Research Article - Interventional Cardiology (2024) Volume 16, Issue 2
Outcomes in Fractional flow reserve guided Percutaneous Coronary Intervention among Indian patients
- Corresponding Author:
- Naveen Jamwal
Department of Cardiology, Dr. Ram Manohar Lohia Institute of Medical Sciences, Uttar Pradesh, India,
E-mail: pratimatripathi.lko@gmail.com
Received date: 26-Mar-2024, Manuscript No. FMIC-24-130924; Editor assigned: 29-Mar-2024, PreQC No. FMIC-24-130924 (PQ); Reviewed date: 12-Apr-2024, QC No. FMIC-24-130924; Revised date: 19-Apr-2024, Manuscript No. FMIC-24-130924 (R); Published date: 26-Apr-2024, DOI: 10.37532/1755- 5310.2024.16(2).820
Abstract
Background: Coronary Artery Disease (CAD) is a leading cause of mortality worldwide. Revascularisation of stenotic segments has been the standard of care. However, the benefit of revascularisation is less evident for lesions that do not cause ischemia, and optimal medical therapy alone is probably just as beneficial in these cases. Fractional Flow Reserve (FFR) is a metric that gauges the physiological importance of coronary stenosis. This method hasn’t achieved widespread adoption amongst the interventional cardiology community, with FFR utilised in only a minority of cases undergoing PCI, especially in India.
Aim: To compare outcomes in FFR-guided vs angiography-guided Percutaneous Coronary Intervention (PCI) among Indian patients with intermediate stenosis (50%- 70%) on coronary angiography.
Methods: This open-label study was conducted on patients with CAD with intermediate stenosis and requiring PCI. A total of 80 patients (40 in each arm) were included. Patients were eligible if they were diagnosed with stable CAD or ACS and had intermediate lesions on coronary angiography. “Cases” underwent FFR-guided PCI while the remaining patients were enrolled as “Controls.”
Results: There was no significant difference in terms of primary outcomes. Anti-anginal medications at 3 and 6 months were significantly higher in the Control group and the EQ-5D score was significantly higher in the FFR group than in the Control group at 3 and 6 months (p<0.05). The mean stent length was significantly higher in the Control group than in the FFR group (P=0.023). The mean number of stents used in the FFR group was significantly lower than the Control group (P=0.033).
Conclusion: Management of intermediate lesions with an FFR-guided strategy was associated with better outcomes regarding angina and functional parameters. FFRguided PCI reduces major adverse cardiovascular events at six months, a finding supporting the evolving strategy of revascularisation of ischemic lesions and medical treatment of physiologically non-ischemic lesions.
Keywords
Fractional Flow Reserve; Angiography; Percutaneous coronary intervention; Coronary artery disease; Myocardial infarction
Introduction
Coronary Artery Disease (CAD) is a leading cause of mortality worldwide [1]. Revascularisation (both percutaneous and surgical) of stenotic segments has been the standard of care. However, the benefit of revascularisation is less evident for lesions that do not cause ischemia, and optimal medical therapy alone is probably just as beneficial in these cases [2].
Identifying which lesions in CAD patients are hemodynamically significant and require angioplasty may be challenging. Due to the high cost and likely complications associated with drug-eluting stents, appropriate usage is essential [3,4]. The capacity of noninvasive stress imaging research to precisely localise lesions that cause ischemia in these patients is limited [5-7]. Coronary stenosis that causes ischemia can be identified with an accuracy greater than 90.0% if the FFR value is 0.80 or less [8]. It has a higher spatial resolution than nuclear studies since each diseased segment is analysed separately [9].
According to current guidelines, FFR is recommended to guide revascularisation in intermediate stenoses without prior indication of myocardial ischemia on non-invasive testing [10]. The primary source of inspiration for these recommendations is the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) trial, which demonstrated lower rates of Major Adverse Cardiac Events (MACE), primarily related to repeat revascularisation, in patients who underwent FFR-guided revascularisation as opposed to angiography-guidance [11].
