Research Article - Neuropsychiatry (2018) Volume 8, Issue 5
Orthostatic Hypotension in Alzheimer’s disease: A Meta-Analysis
- Corresponding Author:
- Zhiyou Cai
Department of Neurology, Chongqing General Hospital, No. 312 Zhongshan First Road, Yuzhong District, Chongqing, People’s Republic of China. 400013
Tel: +86-23-63530157
Fax: +86-23-63530157
Abstract
Objective
To investigate the relationships between orthostatic hypotension (OH) and the risk of AD using meta-analysis.
Methods
The PubMed, Sciencedirect and Web of Science were searched to identify relevant literature published up to October 2016. The RevMan 5.3 software was used for statistical analysis, with odds ratio (OR), risk ratio (RR) and 95% confidence intervals (CI) calculated to evaluate the associations between OH and the risk of AD. Meta-regression and publication bias were also performed.
Results
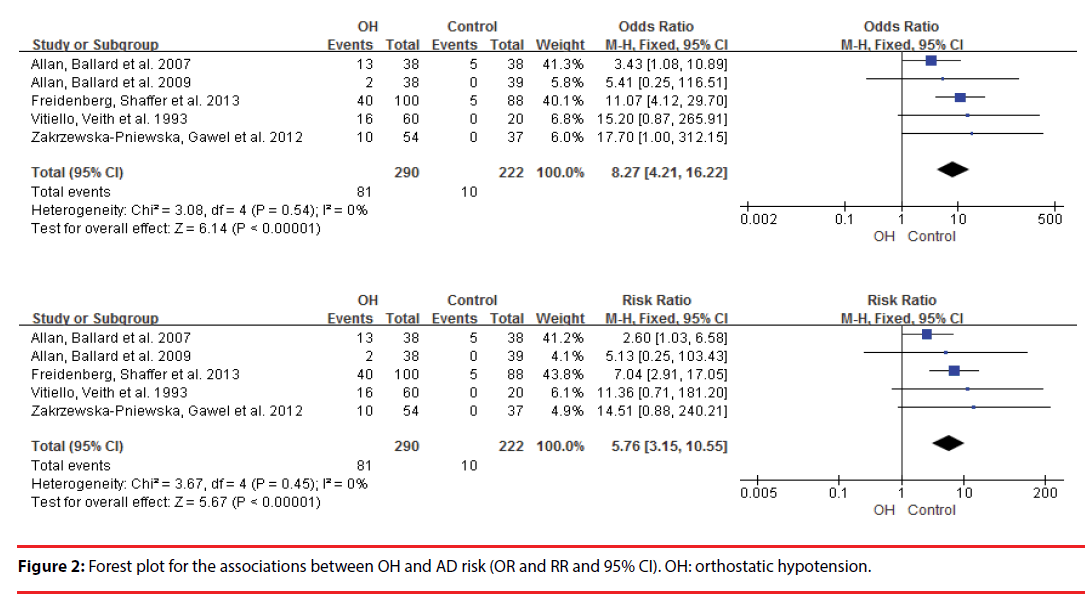
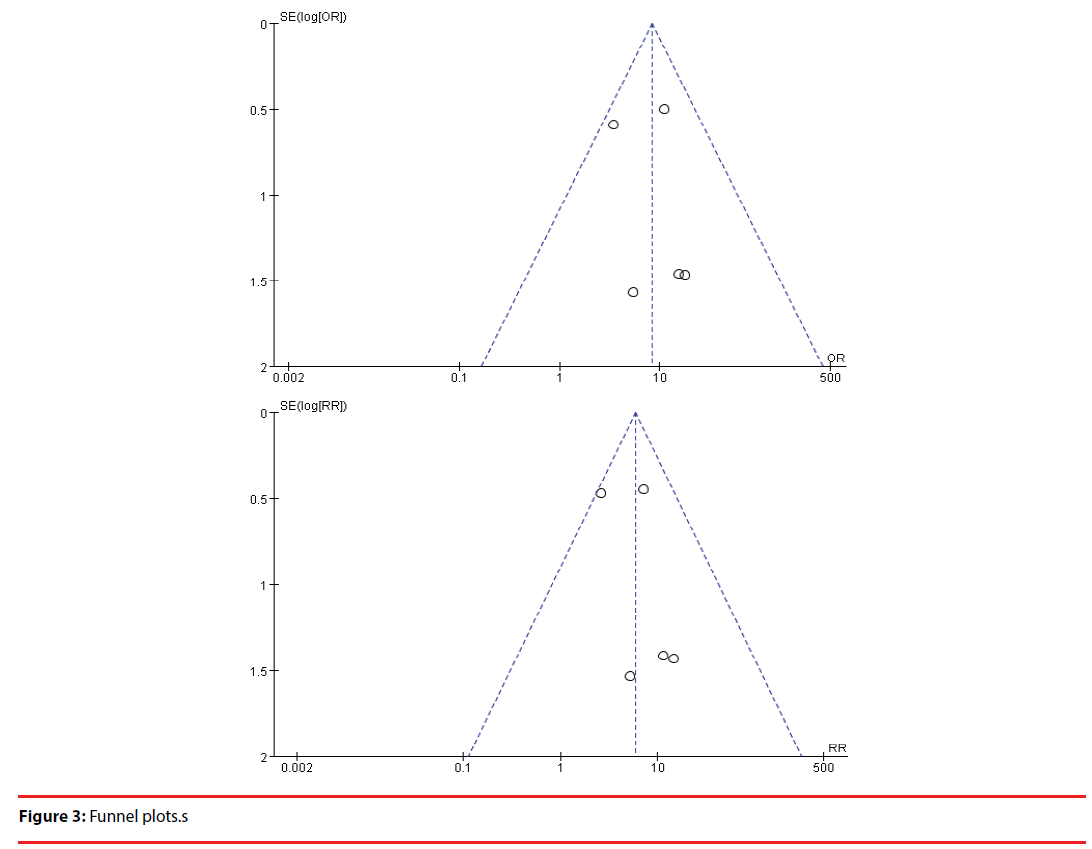
This meta-analysis included 6 studies. OH was associated with increased AD risk (OR=8.27, 95% CI=4.21-16.22, Pï¼Âœ0.00001; RR=8.27, 95% CI=3.15-10.55, Pï¼Âœ0.00001). There was no heterogeneity (OR: P=0.54, I2=0%; RR: P=0.45, I2=0%). The funnel plots were symmetrical. Egger’s linear regression showed that there was no publication bias (P=0. 212).
Conclusion
This meta-analysis showed that OH may be a risk factor for susceptibility to AD. Large-sample multicenter studies are needed to confirm the findings.
Keywords
Alzheimer’s disease, Orthostatic hypotension, Autonomic dysfunction
Introduction
Alzheimer’s disease (AD), an age-related progressive neurodegenerative disorder, is clinically characterized by non-reversible impairment of memory and by disturbances in reasoning, planning, language, and perception [1]. The incidence of AD increases substantially after the age