Research Article - Interventional Cardiology (2020) Volume 12, Issue 3
Outcomes in patients undergoing high-risk PCI using Impella circulatory support: 10-year experience
- Corresponding Author:
- SQ Khan Department of Cardiology, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, B15 2TH Birmingham, United Kingdom E-mail: sohail.khan@uhb.nhs.uk
Abstract
Objectives: The Impella® percutaneous systems (Abiomed, Danvers, USA) are temporary ventricular assist devices, approved for use in high-risk percutaneous coronary intervention (PCI). Impella use in the United Kingdom (UK) is limited by cost, reimbursement, lack of operator experience and paucity of long-term outcome data. We investigated 10-year outcomes of Impella circulatory support in a large quaternary UK hospital.
Methods: Consecutive patients undergoing Impella-assisted PCI between 2008 and 2018 were retrospectively identified and outcome data collected.
Results: Eighty patients underwent Impella-assisted PCI and were predominantly male (73.8%) with a mean age of 71.2 + 13.7 years. Fifty-three (66.3%) presented with non-ST segment elevation myocardial infarction (MI) and 7.5% with ST-segment elevation MI. The majority of patients had severe left ventricular systolic dysfunction (58.8%), multivessel disease (83.8%) and unprotected left main stem disease (52.5%). Ten (12.5%) patients had pre-procedural cardiogenic shock. In-hospital major adverse cardiac and cerebrovascular events (MACCE) and all-cause mortality occurred in 21.3% and 18.8% respectively. Post-procedure stroke and bleeding occurred in 2.5% and 13.8% of patients respectively, with one vascular complication (conservatively managed pseudoaneurysm). Median time to first follow-up visit was 105 (64.5; 282.0) days, at which point MACCE occurred in 18.8% of patients. Pre-procedural cardiogenic shock was a significant predictor of in-hospital MACCE (OR 9.0, C.I. 2.1-37.6, p=0.003).
Conclusion: When used to support high-risk PCI, Impella has an excellent safety profile. These data support the practice of Impella use in this cohort; however a randomised controlled trial is required to determine the efficacy of Impella against unsupported PCI.
Keywords
Impella; Revascularisation; high; risk; PCI
Abbreviation list
ACS: Acute Coronary Syndrome; BCIS-JS: British Cardiovascular Intervention Society Jeopardy Score; CABG: Coronary Artery Bypass Graft; CAD: Coronary Artery Disease; ESC: European Society of Cardiology; IABP: Intra-Aortic Balloon Pump; LV: Left Ventricle; LVEF: Left Ventricular Ejection Fraction; LVSD: Left Ventricular Systolic Dysfunction; MACCE: Major Adverse Cardiac and Cerebrovascular Events; MI: Myocardial Infarction; NSTEMI: Non-ST Segment Elevation Myocardial Infarction; NYHA: New York Heart Association; PCI: Percutaneous Coronary Intervention; QEHB: Queen Elizabeth Hospital Birmingham; STEMI: ST-segment Elevation Myocardial Infarction; TTE: Transthoracic Echocardiogram; UK: United Kingdom
Introduction
In patients with significant left ventricular impairment, advanced coronary artery disease (CAD) is associated with a significant risk of ventricular arrhythmias, heart failure, and sudden death. The impaired ventricle may be hibernating or stunned following an acute infarction and, if found to be viable, revascularisation may improve ventricular function and outcomes. However, these patients represent a particularly high-risk group for intervention [1] and performing complex PCI carries significant risk of coronary hypoperfusion.
Conventionally, intra-aortic balloon pump (IABP) support has been used to augment coronary perfusion. However, recent evidence has demonstrated that IABP does not reduce infarct size [2] or improve outcomes [3,4]. European Society of Cardiology (ESC) guidelines have now downgraded the routine use of IABP in patients with cardiogenic shock to a class III recommendation [5]. Accordingly, other alternatives have been sought to support coronary perfusion during high- risk PCI.