Only a tiny percentage of people with CAD undergo FFR estimation in India, and it is only used in tertiary care facilities. Various factors, including a lipid-rich diet, metabolic syndrome, sedentary lifestyle, and younger age group, impact the natural history and demographic risk profile of Coronary Artery Disease (CAD) in patients from India [12]. Also, studies have suggested smaller coronary artery diameters in them [13]. Thereby, many patients with borderline lesions perhaps undergo unwarranted invasive revascularisation. Comprehensive data regarding the utility of FFR from India needs to be collected.
Despite the advantages of FFR guidance, the general interventional cardiology community has yet to adopt it widely [14]. Furthermore, studies comparing the effectiveness of FFR-guided PCI against angiography-guided PCI in real-world settings have primarily focused on American and European populations. Unfortunately, information outside of these geographic boundaries is scarce. As such, we conducted a study with data from the Indian population to investigate the differing results between angiography-guided and FFR-guided PCI in our patient group.
Materials and Methods
Study design
This open-label observational study was performed in the Department of Cardiology, Dr RMLIMS, a tertiary care teaching hospital in India, from June 2021 to October 2022. The study aimed to compare outcomes in FFR-guided versus angiographyguided PCI in Indian patients with CAD and intermediate stenosis (50-70%) the study protocol approved by the institute’s ethics committee.
Eighty patients (40 in each arm) were included in the study. Patients were eligible if they were diagnosed with stable CAD or Acute Coronary Syndrome (ACS) based on history, clinical examination, ECG findings, and biochemical markers. They had intermediate lesions (50-70% stenosis) on coronary angiography. Written informed consent was obtained from all the participants, and they were available for follow-up for at least six months.
Coronary angiography was performed per the standard protocol, and patients with intermediate lesions were given the option of FFR-guided PCI. Those who provided consent underwent FFR estimation and were enrolled as “Cases,” and the remaining patients underwent angiography-guided PCI and were enrolled as “Controls.”Controls: Underwent PCI or were managed medically based on angiography findings alone.
Controls: Underwent PCI or were managed medically based on angiography findings alone.
Cases (FFR group): Underwent FFR estimation for lesions of intermediate severity based on angiography and proceeded for PCI or medical management accordingly.
The 0.014” Pressure WireTMX (Abbott Medical) and ILUMIEN OPTIS PCI Optimization System (St. Jude Medical) were used to assess FFR. FFR was measured at the peak of intracoronary adenosine-induced hyperemia for the RCA and LCA. The dose of intracoronary adenosine used for LCA was 200 mg, and for RCA was 100 mg. FFR was determined by dividing the mean distal coronary pressure during maximum hyperemia by the mean aortic pressure. After PCI, all patients received dual anti-platelet therapy for at least six months of the study period (as per standard guidelines) unless contraindicated during follow-up:
• Lesions with FFR>0.8 were to be managed with optimal medical therapy alone.
• Lesions with FFR<0.8 were managed with Drug-Eluting Stents and medical therapy.
• Patients in the angiography-only group had PCI in all the indicated lesions.
Ethical considerations were ensured throughout the study, and the data were analysed using SPSS 23. The chi-square test, t-test, nonparametric tests, and correlation coefficients were utilised for data analysis.
Study population
Patients diagnosed as stable CAD or ACS based on history, clinical examination, ECG findings, and biochemical markers with intermediate lesions (50-70%) on coronary angiography were included in this study. Patients with ECG suggestive STElevation Myocardial Infarction (STEMI) were included if the event occurred at least five days before the PCI. Patients who had angiographically significant Left main coronary artery lesion, presented with STEMI within five days from the event, who have highly tortuous or calcified coronary arteries, history of prior CABG and/or contraindication to adenosine or Dual Anti-Platelet Therapy (DAPT) were excluded from the study.
End-points and follow-up
The primary result was the rate of major adverse cardiac events at six months. A significant adverse cardiac event was defined as a composite of death, non-fatal myocardial infarction, and any revascularisation that occurred within six months of the first event. The Canadian Cardiovascular Society classification system’s functional class at three and six months after the procedure, the European Quality of Life-5 dimensions scale’s assessment of the health-related quality of life at three and six months after the procedure, and the number of anti-anginal medications used at follow-up were all examples of secondary end-points. Individual components of the primary end-points were also included.