of 60. With the coming of aging society, the prevalence of AD also increases rapidly [2,3]. The importance of identifying risk factors is therefore evident in the prevention and treatment for AD. In the past decades, abundant in-depth epidemiologic studies have been performed to understand the pathogenesis of AD, many of which indicated that autonomic nervous system (ANS) plays an important role in the development of AD [4,5].
ANS has two main divisions: sympathetic autonomic nervous system (SANS) and parasympathetic autonomic nervous system (PANS). Most organs are controlled primarily by either SANS or PANS. The two divisions work cooperatively to ensure that the body responds appropriately and accurately to different stimulation [6-8]. ANS regulates several basic functions, such as blood pressure, heart and breathing rates, body temperature, digestion, gland secretion (saliva, sweat, and tears), urination, defecation and sexual response. Autonomic dysfunction occurs when the ANS are out of order. Autonomic dysfunction (also called autonomic neuropathy or dysautonomia) may being involved with a small part of the ANS or the entire ANS. The symptoms can range from mild to life-threatening, including orthostatic hypotension, heart rate variability, sweating abnormalities, digestive difficulties, urinary and sexual problems [9,10]. Orthostatic hypotension (OH) is one of the most common presentations of autonomic dysfunction [11]. Increasing results indicate strong associations between OH and the prevalence of cognitive impairment and dementia [12-16]. OH has often been observed to be a risk factor for Alzemer’s dementia [17,18]. This meta-analysis aims to demonstrate the association between OH and the prevalence of AD and identify the impacts of OH on AD. Accordingly, OH is considered a potential cause of AD.
