after IV bolus UFH were measured using Hemotech system. The primary study objective was to determine the number of ACT in the therapeutic range. The secondary objectives were divided into efficacy endpoint (thrombotic events) and safety endpoint (bleeding as defined by the Bleeding Academic Research Consortium criteria).
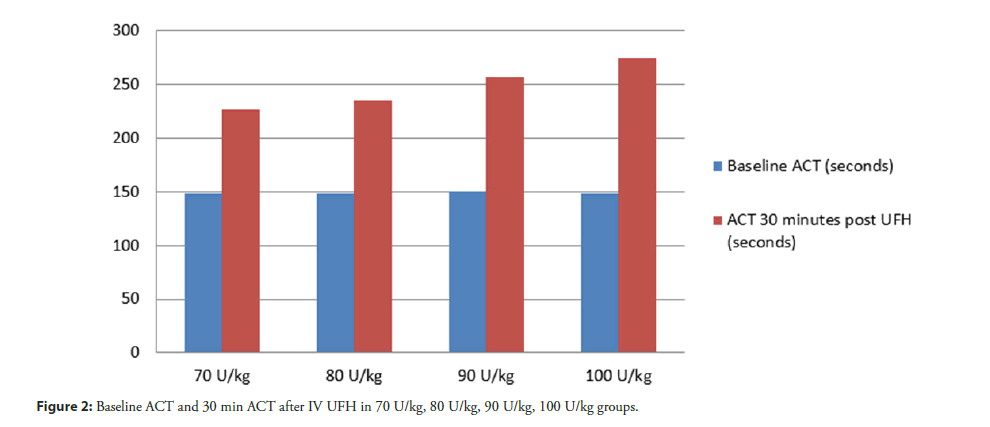
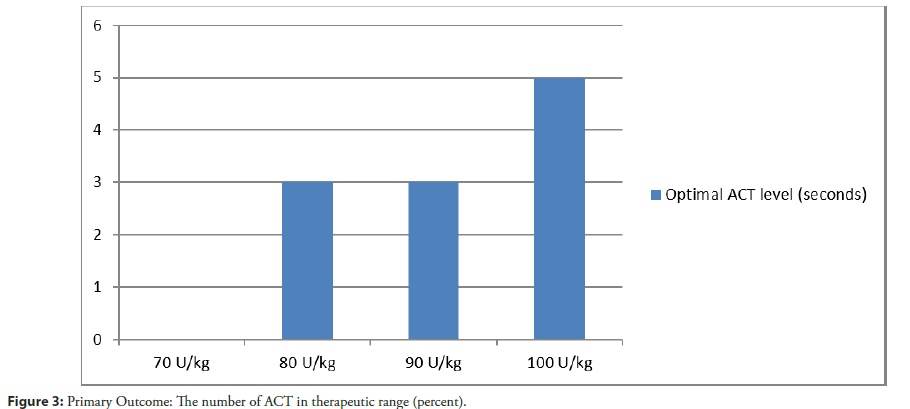
Result: The median of baseline ACT were 148, 148, 150 and 148 sec respectively (χ2=0.106, dF=3, P=0.991). The median of ACT 30 min after IV bolus UFH were 227, 235, 257 and 275 sec respectively (χ2=7.2, dF=5.728, p=0.126). The number of ACT in the therapeutic range was 0 (0%) in 70 U/kg, 3 (30%) in 80 U/kg, 3 (30%) in 90 U/kg, 5 (50%) in 100 U/kg (χ2=6.716, dF=3, p=0.083). There was one event of minor bleeding in the 100 U/kg group. No thrombotic event was observed in this study.
Conclusion: The finding suggested that there is no statistically significant difference among groups. The 100 U/kg IV UFH may tend to achieve the most optimal ACT level in patients who underwent percutaneous coronary intervention.