At three and 6-month periods, hospital appointments were made to check in on patients and evaluate their clinical state, with any new symptoms or significant clinical events. Complete clinical, electrocardiographic, biochemical, and angiographic evaluations (if indicated) were done on patients with any cardiovascular system complaints. Based on the Functional class at 3 and 6 months, the health-related quality of life, the quantity of anti-anginal medicines taken, symptoms and general cardiac health were assessed. Repeat coronary angiography in patients, if indicated, during the followup was performed under the original recommended approach of the angiography guidance or FFR. Information about the patient’s death was collected from hospital records and telephonically from Statistical analysis
Statistical analysis
Determining if there was a significant difference in the 6-month outcome of chief adverse cardiac events between patients who received angiography-guided PCI and those who received FFRguided PCI was the primary goal of the data analysis. A two-sided chi-square test with an alpha threshold of 0.05 and statistical power of 0.80 was used to estimate the sample size, which were 40 instances per group [2]. Categorical variables, encompassing primary end-point and individual components, were presented as proportions and assessed using the chi-square test for comparison. Continuous variables were presented as means along with their corresponding standard deviations and compared using an unpaired t-test. For statistical significance, a P-value of <0.05 was required.
Results
Baseline characteristics and angiographic data
The study included 80 cases, with an equal distribution of 40 patients in each group. The mean age of the patients in the FFR group was 58.1 ± 7.7 years, while in the Control group, it was 56.4 ± 12.1 years. Male patients constituted 77.5% of the FFR group and 67.5% of the Control group.
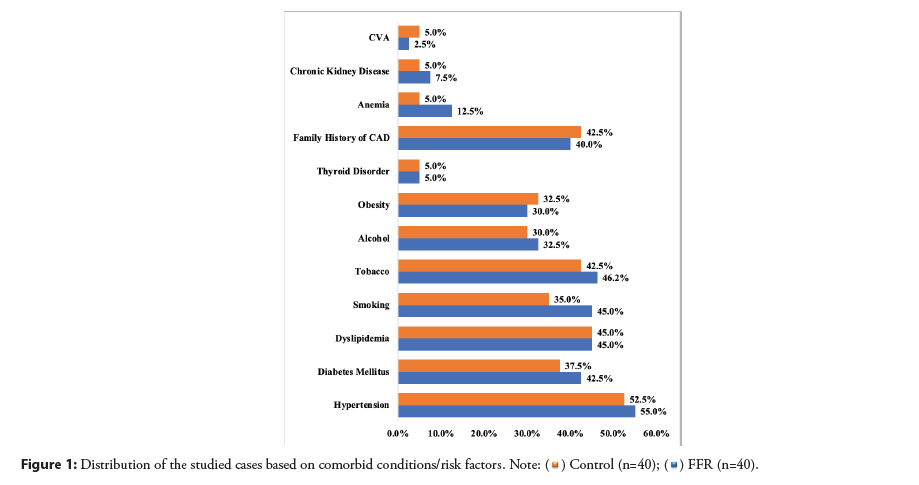
Most of the patients in both groups had hypertension, followed by dyslipidemia, tobacco consumption, family history, and diabetes mellitus, among other factors. Both groups were well-matched. (p>0.05) (Figure 1). CSA and STEMI were the most common presentations in both groups, followed by NSTEMI and UA. Most patients had LVEF>40% and Class II angina.
The EQ-5D Score, a measure of health-related quality of life at admission, was slightly higher in the FFR group (62.1 ± 6.1) compared to the Control group (60.3 ± 5.9) (p>0.05) (Table 1).
| Presentation of CAD | FFR(n=40) | Control(n=40) | p-value | |
|---|---|---|---|---|
| CSA | 15(37.5%) | 13(32.5%) | 0.639 | |
| STEMI | 13(32.5%) | 14(35.0%) | 0.812 | |
| NSTEMI | 8(20.0%) | 8(20.0%) | 1 | |
| UA | 4(10.0%) | 5(12.5%) | 0.723 | |
| Mean LVEF (%) | 54.4 ± 9.0 | 51.9 ± 11.4 | 0.279 | |
| LVEF (%) | = 40 | 6(15.0%) | 8(20.0%) | 0.556 |
| >40 | 34(85.0%) | 32(80.0%) | ||
| Canadian cardiovascular society angina score(at admission) | II | 22(55.0%) | 23(57.5%) | 0.945 |
| III | 12(30.0%) | 12(30.0%) | ||
| IV | 6(15.0%) | 5(12.5%) | ||
| EQ5D Score(at admission) | 62.1 ± 6.1 | 60.3 ± 5.9 | 0.184 | |
Table 1: Baseline characteristics for CAD.