Methods and Materials
▪ Literature search procedure
The following index and databases were included in the identification of relevant studies: PubMed, Sciencedirect and Web of Science. Search terms included Alzheimer’s disease and autonomic nervous system or autonomic dysfunction or dysautonomia or low blood pressure or orthostatic hypotension. The titles and abstracts were first reviewed to find potentially relevant papers. The uncertain paper was reviewed with full text. The literature search procedure was conducted in English. English articles published from January 1987 to October 2016. This metaanalysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [19,20].
▪ Data extraction
Clinical studies were included in this metaanalysis if they were clinical cross-sectional studies, case control, cohort studies or randomized controlled trials. The diagnosis criteria of Alzheimer’s disease conformed with the ICD-10 and DSM-IV for dementia, and was a diagnosis of probable AD according to the NINCDS-ARDA criteria proposed by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ARDA). Clinical OH is diagnosed with an decrease in systolic and/or diastolic blood pressure (minimum of 20 and 10 mmHg, respectively) within 3 minutes of standing from a supine to an erect position [14,21].
▪ Data analysis
This meta-analysis was performed with Review Manager 5.3 (RevMan 5.3) software. The multivariate outcome data (OR, RR and 95% CI) were transformed logarithmically. Potential heterogeneity among the individual studies was evaluated by means of Cochran’s Q statistic and I2 index score, with a significance set at the P-value<0.1 or I2 <50%. Potential publication bias was evaluated using Egger’s test and Begg’s funnel plot.
Results
▪ Literature search
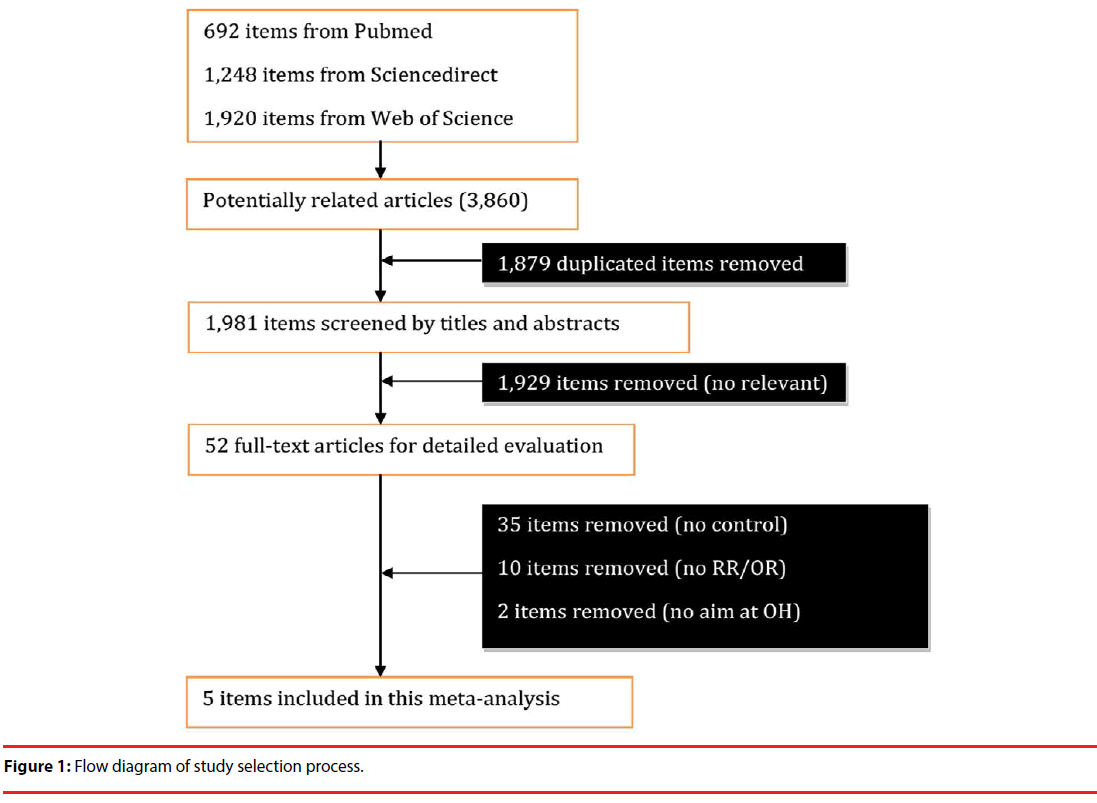
Figure 1 implicates the relevant study search process. 3,860 articles were identified with the described search strategy, from which 52 fulltext articles were retrieved for final review after screening 1,981 items by titles and abstracts (Figure 1). 1,879 duplicate items were first removed. After reading the 52 full-text articles, 35 items were removed due to no control cases, and 10 items excluded because of no RR/OR materials, and 2 items were removed because they had no direct aim at OH research. Finally, 5 studies were included in this meta-analysis [12,22-25] (Table 1).