Impella, a percutaneous ventricular assist device, provides mechanical forward flow from the left ventricle (LV) thereby increasing the cardiac output and coronary perfusion pressure [6]. Impella has emerged as a strategy to protect against haemodynamic instability provoked by procedure- driven ischaemia in high-risk PCI. While there is no standardised definition of high-risk PCI, the most common features considered in previous studies include significant left ventricular systolic dysfunction (LVSD), multi-vessel disease, unprotected left main or last remaining vessel PCI.
The multicentre PROTECT I trial demonstrated the safety and feasibility of Impella support in heart failure patients requiring high-risk PCI [7]. The rate of major adverse cardiac events (MACE) at 30 days was 20%. In the PROTECT II trial, patients with complex CAD requiring haemodynamic support for PCI were randomised to receive circulatory support with IABP or Impella 2.5. While 30-day outcomes were similar, trends for improved outcomes were observed for Impella- supported patients at 90-days, including lower rates of repeat revascularisation and shorter duration of circulatory support [9].
Retrospective registries have demonstrated that Impella improves 1 year survival in patients with acute MI complicated by cardiogenic shock [9] and facilitates more complete revascularisation in elective high-risk PCI, improving LV recovery and survival [10]. A meta-analysis of 2827 patients also found that Impella-supported PCI was associated with good rates of survival and generally low incidence of complication across all indications [11].
However, recent registries have cast doubt over the efficacy of Impella. In a propensity-matched retrospective cohort study of 28,304 patients undergoing PCI for acute MI complicated by cardiogenic shock, Impella was used in 6% of patients and IABP in 30%. Among 1680 propensity- matched pairs, those treated with Impella had a significantly higher risk of in-hospital death and major bleeding [12]. In another retrospective registry of 48,306 patients, a wide variation of outcomes following Impella implantation was seen. Propensity adjusted association analyses revealed that outcomes were better during pre-Impella years and Impella use was associated with increased rate of death, bleeding, and stroke [13].
The purpose of this study was to investigate outcomes of patients undergoing Impella-assisted PCI over a 10-year period in a single quaternary cardiac centre with a typical real- world mixture of acute and elective cases, including patients with cardiogenic shock.
Methods
Study population
The Queen Elizabeth Hospital Birmingham (QEHB) is a major quaternary cardiac centre in the United Kingdom, offering regional cardiac transplantation services. All patients undergoing Impella-assisted PCI between May 2008 and September 2018 at QEHB were retrospectively identified. Clinical, laboratory, echocardiographic, angiographic and procedural data were collected. Complications were recorded until the time of discharge or death in the circumstance of in- hospital mortality.
On admission, the risk profile of all patients was recorded, including age, sex, previous history of acute coronary syndrome (ACS), previous PCI or coronary artery bypass grafting (CABG), diabetes mellitus (fasting blood glucose>7 mmol/L or treated diabetes), dyslipidaemia (low-density lipoprotein>3.0 mmol/L, fasting triglycerides>1.7 mmol/L, or total cholesterol>5 mmol/L), smoking status, hypertension (systolic blood pressure>140 mmHg and/or diastolic blood pressure>90 mmHg or treated hypertension). Diagnoses of stable angina, ST-segment elevation myocardial infarction (STEMI) and non-ST segment MI (NSTEMI) were made according to standard European guideline definitions [14].
Patients were considered for Impella at the discretion of the operator based on perceived high risk. In all non-emergency cases, the final decision to proceed to implantation was based on the consensus opinion of a multidisciplinary team discussion, considering patient factors such as co- morbidities, the degree and pattern of coronary disease, and left ventricular ejection fraction (LVEF).
Echocardiographic, angiographic and procedural analysis
Echocardiographic assessment of LVEF was performed using 2D transthoracic echocardiography (TTE). All TTE scans were reported by an accredited member of the British society of echocardiography. Severe LV dysfunction was defined as an ejection fraction of <35% by Simpson’s Biplane assessment.