Keywords
Percutaneous coronary intervention • Periprocedural ischemic • Hemochron device • Myocardial revascularization • Coagulation cascade
Introduction
PCI has developed a pivotal role in the management of patients with stable or unstable coronary artery disease. The inhibition of the coagulation cascade, platelet activation, adhesion and aggregation are key steps to optimize the result of PCI and prevent periprocedural ischemic complication. UFH is the primary anticoagulant agent for preventing ischemic complications [1]. However, UFH has a poorly predictable effect on the coagulation cascade and a relatively narrow therapeutic window [2,3]. Consequently, the measurement of ACT at the time of PCI has been advocated to mitigate both ischemic and bleeding events during intervention. An intravenous UFH bolus of 70 U/kg to 100 U/kg is recommended to achieve a target ACT of 250 to 300 sec (Hemotech device) or 300 to 350 (Hemochron device) without planned use of Glycoprotein IIb/IIIa Inhibitors (GPI) or 50 U/kg to 70 U/kg bolus to achieve an ACT of 200 to 250 sec when the concomitant use of GPI is anticipated.
2018 ESC/EACTS Guidelines on myocardial revascularization [4], recommend UFH 70 U/kg to 100 U/kg as the standard anticoagulant. However, the optimal dose of UFH is unclear. The aim of this study was to evaluate the rate of optimal ACT levels 30 min after administration of 70 U/kg, 80 U/kg, 90 U/kg and 100 U/kg IV heparin.
Materials and Methods
Study design
This study was a pilot, randomization study in which 70 U/ kg, 80 U/kg, 90 U/kg and 100 U/kg IV UFH were compared in patients undergoing percutaneous coronary intervention at Thammasat University Hospital, Pathum Thani, Thailand, From February 15 2019 to March 15, 2019. The study protocol was approved by the human research ethics committee of Thammasat University (MTU-EC-IM-0-281/61). Written informed consent was obtained from the patient prior to the enrollment. Patients were randomized into 4 groups (70 U/kg, 80 U/kg, 90 U/kg and 100 U/kg IV UFH) on patients undergoing PCI. Data including demographic and risk factors were collected. The SYNTAX score is calculated by a computer program consisting of sequential and interactive self-guided questions. Baseline ACT and ACT 30 min after IV bolus UFH were measured using Medtronic ACT II (Hemotech system) (Figure 1). ACT results were recorded as the average (mean) of the two results if the difference between the two channels is less than 12% of the mean. If the precision is not within range, the patient results cannot be accepted.
Patients
Eligible patients had coronary artery disease which indicated the need for PCI, aged 18 years or older. We excluded patients who received UFH within 4 h, Enoxaparin within 12 h, Fondaparinux within 24 h, Fibrinolytic within 7 days and patients who had a history of active bleeding, hematocrit <20%, required pack red blood cells more than 2 units in 24 h, history of hemorrhagic stroke, history of Heparin Induced Thrombocytopenia (HIT), history of UFH allergy, Coagulopathy (PT>13.8 sec, PTT>29.1 sec, INR>1.4) and mistake of data collection (e.g., error of ACT value, delay ACT collection time). From February 15, 2019 to March 15, 2019, a total of 45 patients were enrolled. 5 patients were excluded from the study. ACT samples were delayed in 4 patients and ACT measurement was erroneous in 1 patient. Eventually, 40 patient candidates for PCI were enrolled in the study. The enrolled patients were randomized into 4 groups (1:1:1:1), 70 U/kg, 80 U/kg, 90 U/kg and 100 U/kg IV UFH (10 patients per group). Randomization was conducted with the use of a block size of four.
UFH administration strategy
A baseline ACT measurement before administrating UFH was performed in all patients undergoing PCI. ACT samples were drawn through the arterial sheath immediately after the insertion. To clear the samples from the flush solution contaminated by UFH, 3 mL of blood were withdrawn from the arterial sheath while UFH was given to the intravenous catheter. There was no UFH in the flush solution. In all groups, the intraprocedural anticoagulant effect of UFH was monitored at 30 min after the first dose of UFH. The optimal level of ACT is 250-300 sec using the Medtronic ACT II system. UFH anticoagulant was reversed with protamine at neutralizing doses, if necessary, according to the operator decision.
Study objective and data collection
The primary study objective was to determine the number of ACT in the therapeutic range. The secondary study objectives were divided into efficacy endpoint (in hospital thrombotic events) and safety endpoint (in hospital bleeding as defined by the Bleeding Academic Research Consortium (BARC) criteria).