| Study or Study group | Country | AD diagnosis | Age | AD | Control | ||
|---|---|---|---|---|---|---|---|
| OH | Total | OH | Total | ||||
| Freidenberg et al. [12] | USA | DSM-IV and NINCDS-ADRDA criteria | Not available | 40 | 100 | 56 | 88 |
| Allan et al. [23] | UK | DSM-IV criteria | Over 65 | 2 | 38 | 0 | 39 |
| Zakrzewska-Pniewska et al. [25] | Poland | NINCDS-ADRDA criteria | 25-91 | 10 | 54 | 0 | 37 |
| Allan et al. [22] | UK | NINCDS-ADRDA criteria | Over 65 | 13 | 38 | 5 | 38 |
| Vitiello et al. [24] | USA | DSM-III and NINCDS-ADRDA criteria | Not available | 16 | 60 | 0 | 20 |
Table 1: Included studies.
This meta-analysis revealed that OH was associated with increased AD risk (OR =8.27, 95% CI =4.21–16.22, P<0.00001; RR= =8.27, 95% CI =3.15–10.55, P<0.00001) (Figure 2). There was no heterogeneity (OR: P=0.54, I2=0%; OR: P=0.54, I2=0%) (Figure 2). Egger’s linear regression showed that there was no publication bias (P=0. 212) and the funnel plots were symmetrical (Figure 3).
Discussion
Orthostatic hypotension (OH) is the most common clinical manifestation in autonomic dysfunction. Multiple research investigations support the role of autonomic dysfunction in the pathogenesis of cognitive impairment and dementia, which seemed similar for AD and vascular dementia [14,26]. The aim of this meta-analysis is to investigate the relation between OH and AD. In this meta-analysis, a statistically significant association between OH and increased AD risk was found, implicating that OH is a potential risk factor for Alzheimer’s dementia.
The signs of dysautonomia in patients with AD were reported by inclding OH, impaired heart rate variability (HRV), constipation and urinary incontinence, clinically characterized by the intensity of mild dysautonomia [25,27,28]. It has been suggested that impaired HRV may be an early sign of AD [28,29]. It was found that OH enhanced long-term risk of developing dementia [14]. Meanwhile, individuals with some dementia-related neurodegenerative disorders possess a greater-than-normal risk of developing OH [30,31]. Allan et al. found that high risk for OH existed in the elderly people with dementia, including AD, vascular dementia, Parkinson disease dementia, and dementia with Lewy bodies [22]. A comprehensive background is that OH is a common cause of cerebral hypoperfusion which is widely implicated in the contribution to cognitive decline and dementia [32-34]. Compelling studies support that OH’s effects on AD may be related to changes in brain blood flow or disruption of blood-brain barrier integrity, while OH results in changes in arterioles and eventually onset of cognitive impairment and dementia [26,35,36]. However, this meta-analysis had no further work to discover the pathogenic role of OH in the occurrence and development of AD.
Several limitations existed in this meta-analysis although there was no publication bias. First, unknown confoundings cannot completely be ruled out since other signs and symptoms is in association with OH, such as light-headedness, vertigo, syncope, generalized weakness, nausea, posterior headache and blurred vision. These patients with OH may be more likely to have other signs of dysautonomia, such as HRV, constipation and urinary incontinence [37,38]. Secondly, a further layer of analysis was not performed due to the small size effect and limited number of included studies. Thirdly, no clinical studies included in this meta-analysis contained treatment data, so it could not be determined whether rectifying OH was an effective measure for the prevention and treatment of AD. Finally, large-sample multicenter research data has been absence until now, and the included studies did not record the severity of OH, the accurate relation between OH and AD is uncertain. Therefore, further analysis is needed to determine the role of OH in AD.
