Case Series - Interventional Cardiology (2022) Volume 14, Issue 5
Overdiagnosis of spontaneous coronary artery dissection in young women: A case series highlighting challenges in angiographic diagnosis
- Corresponding Author:
- Tej Sheth
Department of Cardiology,
McMaster University,
Hamilton Health Sciences,
Ontario,
Canada,
E-mail: shetht@mcmaster.ca
Received date: 29-Aug-2022, Manuscript No. FMIC-22-65188; Editor assigned: 31-Aug-2022, PreQC No. FMIC-22-65188 (PQ); Reviewed date: 14-Sep-2022, QC No. FMIC-22-65188;Revised date: 21-Sep-2022, Manuscript No. FMIC-22-65188 (R);Published date: 28-Sep-2022, DOI: 10.37532/1755-5310.2022.14(5).555
Abstract
Interventional cardiologists sometimes over-diagnose Spontaneous Coronary Artery Dissection (SCAD) in young female patients despite clinical or angiographic findings that suggest ongoing diagnostic uncertainty or an alternative diagnosis. This case series highlights that unresolved symptoms and recurrent events can result when such patients have premature Coronary Atherosclerotic Disease (CAD) that is mislabeled as SCAD and not treated.
Keywords
Atherosclerosis • Coronary angiogram •Intravascular imaging • Optical coherence tomography • Percutaneous coronary intervention • Spontaneous coronary artery dissection
Abbreviations
ACS: Acute Coronary Syndrome; CAD: Coronary Atherosclerotic Disease; DES: Drug Eluting Stent; LAD: Left Anterior Descending Artery; MLA: Minimal Lumen Area; NSTEMI: Non-ST Segment Elevation Myocardial Infarction; OCT: Optical Coherence Tomography; PCI: Percutaneous Coronary Intervention; SCAD: Spontaneous Coronary Artery Dissection
Introduction
There has been increased awareness of the angiographic features of spontaneous coronary artery dissection over the past few decades. These findings may permit definitive diagnosis of SCAD during the index angiogram. Angiographic patterns include type 1 (contrast staining of the arterial wall with appearance of a double lumen), Type 2a (diffuse long smooth tubular with no visible dissection plane and with normal segments proximal and distal, Type 2b (diffuse long smooth tubular lesion extending to the distal tip of the artery), Type 3 (focal tubular lesion due to intramural haematoma that mimic atherosclerosis) and Type 4 (distal vessel occlusion) [1-3]. Among these patterns, type 2b, type 3, and type 4 patterns are not diagnostic of SCAD and may represent normal anatomic variants (Type 2b) or have other causes such as atherosclerosis or embolization (Type 3 or 4). When these angiographic findings are present, angiographers sometimes over-diagnose SCAD in younger female patients when ongoing uncertainty or a definitive alternative diagnosis should be considered.
We present three cases of CAD that were mis-diagnosed and treated as spontaneous coronary artery dissection. All three patients suffered ongoing anginal chest pain that was only relieved once the correct diagnosis was made at repeat invasive angiography and PCI was performed. Key learnings points to help avoid this misdiagnosis are presented.