Statistical analysis
A sample size of N=40 patients (10 patients/group) was determined to be sufficient for investigating the feasibility of the future randomized controlled trial. Continuous variables were presented as mean ± SD or as median (Interquartile Range (IQR)). Categorical variables were presented as frequencies and percentages. Patient characteristics were assessed with the use of a one-way ANOVA test for continuous variables and a Fisher Exact test for categorical variables. Data were tested for normality by the Shapiro-Wilk test. Kruskal-Wallis test was applied if data still did not conform to a normal distribution. Primary and secondary outcomes were compared using the Fisher Exact test. Pearson’s correlation was used to access the relationship between dose of UFH and number of ACT in the therapeutic range. For all analyses, a 2-sided p<0.05 indicated statistical significance. Statistical analysis was performed using Statistical Package for Social Science (version 21.0, SPSS, Inc., Chicago, Illinois).
Results
From February 15, 2019 through March 15, 2019, a total of 40 patients were enrolled at Thammasat University Hospital. The characteristics of the patients at baseline were well balanced among the trial groups (Table 1). The median age among all the patients was 67.5 years and 72.5% of the patients were male. The prevalence of hypertension, diabetes, heart failure or previous MI was similar in all the groups. Among the patients who underwent randomization, 18 out of 40 patients (45%) had acute coronary syndrome. The median SYNTAX score was 24 (interquartile range, 6 to 37). No patient had previous use of oral anticoagulant. Systemic anticoagulant was discontinued as inclusion criteria before they underwent randomization in the trial. A total of 40 patients underwent PCI during the study period. The baseline clinical and procedural characteristics of the study group are summarized in Table 1. The median of baseline ACT in 70 U/kg, 80 U/kg, 90 U/ kg and 100 U/kg IV UFH groups were 148, 148, 150 and 148 sec respectively (χ2=0.106, dF=3, P=0.991) (Table 2). Primary study objective. The number of ACT in the therapeutic range (250-300 sec) in 70 U/kg, 80 U/kg, 90 U/kg and 100 U/kg IV UFH groups were 0 (0%), 3 (30%), 3 (30%), 5 (50%) respectively (χ2=6.716, dF=3, P=0.083) (Figure 2 and Table 3). The median of ACT 30 min after IV UFH were 227, 235, 257 and 275 sec respectively (χ2=7.2, dF=5.728, P=0.126) (Figure 3). 100 U/kg IV UFH may have a tendency to achieve the most optimal ACT level in patient who underwent percutaneous coronary intervention.
| 70 U/kg (N=10) | 80 U/kg (N=10) | 90 U/kg (N=10) | 100 U/kg (N=10) | p-value (N=10) | |
|---|---|---|---|---|---|
| Male | 8 (80%) | 7 (70%) | 8 (80%) | 6 (60%) | 0.865 |
| Age-years | 67.7 ± 8.21 | 64 ± 10.76 | 68.9 ± 13.10 | 64.8 ± 12.23 | 0.733 |
| Smoking | 2 (20%) | 0 (0%) | 2 (20%) | 1 (10%) | 0.726 |