The most common finding on CAG was Double vessel disease in both FFR and Control groups, followed by single vessel disease and triple vessel disease (Table 2).
| FFR(n=40) | Control(n=40) | p-value | ||
|---|---|---|---|---|
| Coronary angiography (CAG) | Single Vessel Disease | 14(35.0%) | 14(35.0%) | 0.688 |
| Double Vessel Disease | 18(45.0%) | 15(37.5%) | ||
| Triple Vessel Disease | 8(20.0%) | 11(27.5%) | ||
Table 2: Coronary Artery Angiography (CAG) and mean FFR findings.
FFR was positive in 10 patients while negative in the rest, i.e., in 30 patients. These ten patients underwent angioplasty with stent deployment (DES) in these lesions. The lesions with negative FFR were managed medically.
In the control group, out of 40 cases, study lesions were managed medically in 25 patients, while PCI with stent was done in 15 patients.
Aspirin was given to all the cases in both groups, followed by statin, Ticagrelor, Prasugrel, and Clopidogrel, and both groups were wellmatched (p>0.05) (Table 3).
| Drugs | FFR(n=40) | Control(n=40) | p-value |
|---|---|---|---|
| Beta Blockers | 29(72.5%) | 32(80.0%) | 0.431 |
| CCB | 19(47.5%) | 16(40.0%) | 0.499 |
| Nitrates | 33(82.5%) | 33(82.5%) | 1.00 |
| ACEi/ARB | 30(75.0%) | 29(72.5%) | 0.798 |
| Statin | 37(92.5%) | 38(95.0%) | 0.644 |
| Aspirin | 40(100.0%) | 40(100.0%) | 1.00 |
| Clopidogrel | 6(15.0%) | 7(17.5%) | 0.762 |
| Prasugrel | 12(30.0%) | 11(27.5%) | 0.804 |
| Ticagrelor | 12(30.0%) | 16(40.0%) | 0.348 |
Table 3: Distribution of the studied cases based on the medication.
Primary end point
Complete 3- and 6-month follow-up data were obtained for all patients. Primary end-point (composite of death, MI, and repeat revascularisation) happened in 2(5%) patients in the FFR group and 4(10%) patients in the Control group (P=0.369). Therefore, the difference was not statistically significant regarding the primary outcome.
Secondary end points
All-cause mortality at six months was 2.5% (1 death, which had cardiac cause) in the FFR group and 5% (2 deaths, both of which had cardiac causes) in the Control group (P=0.556). Non-fatal myocardial infarction occurred in 1 patient (2.5%) in the FFR group and 2(5%) in the control group (P=0.556). None of the patients in the FFR group and 2(5%) in the control group required repeat revascularisation (P=0.152). At six months, 28(70.0%) of the patients in the FFR group were free from angina, as compared with 15(37.5%) in the control group (P=0.029) (Table 4).
| Outcome | FFR(n=40) | PCI(n=40) | p-value |
|---|---|---|---|
| Death | 1(2.5) | 2(5.0) | 0.556 |
| Non-Fatal Myocardial Infraction | 1(2.5) | 2(5.0) | 0.556 |
| Repeat Revascularization | 0(0.0) | 2(5.0) | 0.152 |
Table 4: Outcomes in terms of death, non-fatal myocardial infraction, repeat revascularization.
The mean stent length was significantly higher in the Control group than in the FFR group, 43.5 mm and 29.5 mm, respectively (p=0.023). The mean number of stents used in the FFR group (1.28 ± 1.3) was significantly lower than the Control group (1.95 ± 1.45) (p=0.033).