Case Presentation
Case 1
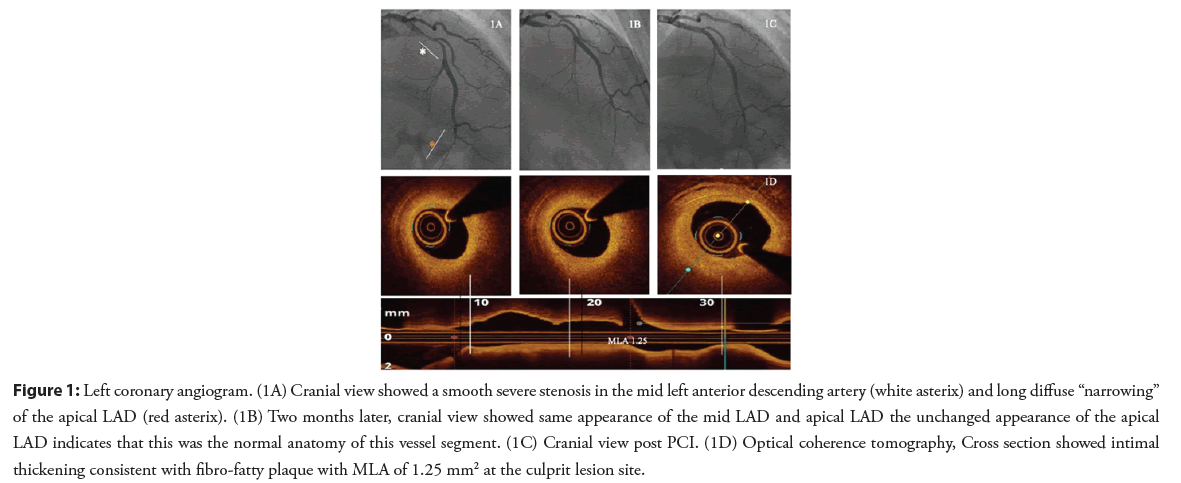
A 31-year-old female patient, with no cardiovascular risk factors, presented with exertional angina of one month duration. The patient underwent an exercise treadmill test which was clinically and electrically positive. Coronary angiography showed a smooth severe stenosis in the mid Left Anterior Descending artery (LAD) and a second distal area of suspected long diffuse “narrowing” in the apical LAD (Figure 1A). The patient was diagnosed as having Spontaneous Coronary Artery Dissection (SCAD) type 3 in the mid segment and SCAD type 2b in the apical segment and was discharged on medical therapy with follow up in SCAD clinic. No imaging was performed at the time of this angiogram.
After 6 weeks, the patient was seen in the regional SCAD clinic. She described persistent Canadian Cardiovascular Society class 3 exceptional chest discomforts. A second angiogram was performed and showed the same appearance of the mid LAD lesion, making SCAD a very unlikely cause (Figure 1B). The appearance of the apical LAD was also unchanged indicating that this was the normal anatomy of this vessel segment rather than Type 2B pattern of SCAD. Optical Coherence Tomography (OCT) was performed and showed a fibro-fatty atherosclerotic lesion of the mid LAD. Percutaneous Coronary Intervention (PCI) was performed with one Drug Eluting Stent (DES) (Figures 1C and 1D). Following this intervention, the patient’s angina resolved, and she remained asymptomatic at one year of follow-up.
Figure 1: Left coronary angiogram. (1A) Cranial view showed a smooth severe stenosis in the mid left anterior descending artery (white asterix) and long diffuse “narrowing” of the apical LAD (red asterix). (1B) Two months later, cranial view showed same appearance of the mid LAD and apical LAD the unchanged appearance of the apical LAD indicates that this was the normal anatomy of this vessel segment. (1C) Cranial view post PCI. (1D) Optical coherence tomography, Cross section showed intimal thickening consistent with fibro-fatty plaque with MLA of 1.25 mm2 at the culprit lesion site.
Case 2
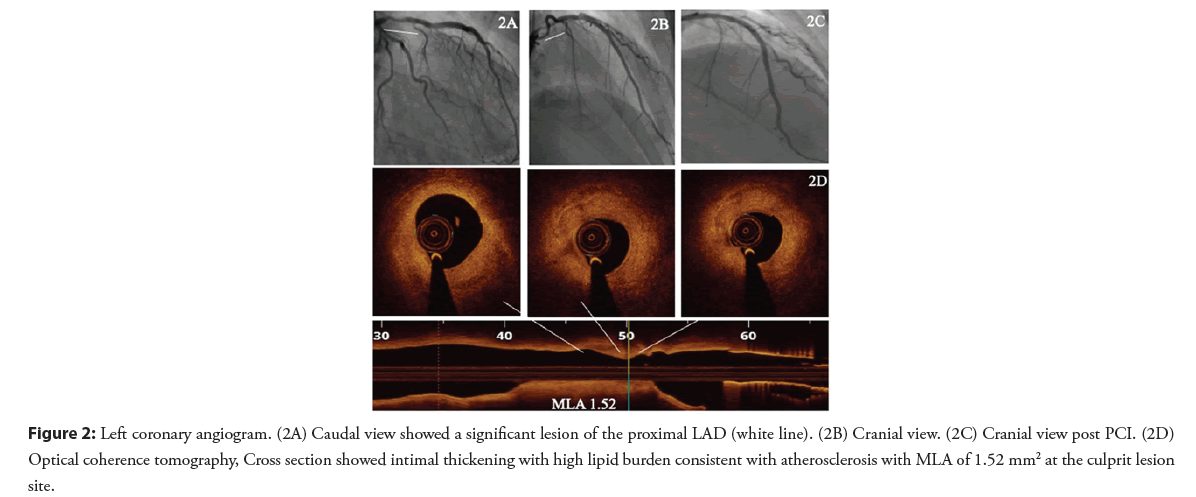
A 29-year-old female patient newly diagnosed with hypertension and type 1 diabetes mellitus presented with chest pain with significant troponin elevation. She underwent a coronary angiogram that showed a focal stenosis of the proximal LAD (Figures 2A and 2B). At that time, her ejection fraction was 35% and she was discharged on medical therapy for heart failure and treated as SCAD.