Coronary artery disease burden was graded using the British Cardiovascular Intervention Society Jeopardy Score (BCIS-JS) [15] and Syntax scores I [16] and II [17]. Multivessel disease was defined as the presence of more than 1 vessel with angiographically significant lesions.
Prior to PCI, Impella 2.5 or Impella CP® devices were inserted via the femoral arterial approach using fluoroscopic guidance to locate the femoral head and the puncture made with or without the use of ultrasound guidance, according to operator preference. More recently, our centre has adopted the routine use of a 4F Transitionless-Tip Design Micropuncture® Set (Cook Medical, Indiana USA), with ultrasound guidance for the initial puncture, to reduce potential vascular complications. This can then be upsized to a 6-8 Fr sheath for pre-closure with 2 Perclose ProGlide® Suture-Mediated Closure System devices before the introduction of a peel-away 13 or 14 Fr Impella sheath. A 6 Fr pigtail or diagnostic Judkins right catheter was advanced into the LV over a 0.035 guidewire before exchanging for the Impella catheter. Impella-support was initiated and maintained throughout PCI.
Demographic and procedural data related to PCI were recorded contemporaneously in an in-house PCI database, which is used for prospective national recording of operator and procedural outcomes. The data completeness was very high, with data only missing in those who died before information could be attained.
Clinical follow-up
Major adverse cardiac and cerebrovascular events (MACCE) were defined as a composite of death, non-fatal MI, stroke and unplanned revascularisation. Post-procedure in-hospital occurrence of MACCE, vascular complications, bleeding and renal failure were recorded.
During routine follow-up visits, occurrence of MACCE was recorded, together with admissions for heart failure, Canadian Cardiovascular Society (CCS) angina grade and New York Heart Association (NYHA) functional classification.
Statistical analysis
Data distribution was assessed by Shapiro-Wilk test. Continuous variables were expressed as mean ± standard deviation or median with interquartile range if variables followed a normal or non-normal distribution, respectively. Dichotomous variables were expressed as percentages. Logistic and Cox regression analysis were performed to identify predictors of MACCE during admission and at follow- up respectively. Variables showing a p-value <0.05 at the univariate model were then included in the multivariate model. Variables showing a p-value of <0.05 at the multivariate model were considered independent predictors of MACCE. Long-term survival Kaplan Meier analysis was performed according to national registry death data. All analyses were performed using SPSS software version 23.0 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline Characteristics
A total of 80 consecutive patients were retrospectively identified to have undergone Impella-assisted PCI between May 2008 and September 2018. Patients had a mean age of 71.2 + 13.7 years (Table 1) and were of predominantly male sex (73.8%). The most prevalent cardiovascular risk factor was hypertension (59;73.8%) but other important risk factors included dyslipidaemia (46;57.5%), smoking (43;53.8%), diabetes mellitus (33;41.3%) and family history of CAD (32;40%). Prior ACS occurred in 41(51.3%) patients and 13(16.3%) patients had a history of previous PCI.
| Patient Population Baseline Characteristics (n= 80) | |
|---|---|
| Age [years ± SD] | 71.2 ± 13.7 years |
| Sex (male) [n, (%)] | 59 (73.8%) |
| Hypertension [n, (%)] | 59 (73.8%) |
| Diabetes mellitus n, (%)] | 33 (41.3%) |
| Smoking habit [n, (%)] | 43 (53.8%) |
| Dyslipidaemia [n, (%)] | 46 (57.5%) |
| Family history of CAD [n, (%)] | 32 (40%) |
| BMI (Kg/m²) [median (IQR)] | 26.93 [24.06; 29.42] |
| Previous ACS [n, (%)] | 41 (51.3%) |
| Previous PCI [n, (%)] | 13 (16.3%) |
| Previous CABG [n, (%)] | 4 (5%) |
| Stable angina | 21 (26.3%) |
| NSTE-ACS | 53 (66.3%) |
| STEMI | 6 (7.5%) |
| Peak CK [median (IQR)] | 196 [106.00;508.50] |
| LVEF (Mean ± SD) | 32.7% ± 16.6% |
| Severe LV systolic dysfunction | 47 (58.8%) |
| Multivessel disease [n, (%)] | 67 (84.4%) |
| Unprotected left main disease [n, (%)] | 45 (56.3%) |
| Last remaining vessel | 12 (15%) |
| Pre-procedural cardiogenic shock | 10 (12.5%) |
SD=Standard Deviation; CAD =Coronary Artery Disease; BMI=Body Mass Index; IQR=Interquartile Range; ACS=Acute Coronary Syndrome; PCI=Percutaneous Coronary Intervention; CABG=Coronary Artery Bypass Graft; NSTE-ACS=Non-ST Elevation Acute Coronary Syndrome; STEMI=ST Elevation Myocardial Infarction; CK=Creatine Kinase; LVEF=Left Ventricular Ejection Fraction; LV=Left Ventricle
Table 1: Baseline characteristics of the patient population.