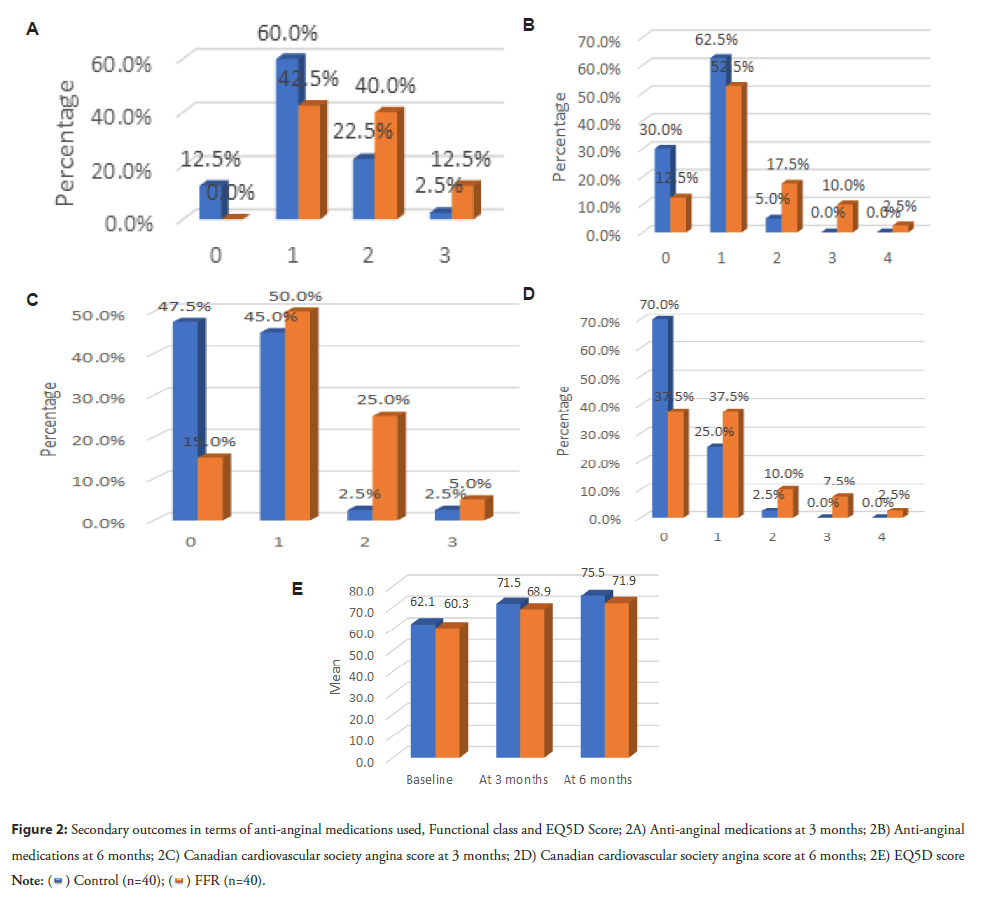
Anti-anginal medications at 3 and 6 months were significantly higher in the Control group, and the EQ-5D score was significantly higher in the FFR group than in the Control group at 3 and 6 months (p<0.05) (Table 5).