In summary, findings from this meta-analysis of published case-control and cohort studies suggest a strong association between OH and increased AD risk, implicating that OH is a potential risk factor for Alzheimer’s dementia. Future clinical studies are needed to confirm these findings.
Acknowledgments
This work was supported by the Chongqing Health and Family Planning Scientific Research Project (ZY201702042), and the Nature Science Foundation of Chongqing General Hospital to Dr. Zhiyou Cai.
Conflict of Interest
None declared.
References
- Studart AN, Nitrini R. Subjective cognitive decline: The first clinical manifestation of Alzheimer's disease? Dement. Neuropsychol 10(3), 170-177 (2016).
- Meunier B. Age and Alzheimer's Disease. Nutrients 8(6) 2016.
- Gavett BE, John SE, Gurnani AS, et al. The Role of Alzheimer's and Cerebrovascular Pathology in Mediating the Effects of Age, Race, and Apolipoprotein E Genotype on Dementia Severity in Pathologically-Confirmed Alzheimer's Disease. J. Alzheimers. Dis 49(2), 531-545 (2016).
- Struhal W, Mahringer C, Lahrmann H, et al. Heart Rate Spectra Confirm the Presence of Autonomic Dysfunction in Dementia Patients. J. Alzheimers. Dis 54(2), 657-667 (2016).
- Affoo RH, Foley N, Rosenbek J, et al. Swallowing dysfunction and autonomic nervous system dysfunction in Alzheimer's disease: a scoping review of the evidence. J. Am. Geriatr. Soc 61(12), 2203-2213 (2013).
- Alito AE, Romeo HE, Baler R, et al. Autonomic nervous system regulation of murine immune responses as assessed by local surgical sympathetic and parasympathetic denervation. Acta. Physiol. Pharmacol. Latinoam, 37(3), 305-319 (1987).
- van den Berg MM, Maas J, Muller R, et al. Autonomic Nervous System Responses to Viewing Green and Built Settings: Differentiating Between Sympathetic and Parasympathetic Activity. Int. J. Environ. Res. Public. Health 12(12), 15860-15874 (2015).
- Schneider U, Schleussner E, Fiedler A, et al. Fetal heart rate variability reveals differential dynamics in the intrauterine development of the sympathetic and parasympathetic branches of the autonomic nervous system. Physiol. Meas 30(2), 215-226 (2009).
- Freeman R, Chapleau MW. Testing the autonomic nervous system. Handb. Clin. Neurol 115(1), 115-136 (2013).
- Thaisetthawatkul P, Boeve BF, Benarroch EE, et al. Autonomic dysfunction in dementia with Lewy bodies. Neurology 62(10), 1804-1809 (2004).
- Cohen JA, Miller L, Polish L. Orthostatic hypotension in human immunodeficiency virus infection may be the result of generalized autonomic nervous system dysfunction. J. Acquir. Immune. Defic. Syndr 4(1), 31-33 (1991).
- Freidenberg DL, Shaffer LE, Macalester S, et al. Orthostatic hypotension in patients with dementia: clinical features and response to treatment. Cogn. Behav. Neurol 26(3), 105-120 (2013).
- Momeyer MA. Orthostatic hypotension in older adults with dementia. J. Gerontol. Nurs, 40(6), 22-29 (2014).
- Wolters FJ, Mattace-Raso FU, Koudstaal PJ, et al. Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study. PLoS. Med, 13(10), e1002143 (2016).
- Oishi E, Sakata S, Tsuchihashi T, et al. Orthostatic Hypotension Predicts a Poor Prognosis in Elderly People with Dementia. Intern. Med 55(15), 1947-1952 (2016).
- Soennesyn H, Nilsen DW, Oppedal K, et al. Relationship between orthostatic hypotension and white matter hyperintensity load in older patients with mild dementia. PLoS. One 7(12), e52196 (2012).
- Sonnesyn H, Nilsen DW, Rongve A, et al. High prevalence of orthostatic hypotension in mild dementia. Dement. Geriatr. Cogn. Disord 28(4), 307-313 (2009).