| Alcohol | 0 (0%) | 0 (0%) | 0 (0%) | 1 (10%) | 1 |
| FH of CAD | 1 (10%) | 3 (30%) | 0 (0%) | 1 (10%) | 0.357 |
| Weight | 62 ± 11.86 | 72 ± 15.59 | 72 ± 11.65 | 67 ± 13.83 | 0.134 |
| Height | 164 ± 6.93 | 167 ± 9.27 | 163 ± 8.01 | 160 ± 12.30 | 0.5 |
| BMI | 23 ± 2.75 | 26 ± 8.37 | 27 ± 4.49 | 23 ± 2.71 | 0.213 |
| BSA | 1.66 ± 0.18 | 1.79 ± 0.14 | 1.77 ± 0.16 | 1.64 ± 0.24 | 0.183 |
| Hypertension | 6 (60%) | 3 (30%) | 7 (70%) | 7 (70%) | 0.257 |
| Diabetes | 6 (60%) | 3 (30%) | 4 (40%) | 6 (60%) | 0.538 |
| Dyslipidemia | 7 (70%) | 3 (30%) | 4 (40%) | 7 (70%) | 0.218 |
| Heart failure | 0 (0%) | 1 (10%) | 0 (0%) | 0 (0%) | 1 |
| Myocardial infarction | 2 (20%) | 3 (30%) | 2 (20%) | 0 (0%) | 0.457 |
| Previous PCI | 2 (20%) | 2 (20%) | 1 (10%) | 1 (10%) | 1 |
| Previous CABG | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Renal insufficiency | 3 (30%) | 3 (30%) | 1 (10%) | 1 (10%) | 0.568 |
| ESRD | 2 (20%) | 2 (20%) | 0 (0%) | 0 (0%) | 0.3 |
| Prior stroke | 2 (20%) | 1 (10%) | 1 (10%) | 1 (10%) | 1 |
| Clinical presentation | |||||
| Stable CAD | 6 (60%) | 8 (80%) | 4 (40%) | 4 (40%) | 0.444 |
| Unstable angina | 0 (0%) | 0 (0%) | 1 (10%) | 1 (10%) | |
| NSTEMI | 3 (30%) | 1 (10%) | 4 (40%) | 5 (50%) | |
| STEMI | 1 (10%) | 1 (10%) | 1 (10%) | 0 (0%) | |
| Aspirin | 10 (100%) | 10 (100%) | 10 (100%) | 10 (100%) | 1 |
| Clopidogrel | 9 (90%) | 8 (80%) | 8 (80%) | 9 (90%) | 1 |
| Ticagrelor | 1 (10%) | 1 (10%) | 1 (10%) | 1 (10%) | 1 |
| Prasugrel | 0 (0%) | 1 (10%) | 0 (0%) | 0 (0%) | 1 |
| Beta-blocker | 5 (50%) | 4 (40%) | 5 (50%) | 8 (80%) | 0.332 |
| Statin | 9 (90%) | 10 (100%) | 9 (90%) | 10 (100%) | 1 |
| ACEI/ARB | 4 (40%) | 7 (70%) | 6 (60%) | 3 (30%) | 0.331 |
| Insulin | 1 (10%) | 2 (20%) | 1 (10%) | 1 (10%) | 1 |
| Oral hypoglycemic drug | 3 (30%) | 1 (10%) | 1 (10%) | 2 (20%) | 0.804 |
| Hb | 11.21 ± 1.91 | 11.67 ± 2.17 | 12.22 ± 2.06 | 12.67 ± 2.26 | 0.422 |
| Platelets × 103 | 240 ± 73 | 247 ± 62 | 231 ± 45 | 286 ± 105 | 0.382 |
| PT | 13.22 ± 1.72 | 13.03 ± 0.91 | 12.79 ± 0.77 | 13.12 ± 1.37 | 0.885 |
| PTT | 26.57 ± 5.46 | 24.78 ± 1.27 | 26.11 ± 4.02 | 24.8 ± 2.77 | 0.616 |
| INR | 1.06 ± 0.13 | 1.05 ± 0.09 | 1.04 ± 0.04 | 1.07 ± 0.11 | 0.912 |
| EF (%) approach | 42 ± 9.74 | 53 ± 18.55 | 54 ± 13.42 | 54 ± 14.5 | 0.201 |
| Radial | 5 (50%) | 6 (60%) | 6 (60%) | 7 (70%) | 0.969 |
| Femoral | 5 (50%) | 4 (40%) | 4 (40%) | 3 (30%) | |
| Puncture attempt | 1 ± 0 | 1.1 ± 0.32 | 1.2 ± 0.41 | 1.3 ± 0.48 | 0.288 |
| 70 U/kg | 80 U/kg | 90 U/kg | 100 U/kg | p-value | |
| (N=10) | (N=10) | (N=10) | (N=10) | ||
| Sheath size | 6.1 ± 0.32 | 6.3 ± 0.48 | 6 ± 0 | 6.15 ± 0.36 | 0.288 |
| Vessel involvement | |||||
| SVD | 0 (0%) | 2 (20%) | 1 (10%) | 1 (10%) | 0.319 |
| DVD | 4 (40%) | 1 (10%) | 4 (40%) | 0 (0%) | |
| TVD | 5 (50%) | 6 (60%) | 4 (40%) | 8 | |
| TVD + left main disease | 1 (10%) | 1 (10%) | 1 (10%) | 1 (10%) | |
| % stenosis | 87 ± 11.30 | 80 ± 9.83 | 81 ± 9.98 | 83 ± 9.77 | 0.442 |