| Outcomes | FFR(n=40) | CONTROL(n=40) | p-value | |
|---|---|---|---|---|
| Anti-anginal medications at 3 months | 0 | 5(12.5%) | 0(0.0%) | 0.012 |
| 1 | 24(60.0%) | 17(42.5%) | ||
| 2 | 9(22.5%) | 16(40.0%) | ||
| 3 | 1(2.5%) | 5(12.5%) | ||
| Anti-anginal medications at 6 months | 0 | 12(30.0%) | 5(12.5%) | 0.026 |
| 1 | 25(62.5%) | 21(52.5%) | ||
| 2 | 2(5.0%) | 7(17.5%) | ||
| 3 | 0(0.0%) | 4(10.0%) | ||
| 4 | 0(0.0%) | 1(2.5%) | ||
| Canadian cardiovascular society angina score at 3 months | 0 | 19(47.5%) | 6(15.0%) | 0.002 |
| 1 | 18(45.0%) | 20(50.0%) | ||
| 2 | 1(2.5%) | 10(25.0%) | ||
| 3 | 1(2.5%) | 2(5.0%) | ||
| Canadian cardiovascular society angina score at 6 months | 0 | 28(70.0%) | 15(37.5%) | 0.029 |
| 1 | 10(25.0%) | 15(37.5%) | ||
| 2 | 1(2.5%) | 4(10.0%) | ||
| 3 | 0(0.0%) | 3(7.5%) | ||
| 4 | 0(0.0%) | 1(2.5%) | ||
| EQ5D score | Baseline | 62.1 ± 6.1 | 60.3 ± 5.9 | 0.184 |
| At 3 months | 71.5 ± 4.1 | 68.9 ± 4.8 | 0.0125 | |
| At 6 months | 75.5 ± 4.9 | 71.9 ± 7.2 | 0.0121 | |
Table 5: Outcomes in terms of Anti-anginal medications and Canadian cardiovascular society angina score.
Discussion
This study demonstrated that routine FFR measurement during PCI did not significantly lower the primary composite end-point of death, myocardial infarction, and repeat revascularisation at six months in patients with CAD compared to the standard strategy of PCI guided by angiography. Additionally, there was no discernible decrease in the death and myocardial infarction rates combined. The strategy guided by FFR resulted in a noteworthy reduction in secondary outcomes, including improvements in functional class at both the three- and six-month post-procedure assessments (based on the Canadian Cardiovascular Society classification system), enhancements in health-related quality of life evaluated using European quality of life five dimensions scale at three- and sixmonths, as well as a decreased reliance on anti-anginal medications during the follow-up period. In terms of angiographic findings, this study included patients with single vessel and multivessel CAD instead of only multivessel CAD patients as compared to the previous study by Tonino, et al., [2].
The number of stents used and the mean length were significantly higher in the control group than in the FFR group (p=0.033). The control group had a higher average number of stents (1.95) and longer mean stent length (45.35 mm) compared to the FFR group (1.28 stents and 29.5 mm, respectively). Tonino, et al., [2], reported similar findings in their study in which the average number of stents per patient was 1.9 mm and 2.7 mm, respectively (p<0.001). In their study, the mean stent length was 37.9 mm in FFR and 51.9 mm in angiography, which was statistically significant. Sengottuvelu, et al., [15], observed that 81 vessels with an intermediate lesion in 59 cases required 26 stents less when the FFR data was added to an angiogram.
The outcomes showed that death was 5.0% in PCI and 2.5% in the FFR group, and repeat revascularisation was 5.0% in PCI, whereas 0.0% in the FFR group. Non-fatal myocardial infarction was 0% in PCI and 2.5% in FFR, with a statistically insignificant difference. Our findings were comparable to those of Pijls, et al., [16], which observed that the group guided by angiography exhibited a mortality or myocardial infarction rate of 12.9%. In contrast, the FFR-guided group demonstrated a lower rate of 8.4%. In cases where lesions were deferred due to FFR values surpassing 0.80, myocardial infarction occurred at a mere 0.2%, and revascularisation was observed in 3.2% of cases within two years. Li, et al., [17], reported that patients who underwent interventions guided by FFR exhibited a slightly lower incidence of death or myocardial infarction when contrasted with individuals who received angiography-guided interventions.
Anti-anginal medications and Canadian Cardiovascular Society angina score and EQ-5D were found to be significantly higher in the FFR group than the control group at three- and six months. According to their research, Tonino, et al., [2], reported that patients without angina, anti-anginal drugs, and EQ-5D scores did not significantly differ between the two groups under study. At one year, 78.0% of angiography patients and 81.0% of the FFR group were angina-free (P=0.20) (Figure 2).
Figure 2: Secondary outcomes in terms of anti-anginal medications used, Functional class and EQ5D Score; 2A) Anti-anginal medications at 3 months; 2B) Anti-anginal medications at 6 months; 2C) Canadian cardiovascular society angina score at 3 months; 2D) Canadian cardiovascular society angina score at 6 months; 2E) EQ5D score 
This study compared two strategies for treating CAD patients. The conventional approach encompassed PCI guided solely by angiographic observations, whereas the alternative approach incorporated FFR measurements alongside angiography. The study revealed that the FFR-guided strategy yielded superior outcomes.