Twenty-one (26.3%) patients presented with stable angina, 53 (66.3%) with non-ST segment elevation myocardial infarction (NSTEMI) and 6 (7.5%) with ST-segment elevation myocardial infarction (STEMI). Ten (12.5%) patients had pre-procedural cardiogenic shock. The mean left ventricular ejection fraction (LVEF) was 32.7 + 16.6%. Forty-seven (58.8%) patients had severely impaired LV systolic function.
Sixty-seven (83.8%) patients had multivessel disease, 42(52.5%) unprotected left main stem disease and 12 (15%) a last patent remaining vessel. The median BCIS-JS (Table 2) was 10 (8.00;12.00), representing significant myocardial jeopardy.
| PCI Characteristics and Procedural Data (n= 80) | |
|---|---|
| PCI access | |
| Single femoral | 32 (40%) |
| Single radial | 44 (55%) |
| Radial + Femoral | 4 (5%) |
| Haemostasis technique | |
| Compression device | 39 (48.8%) |
| Closure device | 41 (51.2%) |
| BCIS-Jeopardy Score [median (IQR)] | 10 [8.00; 12.00] |
| Syntax I score [median (IQR)] | 26.37 [20.25; 32.5] |
| Syntax II score [median (IQR)] | 50.3 [41.95; 62.20] |
| Bifurcation | 51 (63.8%) |
| Rotational atherectomy | 26 (32.5%) |
| Laser | 3 (3.8%) |
| Cutting Balloon | 1 (1.3%) |
| Glycoprotein IIb-IIIa Inhibitors | 7 (8.8%) |
| IVUS | 31 (38.8%) |
| OCT | 3 (3.8%) |
| Attempted CTO procedure | 4 (5%) |
Table 2: Characteristics of Impella-assisted percutaneous coronary intervention procedures. PCI=Percutaneous Coronary Intervention; IQR=Interquartile Range; BCIS=British Cardiovascular Intervention Society
Median Syntax I and II scores (Table 2) were 26.37 (20.25;32.5) and 50.3 (41.95;62.20) respectively.
Of the 80 Impella implantations, 36(45%) were Impella 2.5 which were used up until Impella CP became available which was subsequently implanted in 44 (55%) patients. Impella CP provides a peak forward flow rate of up to 3.5 L/min compared to 2.5 L/min with Impella 2.5.
Procedural data
PCI access was obtained via the radial artery in the majority of cases (55%), however a significant proportion of patients (40%) underwent PCI via the femoral approach.
The significant coronary disease burden of this cohort of patients often necessitated complex PCI techniques. Fifty-six (70%) patients required PCI with multiple stents and 32(40%) patients required 3 or more stents. Fifty-one patients (63.8%) required bifurcation PCI, 26(32.5%) rotational atherectomy, 3(3.8%) laser atherectomy and 1(1.3%) cutting balloon. In 4(5%) patients, a chronic total occlusion (CTO) PCI was attempted. Intravascular imaging was used to guide PCI in 34(43%) of patients (Table 2).