| Target lesion | |||||
| Left main | 0 (0%) | 1 (10%) | 1 (10%) | 2 (20%) | 0.683 |
| LAD | 5 (50%) | 7 (70%) | 6 (60%) | 7 (70%) | |
| LCx | 3 (30%) | 4 (40%) | 1 (10%) | 6 (60%) | |
| RCA | 5 (50%) | 3 (30%) | 4 (40%) | 2 (20%) | |
| TIMI flow pre PCI | |||||
| 0 | 3 (30%) | 2 (20%) | 2 (20%) | 2 (20%) | 0.995 |
| 1 | 1 (10%) | 0 (0%) | 0 (0%) | 1 (10%) | |
| 2 | 1 (10%) | 1 (10%) | 2 (20%) | 1 (10%) | |
| 3 | 5 (50%) | 7 (70%) | 6 (60%) | 6 (60%) | |
| Bifurcation | 6 (60%) | 4 (40%) | 6 (60%) | 4 (40%) | 0.566 |
| Intravascular imaging | 6 (60%) | 8 (80%) | 9 (90%) | 7 (70%) | 0.622 |
| IVUS | 6 (60%) | 7 (70%) | 7 (70%) | 7 (70%) | 1 |
| OCT | 0 (0%) | 1 (10%) | 2 (20%) | 1 (10%) | 0.891 |
| Thrombus | 1 (10%) | 1 (10%) | 1 (10%) | 2 (20%) | 1 |
| Lipid plaque | 1 (10%) | 2 (20%) | 2 (20%) | 1 (10%) | 0.867 |
| TIMI flow post PCI | |||||
| 0 | 3 (30%) | 1 (10%) | 1 (10%) | 2 (20%) | 0.615 |
| 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (10%) | |
| 3 | 7 (70%) | 9 | 9 | 77 (70%) | |
| Syntax score | 19.4 (14.49) | 29.3 (14.73) | 23.2 (22.86) | 20.9 (19.23) | 0.635 |
| FFR | 1 (10%) | 1 (10%) | 1 (10%) | 0 (0%) | 1 |
| Number of stent | 2 (1.16) | 2.2 (0.92) | 1.7 (1.16) | 2.7 (1.83) | 0.391 |
| DES size | 2.44 (0.91) | 2.99 (0.58) | 2.73 (1.06) | 2.56 (0.90) | 0.564 |
| DES lenght | 56 (33.49) | 49 (29.77) | 50 (33.13) | 36 (18.75) | 0.498 |
Note: Values shown are n (%); mean ± SD or median (interquartile range); p-value from Fisher’s Exact test for categorical data and one-way ANOVA for continuous data.
Abbreviations: ACEI: Angiotensin Converting Enzyme Inhibitor; ARB: Angiotensin Receptor Blocker; BMI: Body Mass Index; BSA: Body Surface Area; CABG: Coronary Bypass Graft; CAD: Coronary Artery Disease; DES: Drug Eluting Stent; DVD: Double Vessel Disease; EF: Ejection Fraction; ESRD: End Stage Renal Disease; FFR: Fractional Flow Reserve PCI; FH: Familial History; Hb: Hemoglobin; IVUS: Intravascular Ultrasound Percutaneous Coronary Intervention; LAD: Left Anterior Descending Artery; LCx: Left Circumflex Artery; NSTEMI: Non ST Elevation Myocardial Infarction; OCT: Optical Coherence Tomography; PCI: Percutaneous Coronary Intervention; PT: Prothrombin Time; PTT: Partial Thromboplastin Time; RCA: Right Coronary Artery; STEMI: ST Elevation Myocardial Infarction; SVD: Single Vessel Disease; TIMI: Thrombolysis In Myocardial Infarction and TVD: Triple Vessel Disease.
Table 1: Clinical and procedural characteristics in the overall study population in 70 U/kg, 80 U/kg, 90 U/kg, 100 U/kg.
| 70 U/kg (N=10) | 80 U/kg (N=10) | 90 U/kg (N=10) | 100 U/kg (N=10) | p-value | |
|---|---|---|---|---|---|
| ACT baseline | 148 (138,160) | 148 (139,154) | 150 (115,155) | 148 (137,152) | 0.991 |
| ACT 30 min | 227 (216,319) | 235 (222,255) | 257 (243,301) | 275 (253,316) | 0.126 |
Note: Values shown are median (interquartile range); p-value from Kraskal Walis’s test.