- Isik AT, Soysal P, Usarel C. Effects of Acetylcholinesterase Inhibitors on Balance and Gait Functions and Orthostatic Hypotension in Elderly Patients With Alzheimer Disease. Am. J. Alzheimers. Dis. Other. Demen, 31(7), 580-584 (2016).
- Vrabel M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Oncol. Nurs. Forum, 42(5), 552-554 (2015).
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 3(3), e123-130 (2009).
- Shi X, Wray DW, Formes KJ, et al. Orthostatic hypotension in aging humans. Am. J .Physiol. Heart. Circ. Physiol 279(4), H1548-1554 (2000).
- Allan LM, Ballard CG, Allen J, et al. Autonomic dysfunction in dementia. J. Neurol. Neurosurg. Psychiatry, 78(7), 671-677 (2007).
- Allan LM, Ballard CG, Rowan EN, et al. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS. One, 4(5), e5521 (2009).
- Vitiello B, Veith RC, Molchan SE, et al. Autonomic dysfunction in patients with dementia of the Alzheimer type. Biol. Psychiatry 34(7), 428-433 (1993).
- Zakrzewska-Pniewska B, Gawel M, Szmidt-Salkowska E, et al. Clinical and functional assessment of dysautonomia and its correlation in Alzheimer's disease. Am. J. Alzheimers. Dis. Other. Demen 27(8), 592-599 (2012).
- Siennicki-Lantz A, Lilja B, Elmstahl S. Orthostatic hypotension in Alzheimer's disease: result or cause of brain dysfunction? Aging (Milano), 11(3), 155-160 (1999).
- Kasanuki K, Iseki E, Fujishiro H, et al. Impaired heart rate variability in patients with dementia with Lewy bodies: Efficacy of electrocardiogram as a supporting diagnostic marker. Parkinsonism. Relat. Disord 21(7), 749-754 (2015).
- Mellingsaeter MR, Wyller TB, Ranhoff AH, et al. Reduced sympathetic response to head-up tilt in subjects with mild cognitive impairment or mild Alzheimer's dementia. Dement. Geriatr. Cogn. Dis. Extra 5(1), 107-115 (2015).
- Negami M, Maruta T, Takeda C, et al. Sympathetic skin response and heart rate variability as diagnostic tools for the differential diagnosis of Lewy body dementia and Alzheimer's disease: a diagnostic test study. BMJ. Open 3(3) (2013).
- Dias FL, Silva RM, Moraes EN, et al. Clinical and autonomic profile of patients with Alzheimer's disease and mixed dementia patients. Rev. Assoc. Med. Bras (1992) 59(5), 435-441 (2013).
- Collins O, Dillon S, Finucane C, et al. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol. Aging, 33(10), 2324-2333 (2012).
- Passant U, Warkentin S, Karlson S, et al. Orthostatic hypotension in organic dementia: relationship between blood pressure, cortical blood flow and symptoms. Clin. Auton. Res 6(1), 29-36 (1996).
- Perlmuter LC, Sarda G, Casavant V, et al. A review of orthostatic blood pressure regulation and its association with mood and cognition. Clin. Auton. Res 22(2), 99-107 (2012).
- Robertson AD, Messner MA, Shirzadi Z, et al. Orthostatic hypotension, cerebral hypoperfusion, and visuospatial deficits in Lewy body disorders. Parkinsonism. Relat. Disord 22(1), 80-86 (2016).
- van Beek AH, Sijbesma JC, Jansen RW, et al. Cortical oxygen supply during postural hypotension is further decreased in Alzheimer's disease, but unrelated to cholinesterase-inhibitor use. J. Alzheimers. Dis 21(2), 519-526 (2010).
- Feldstein CA. Association between chronic blood pressure changes and development of Alzheimer's disease. J. Alzheimers. Dis 32(3), 753-763 (2012).
- Monsuez JJ, Beddok R, Mahiou A, et al. [Orthostatic hypotension: epidemiology and mechanisms]. Presse. Med 41(11), 1092-1097 (2012).
- Perlmuter LC, Sarda G, Casavant V, et al. A review of the etiology, associated comorbidities, and treatment of orthostatic hypotension. Am. J. Ther 20(3), 279-291 (2012).