At procedure end, the Impella pump flow rate was gradually decreased and eventually removed, provided the patient was hemodynamic stable.
There was a roughly even split between the use of a closure device or compression alone. Two 6 Fr Perclose ProGlide® Suture-mediated Closure Systems (Abbott, Illinois USA) were used in 41(51.2%) cases while manual pressure or Femstop® (Abbott, Illinois USA) compression system were applied in 39(48.8%) of cases (Table 2).
Although there were no instances of significant haemolysis, post-procedure bleeding occurred in 11 patients (Table 3). Of these, 8 were categorised as Bleeding Academic Research Consortium (BARC) 3a, indicating a reduced haemoglobin of 3-5 g/dL requiring transfusion. all but one of these cases were non-access site bleeding and unrelated to Impella implantation. One case involved non-access site bleeding with Impella in situ and only one case involved the Impella access site with the Impella in situ. Three cases were categorised as BARC 3b due to blood loss resulting in a haemoglobin reduction of >5 g/dL. All 3 cases involved non-access site bleeding.
| Outcomes in-hospital and at follow up (n= 80) | |
|---|---|
| IN HOSPITAL OUTCOMES | |
| MACCE | 17 (21.3%) |
| All-cause mortality | 15 (18.8%) |
| Non-fatal myocardial infarction | 0 (0%) |
| Stroke | 2 (2.5%) |
| Unplanned Revascularization | 0 (0%) |
| Vascular complications | 1 (1.3%) |
| Bleeding | 11 (13.8%) |
| BARC classification 3a | 8 |
| BARC classification 3b | 3 |
| Renal failure delaying discharge | 17 (21.3%) |
| FOLLOW UP OUCOMES | |
| Median time to follow-up in survivors (days, IQR) | 105 [64.5; 282.0] |
| MACCE | 15 (18.8%) |
| All-cause mortality | 13 (16.3%) |
| Non-fatal myocardial infarction | 0 (0%) |
| Stroke | 1 (1.3%) |
| Unplanned Revascularization | 1 (1.3%) |
| Hospital admission for heart failure | 3 (3.8%) |
| CCS class [median (IQR)] | 0 (0; 0) |
| NYHA class [median (IQR)] | 1 (1; 2) |
| Mean survival time [months (C.I.)] | 21 (14.4;29.0) |
MACCE=Major Adverse Cardiovascular and Cerebrovascular Events; BARC=Bleeding Academic research Consortium; IQR=Interquartile Range; CCS=Canadian Cardiovascular Society; NYHA=New York Heart Association
Table 3: Outcome data for all Impella-assisted PCI.
Outcomes
Of the 80 Impella-assisted PCI cases performed, 65(81.2%) patients survived to discharge. Eight (10%) patients had a cardiac arrest during the procedure, among whom 4 died in-hospital (mean survival 4 days post-cardiac arrest) and the remaining 4 patients died in the community (mean survival 434 days post-cardiac arrest). Overall, the rate of in-hospital all-cause mortality and MACCE was 18.8% and 21.3% respectively. While there were no cases of non-fatal MI or further unplanned revascularisation procedures, there were 2(2.5%) cases of stroke. One vascular complication was recorded (a femoral access site pseudoaneurysm) which was conservatively managed with no sequelae.
Median time to first follow-up visit for survivors was 105 (64.5;282.0) days at which point 15(18.8%) patients had developed MACCE (Table 3). Three (3.8%) patients had hospital admissions for heart failure. Median NYHA was 1(1;2) and median CCS was 0(0;0). Mean survival time was 21 months (C.I. 14.4-29.0).