Abbreviations: ACT: Activated Clotting Time; UFH: Unfractionated Heparin.
Table 2: ACT level at baseline and 30 min after IV UFH in 70 U/kg, 80 U/kg, 90 U/kg, 100 U/kg groups.
| 70 U/kg (N=10) | 80 U/kg (N=10) | 90 U/kg (N=10) | 100 U/kg (N=10) | p-value | |
|---|---|---|---|---|---|
| Primary Outcome | |||||
| Optimal ACT | 0 (0%) | 3 (30%) | 3 (30%) | 5 (50%) | 0.083 |
| Secondary Outcomes | |||||
| MACE | |||||
| Cardiovascular death | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Procedural MI | 0 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Stroke/systemic emboli | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Bleeding | 0 (0%) | 0 (0%) | 0 (0%) | 1 (10%) | 1 |
| Puncture site complication | 0 (0%) | 0 (0%) | 1 (10%) | 1 (10%) | 1 |
Note: Values shown are n (%); p value from Fisher’s Exact test.
Abbreviations: ACT: Activated Clotting Time; MACE: Major Adverse Cardiac Events; UFH: Unfractionated Heparin; MI: Myocardial Infarction.
Table 3: Primary and secondary outcomes in 70 U/kg, 80 U/kg, 90 U/kg, 100 U/kg.
Secondary study objectives, outcomes were divided into an efficacy endpoint (thrombotic events) and a safety endpoint (bleeding as defined by the Bleeding Academic Research Consortium (BARC) criteria). For the efficacy, there was no thrombotic event in this study. For the safety endpoint, there was one <5 centimeters of groin hematomas in the 100 U/kg group (BARC class II) caused by multiple puncture attempts. No significant differences in major adverse cardiac events were observed among the groups.
Discussion
The standard test for evaluation of the degree of UFH activity is Activated Partial Thromboplastin Clotting Time (aPTT), but it needs laboratory equipment and trained staff and it cannot be done as a bedside test. On the other hand, high dose of UFH used in PCI results in aPTT values beyond the measurable range [5]. ACT does not have these disadvantages. Early studies showed a linear relationship between UFH and ACT levels, but the slope of this line varies from patient to patient [6].
Administration of UFH according to body weight and its monitoring by intraprocedural arterial ACT is the method mostly used in percutaneous coronary intervention. Currently UFH is the recommended anticoagulation therapy during percutaneous coronary intervention with a targeted ACT ranging between 250- 300 sec by Hemotech method throughout the procedure. The cost and availability of rapid “point of care” test for dose adjustment makes UFH a valid therapeutic option for this procedure [7].
From the current American College of Cardiology/American Heart Association (ACC/AHA) and European Society of Cardiology (ESC) guideline UFH 70-100 U/kg is the dosage of UFH to be given before PCI [8]. However, no study shows the best dosage between 70-100 U/kg that is the most optimal dosage of UFH before PCI. To our knowledge, this may be the first randomized controlled pilot study to show the best dosage of UFH during PCI.
Optimization of UFH dosing has been having much controversy over the level of optimal ACT for PCI. There is too much patient to patient variation between UFH dosing and extent of ACT. On the other hand, there are some debates about a linear relationship between achieved ACT and ischemic or bleeding endpoints. In the ESPIRIT trial, the increase of ischemic events did not increase as ACT decreased, at least to a level of 200s. The increase in major or minor bleeding corresponding with increasing ACT has no statistical significance in this trial [9]. Chew et al in a meta-analysis showed that the risk of ischemic events was progressively reduced with increasing ACT level. However, the optimal level of ACT should be achieved to prevent ischemic or bleeding events.
There were factors that may cause analytical error; we have corrected these before beginning the study. First, for UFH contamination, we collected baseline ACT samples from the arterial sheath while UFH was given through the intravenous catheter. There was no UFH in the flush solution. Second, ACT was measured using the Medtronic ACT II system (Hemotech). This is an important concept because ACT values are device-specific. All available devices have acceptable reproducibility, but reference and therapeutic ACT ranges vary considerably. Therefore, every ACT test should be regarded as unique.