The study encompassed diverse patients with varying clinical manifestations of CAD, including ACS-STEMI, NSTEMI, and unstable angina. Additionally, individuals with CSA were also included in the analysis. Patients with both multivessel and singlevessel disease were included.
The study found that 75.0% of patients with intermediate lesions on coronary angiography had FFR measurements below 0.8, indicating that these lesions were not hemodynamically significant and could be managed medically without stent placement.
The frequency of events recorded during angiography within this study closely resembled that observed in other recent investigations assessing the application of drug-eluting stents in cases of coronary artery disease [18-21].
We attempted to investigate standard PCI practices in this study. The study excluded patients with left main disease who were considered substantial based on angiography to concentrate primarily on routine practices. Additionally excluded were those having a STEMI from a recent myocardial infarction. FFR measurements may produce unreliable results in these circumstances. However, patients five days or more past the acute episode were included in the latter group.
Limitations of the Study
This was a single-centre study done in a tertiary care setting with a limited sample size from a single state in India. Hence, the results cannot be generalised to all populations. The data are restricted to a six-month follow-up period. Theoretically, lesions in FFR that are managed medically could progress, leading to events later in life. Some differences in results compared to landmark trials can be attributed to longer follow-ups in those studies. Also, patients with the left main disease are omitted.
Conclusion
The use of FFR guidance during angioplasty leads to changes in treatment decisions for most patients, resulting in fewer stent implantations or even avoiding stent placement altogether. Our study showed that patients who received FFR-guided treatment had positive long-term outcomes, consistent with findings from previous clinical trials. These findings offer significant evidence that advocates for incorporating FFR into standard clinical practice.
For individuals diagnosed with coronary artery disease undergoing drug-eluting stent placement via PCI, the study compares two approaches: One involving the routine assessment of FFR alongside angiographic guidance and the other solely guided by angiography for the PCI procedure. This combined approach reduces the occurrence of major adverse events within six months. These findings support the evolving strategy of revascularisation for ischemic lesions while opting for medical treatment for nonischaemic lesions.
Learning Points
What is already known: FFR is recommended in evaluating the physiological significance of intermediate stenosis on coronary angiography.
What this study adds: This study adds to the already existing evidence, albeit in the Western population, about the benefit of FFR in the Indian population with CAD with intermediate stenosis in terms of reducing MACE, anti-anginal medications, quality of life and the number and mean stent length.
Article Highlights
Research background
FFR assesses the physiological significance of coronary stenosis. It measures the ratio of the maximum blood flow achieved in a constricted artery to the ideal blood flow in a healthy artery. Measurement of it during coronary angiography is straightforward.
Research motivation
Most of the guidelines recommend estimation of FFR in intermediate coronary artery stenosis. Regrettably, there is a lack of available information in India.
Research objectives
This study aimed to investigate the benefit of FFR-guided PCI in patients with intermediate coronary artery stenosis undergoing PCI in terms of MACE, Functional class, quality of life, and antianginal medications used.
Research methods
We conducted an open-label observational study in 80 patients undergoing PCI. Patients were eligible if they were diagnosed with stable CAD or ACS and had intermediate lesions on coronary angiography. “Cases” underwent FFR-guided PCI while the remaining patients were enrolled as “Controls.”
Research results
Regarding the primary outcomes, there was no discernible difference. The Control group had significantly greater levels of anti-anginal drugs at three and six months, while the FFR group had significantly higher EQ-5D scores than the Control group (p<0.05). The mean stent length was significantly higher in the Control group than in the FFR group (p=0.023). The FFR group’s mean stent usage was notably less than that of the Control group (p=0.033).
Research conclusions
Management of intermediate lesions with an FFR-guided strategy was associated with better outcomes regarding angina and functional parameters. Major adverse cardiovascular events are reduced after six months of FFR-guided PCI, consistent with the Management of intermediate lesions with an FFR-guided strategy was associated with better outcomes regarding angina and functional parameters. Major adverse cardiovascular events are reduced after six months of FFR-guided PCI, consistent with the emerging approach of revascularisation for ischemic lesions and medicinal management for physiologically non-ischemic lesions.