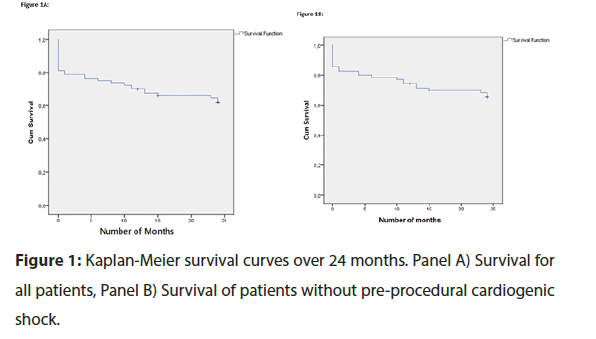
Figure 1A shows Kaplan-Meier survival curve over 24 months for all patients treated with Impella over the 10-year period. The most significant fall in survival is attributable to in- hospital mortality. Overall all-cause mortality over the 10-year period was 35%.
Tables 4 and 5 demonstrate an analysis of risk factors for MACCE by logistic and Cox regression respectively. Pre- procedural cardiogenic shock was the only statistically significant predictor of in-hospital MACCE (OR 9.0, C.I. 2.1- 39.6, p=0.003).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| (R2 = 0.422) | ||||
| OR (95% C.I.) | P | OR (95% C.I.) | P | |
| All patients | ||||
| Pre-procedural cardiogenic shock | 9.0 (2.1; 37.6) | 0.003 | ||
| Patients without pre-procedural cardiogenic shock: | ||||
| Creatinine (post-PCI) | 1.006 (1.001; 1.011) | 0.029 | NS | |
| Syntax II score | 1.064 (1.008; 1.124) | 0.026 | 1.088 (1.015; 1.166) | 0.018 |
OR=Odds Ratio; C.I.=Confidence interval; R2=Coefficient of determination; NS=Not significant
Table 4: Predictors of in-hospital MACCE in all patients and in patients without pre-procedural cardiogenic shock by univariate and multivariate Logistic regression analysis.
| Univariate analysis | ||
|---|---|---|
| HR (95% C.I.) | P | |
| All patients | ||
| Severe LVSD | 4.765 (0.999; 22.732) | 0.05 |
| Patients without pre-procedural cardiogenic shock: | ||
| Severe LVSD | 9.460 (1.169; 76.521) | 0.035 |
HR=Hazard Ratio; C.I.=Confidence interval; LVSD=Left Ventricular Systolic Dysfunction.
Table 5: Predictors of MACCE at follow up in all patients and in patients without pre-procedural cardiogenic shock by Cox regression analysis.
A sub-group analysis was performed amongst patients without cardiogenic shock in order to investigate predictors of in-hospital and follow-up MACCE in this cohort. Logistic regression analysis revealed that post-PCI serum creatinine (OR 1.006, C.I. 1.001–1.011, p=0.029) and Syntax II score (OR 1.064, C.I. 1.008–1.124, p=0.026) were statistically significant predictors of in-hospital MACCE in the univariate model. Following multivariate analysis, the Syntax II score (OR 1.088, C.I. 1.015–1.166, p=0.018) was the only independent predictor of in-hospital MACCE (Table 4).
A total of 26 patients had follow up echocardiograms within 1 year of Impella implantation (mean 166 days post-Impella implantation). An improvement of LVEF by > 10% was observed in 12(46%) patients (mean improvement 24%) while LVEF remained similar (<10% change) in 12 patients. The LVEF worsened by > 10% in 2 patients (mean decrease 16%).
Discussion
This observational study describes the outcomes of patients undergoing Impella-assisted PCI in a single centre in the UK. While the total study number of 80 patients is relatively small, it highlights the limited use of Impella circulatory support in modern day clinical practice, even in a large quaternary cardiac centre. According to the British Cardiovascular Intervention Society (BCIS) national annual audit reports, the 38 Impella-assisted PCI cases performed at our centre since 2014, represents a fifth of all Impella-assisted PCIs performed in the UK in that timeframe. The significant proportion of UK Impella cases implanted at QEHB may be reflective of its status as a transplant centre with access to Impella. The single payer government-run healthcare model in the UK does not reimburse hospitals for Impella implantation, contrary to other insurance-based healthcare systems worldwide. This further discourages Impella use in non-transplant UK PCI centres where Impella is not otherwise available.