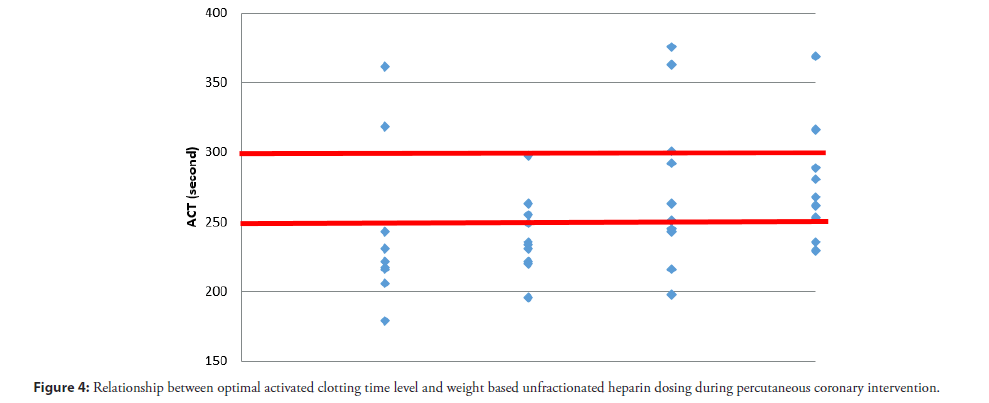
These results show that 100 U/kg of IV UFH during PCI has a tendency to achieve the most optimal level. A Pearson correlation analysis revealed a statistically significant moderate positive relationship between optimal ACT levels and weight based UFH dosing, r=0.38, p=0.017 (Figure 4). The 100 U/kg IV UFH may increase the small risk of minor bleeding (groin hematoma <5 cm). No thrombotic event was observed in this study. One patient, who had bleeding, achieved ACT in the targeted range (268 sec). Bleeding was caused by multiple puncture attempts. Because this is a pilot study, we need a randomized controlled trial in the future.
Conclusion
As an intravenous UFH bolus of 70 to 100 U/kg is recommended to achieve a target ACT of 250 to 300 s (Hemotech device). The 100 U/kg of intravenous unfractionated heparin may have a tendency to achieve the most optimal ACT level in patient who underwent percutaneous coronary intervention without significant increase risk of bleeding and thrombosis. Larger RCTs are needed to examine the optimal activated clotting time Level and weight based unfractionated heparin dosing during percutaneous coronary intervention
References
- Chew DP, Bhatt DL, Lincoff AM, et al. Defining the optimal activated clotting time during percutaneous coronary intervention: Aggregate results from 6 randomized, controlled trials. Circulation. 103(7): 961-966 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Valgimigli M, Frigoli E, Leonardi S, et al. Bivalirudin or unfractionated heparin in acute coronary syndromes. N Engl J Med. 373(11):997-1009 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Gargiulo G, Moschovitis A, Windecker S, et al. Developing drugs for use before, during and soon after percutaneous coronary intervention. Expert Opin Pharmacother. 17(6):803-818 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. The task force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome). 20(7-8):1S-61S (2019).
[CrossRef] [Google Scholar] [PubMed]
- Marmur JD. Direct versus indirect thrombin inhibition in percutaneous coronary intervention. J Invasive Cardiol.14:8B-18B (2002).
[Google Scholar] [PubMed]
- Mulry CC, Le Veen RF, Sobel M, et al. Assessment of heparin anticoagulation during peripheral angioplasty. J Vasc Interv Radiol. 2(1):133-139 (1991).
[CrossRef] [Google Scholar] [PubMed]
- Bernelli C, Chieffo A, Montorfano M, et al. Usefulness of baseline activated clotting time-guided heparin administration in reducing bleeding events during transfemoral transcatheter aortic valve implantation. JACC Cardiovasc Interv.7(2):140-151 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 130(25):2354-2394 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Tolleson TR, O’Shea JC, Bittl JA, et al. Relationship between heparin anticoagulation and clinical outcomes in coronary stent intervention: Observations from the ESPRIT trial. J Am Coll Cardiol. 41(3):386-393 (2003).
[CrossRef] [Google Scholar] [PubMed]