Research perspectives
FFR guidance changes treatment decisions, resulting in fewer stent implantations or even avoiding stent placement altogether. Patients who received FFR-guided treatment had positive outcomes, consistent with findings from previous clinical trials. This advocates for the incorporation of FFR into standard clinical practice.
References
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3(11):E442 (2006).
- Tonino PAL, Bruyne BD, Pijls NHJ, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 360(3):213-224 (2009).
- Ong AT, van Domburg RT, Aoki J, et al. Sirolimus-eluting stents remain superior to bare-metal stents at two years: Medium-term results from the Rapamycin-Eluting Stent Evaluated at Rotterdam Cardiology Hospital (RESEARCH) registry. J Am Coll Cardiol. 47(7):1356-1360 (2006).
- Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: An observational study of drug-eluting versus baremetal stents. J Am Coll Cardiol. 48:2584-2591 (2006).
- Lima RS, Watson DD, Goode AR, et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 42(1):64-70 (2003).
- Fischer JJ, Samady H, McPherson JA, et al. Comparison between visual assessment and quantitative angiography versus fractional flow reserve for native coronary narrowings of moderate severity. Am J Cardiol. 90(3):210-215 (2002).
- Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary artery stenoses. N Engl J Med. 334(26):1703-1708 (1996).
- De Bruyne B, Pijls NH, Bartunek J, et al. Fractional flow reserve in patients with prior myocardial infarction. Circulation. 104:157-162 (2001).
- Pijls NH. Optimum guidance of complex PCI by coronary pressure measurement. Heart. 90(9):1085-1093 (2004). [CrossRef]
- Lawton JS, Tamis-Holland JE, Bangalore S, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 145(3):E4-E17 (2022).
- van Nunen LX, Zimmermann FM, Tonino PAL, et al. Fractional flow reserve versus angiography for the guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomized controlled trial. Lancet. 386(10006):1853-1860 (2015).
- Botman KJ, Pijls NH, Bech JW, et al. Percutaneous coronary intervention or bypass surgery in multivessel disease? A tailored approach based on coronary pressure measurement. Catheter Cardiovasc Interv. 63(2):184-191 (2004).
- Lip GY, Rathore VS, Katira R, et al. Do Indo-Asians have smaller coronary arteries? Postgrad Med J. 75(886):463-466 (1999).
- Harle T, Zeymer U, Hochadel M, et al. Real-world use of fractional flow reserve in Germany: Results of the prospective ALKK coronary angiography and PCI registry. Clin Res Cardiol. 106(2)140-150 (2017).
- Sengottuvelu G, Chakravarthy B, Rajendran R, et al. Clinical usefulness and cost effectiveness of fractional flow reserve among Indian patients (FIND study). Catheter Cardiovasc Interv. 88(5):E139-E144 (2016).
- Pijls NHJ, Fearon WF, Tonino PAL, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease. J Am Coll Cardiol. 56(3):177-184 (2010).
- Li J, Elrashidi MY, Flammer AJ, et al. Long-term outcomes of fractional flow reserve-guided vs angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 34(18):1375-1383 (2013).
- Hannan EL, Wu C, Walford G, et al. Drug-eluting stents vs coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 358(4):331-341 (2008).
- Stankovic G, Cosgrave J, Chieffo A, et al. Impact of sirolimus-eluting and paclitaxel-eluting stents on outcome in patients with diabetes mellitus and stenting in more than one coronary artery. Am J Cardiol. 98(3):362-366 (2006).
- Tanimoto S, Daemen J, Tsuchida K, et al. Two-year clinical outcome after coronary stenting of small vessels using 2.25-mm sirolimus- and paclitaxel-eluting stents: Insight into the RESEARCH and T-SEARCH registries. Catheter Cardiovasc Interv. 69(1):94-103 (2007).
- Win HK, Caldera AE, Maresh K, et al. Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. JAMA. 297:2001 (2007).