Over the 10-year period observed, transradial PCI was the preferred method of access in 88.4% of all PCIs at the QEHB. Despite this, radial access was only utilised in 55% of Impella- assisted PCI, reflecting the strong preference for a trans- femoral approach in complex high-risk cases, even in the modern radial-dominant era.
The 80 Impella-assisted PCI procedures between 2008 and 2018 represent 0.85% of the total PCI procedures performed at the QEHB in this time period. On average, there were 8 Impella-assisted PCI cases per year and since 2010, a static number of Impella-assisted cases were performed per year (approximately 8 per year). This low annual volume of procedures may have impacted outcomes due to limited operator experience.
Impella support enabled successful revascularisation with only a single case of unplanned repeat revascularisation over the follow-up period. Incidences of stroke and vascular complications were also low, demonstrating the safety of Impella-assisted PCI. The latter may be partly attributable to the ultra-sounded-guided micro puncture technique, which is now common practice in our centre. It is particularly notable that there was only 1 vascular complication (a pseudoaneurysm) which was successfully managed conservatively.
As expected in a cohort with an inherently adverse risk profile, there was a significant rate of in-hospital and follow- up MACCE, of which all-cause mortality was the major component. A total of 28 patients developed MACCE over the 10-year period, which represents 35% of the patients undergoing Impella-assisted PCI. It is worth noting that the present study included patients with pre-procedural cardiogenic shock, which adversely impacted outcomes. In comparison, a recent retrospective registry in which patients with MI and cardiogenic shock were excluded, demonstrated MACCE occurrence in 24% of patients at 14-month follow up (10). In another similar multicentre observational registry, the 1-year all-cause mortality rate amongst patients with and without cardiogenic shock was found to be 57% and 15.6% respectively [18]. The findings from these recent comparatively similar registries imply that the occurrence rate of MACCE in the present study is within expected limits. The IMP-IT registry [18] also demonstrated the significant impact of cardiogenic shock on all-cause mortality in patients undergoing Impella-assisted PCI. These findings are reflected in our study, in which pre-procedural cardiogenic shock was the only strong statistically significant predictor of in-hospital MACCE.
In a subgroup analysis of patients without pre-procedural cardiogenic shock, post-PCI creatinine and Syntax II score were predictors of in-hospital MACCE. Syntax II score comprises a number of important clinical and anatomical risk factors to predict 4-year mortality in patients undergoing proposed coronary revascularisation. Although the Syntax II score was not validated in patients undergoing Impella-assisted PCI [17], this study found that it was the only independent predictor of MACCE in stable Impella-assisted PCI patients (without pre- procedural cardiogenic shock).
At follow-up, severe LVSD was an independent predictor of MACCE among patients without cardiogenic shock. Despite this, survivors seen at follow up tended to be asymptomatic with an extremely low incidence of hospitalisation for heart failure (3.8%) compared with the IMP-IT registry [18]. The low incidence of heart failure hospitalisation may be related to improvement in LVEF. This study found that up to 46% of patients who had follow up echocardiography within 1 year of Impella implantation had > 10% (mean 24%) improvement in LVEF.
Conclusion
In conclusion, outcome data in survivors suggests that Impella circulatory support may be used safely in the setting of complex PCI such as those with severe LVSD, multivessel disease, unprotected left main disease and PCI to the last remaining vessel. Without a control comparator group in this study, we cannot comment on the efficacy of Impella. A randomised controlled trial is urgently warranted to fully assess effectiveness in this cohort of patients. Until then, national guidelines should consider that Impella circulatory support is seldom used in most UK centres and should remain limited to centres with appropriate experience and expertise.
Limitations of the Study
The authors recognise that there are several limitations of the study. Firstly, the study was conducted retrospectively due to the nature of investigating 10-year historical outcomes of Impella use. Accordingly, there are missing procedural data (such as length of time of Impella support) and treatment heterogeneity (a mixture of Impella CP and Impella 2.5 employed within the study cohort). We also appreciate that the total number of patients studied is relatively small due to the limited use of Impella in the UK. The small number of Impella implantations per year contributes to operator unfamiliarity which could have impacted outcomes. The cohort investigated is heterogeneous due to a real-world mixture of patients with and without cardiogenic shock. Sub-group analysis has therefore been performed on a small sample size.
Acknowledgements
Dr. H. Sharma and Dr. V. Vetrugno contributed equally as first authors to this work
Conflicts of interest
All authors have no financial/proprietary interest in the subject matters of manuscript.
Funding
No funding was received for this work.
References
- Keelan PC, Johnston JM, Koru-Sengul T, Detre KM, et al. Comparison of in-hospital and one-year outcomes in patients with left ventricular ejection fractions ≤40%, 41% to 49%, and ≥50% having percutaneous coronary revascularization. Am J Cardiol. 91(10): 1168-1172 (2003).
- Patel MR, Smalling RW, Thiele H, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: The CRISP AMI randomized trial. JAMA-J Am Med Assoc. 306(12): 1329-1337 (2011).
- Thiele H, Zeymer U, Neumann F-J, et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N Engl J Med. 367(14): 1287-1296 (2012).
- Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): Final 12-month results of a randomised, open-label trial. Lancet. 382(9905): 1638-1645 (2013).
- Windecker S, Kolh P, Alfonso F, et al. 2014 EG on myocardial revascularization: TTF on MR of the ES of C (ESC) and the EA for C-TS (EACTS) D with the special contri. Ehu278. 2541–619 (2014)
- Sauren LDC, Accord RE, Hamzeh K, et al. Combined Impella and Intra-aortic Balloon Pump Support to Improve Both Ventricular Unloading and Coronary Blood Flow for Myocardial Recovery: An Experimental Study. Artif Organs. 31(11): 839-42 (2007).
- Dixon SR, Henriques JPS, Mauri L, et al. A Prospective Feasibility Trial Investigating the Use of the Impella 2.5 System in Patients Undergoing High-Risk Percutaneous Coronary Intervention (The PROTECT I Trial). Initial U.S. Experience. JACC Cardiovasc Interv. 2(2):91-96 (2009).
- O’Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 126(14): 1717-1727 (2012).
- Loehn T, O’Neill WW, Lange B, et al. Long-term survival after early unloading with Impella CP ® in acute myocardial infarction complicated by cardiogenic shock. Eur Hear J Acute Cardiovasc Care. (2018).
- Burzotta F, Russo G, Ribichini F, et al. Long-Term Outcomes of Extent of Revascularization in Complex High Risk and Indicated Patients Undergoing Impella-Protected Percutaneous Coronary Intervention: Report from the Roma-Verona Registry. J Interv Cardiol. (2019).
- Davila CD, Sharma S, Krishnamoorthy P, et al. Prevalence and clinical correlates of extended mechanical support in patients undergoing high-risk percutaneous coronary intervention in current clinical practice: Insights from the cVAD registry. Cardiovasc Revascularization Med. (2019).
- Dhruva SS, Ross JS, Mortazavi BJ, et al. Association of Use of an Intravascular Microaxial Left Ventricular Assist Device vs Intra-aortic Balloon Pump with In-Hospital Mortality and Major Bleeding among Patients with Acute Myocardial Infarction Complicated by Cardiogenic Shock. JAMA-J Am Med Assoc. 323(8): 734–745 (2020).
- Amin AP, Spertus JA, Curtis JP, et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention with Mechanical Circulatory Support. Circulation. (2020).
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of. Eur Heart J. 37(3): 267–315 (2016).
- Perera D, Stables R, Booth J, et al. The Balloon pump-assisted Coronary Intervention Study (BCIS-1): Rationale and design. Am Heart J. 158(6): (2009)
- Sianos G, Morel M, Kappetein AP, et al. SYNTAX Score. 219-227 (2005).
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of. Eur Heart J. 37(3): 267–315 (2016).
- Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of. Eur Heart J. 37(3): 267–315 (2016).