Two months later, she presented with progressive chest pain without troponin elevation or ECG abnormalities. She was admitted as unstable angina and a repeat coronary angiogram was performed showing persistent proximal LAD stenosis. OCT findings were consistent with an atherosclerotic lesion. PCI was performed with implantation of one drug eluting stent (Figures 2C and 2D).
Figure 2: Left coronary angiogram. (2A) Caudal view showed a significant lesion of the proximal LAD (white line). (2B) Cranial view. (2C) Cranial view post PCI. (2D) Optical coherence tomography, Cross section showed intimal thickening with high lipid burden consistent with atherosclerosis with MLA of 1.52 mm2 at the culprit lesion site.
Case 3
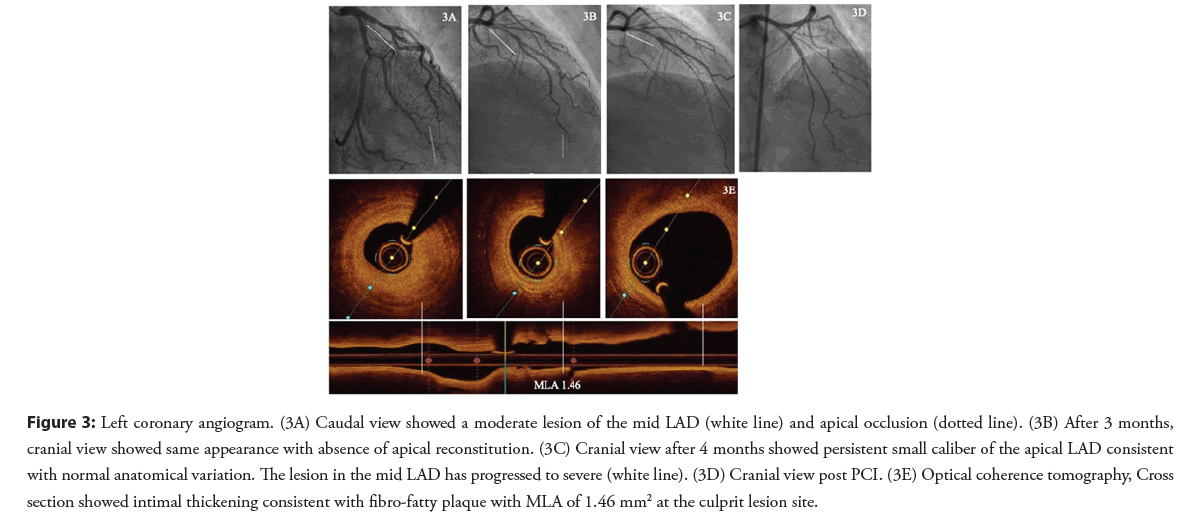
A 41-year-old female patient with hypertension and family history of premature CAD presented with NSTEMI. Coronary angiography demonstrated a moderate lesion in the mid segment and apical LAD occlusion. An unsuccessful attempt was made to wire the apical LAD occlusion and the final diagnosis at the time was type 4 SCAD (Figure 3A).
After three months and despite medical therapy, the patient was still complaining of chest pain on exertion. A coronary angiogram showed persistence of the moderate mid LAD stenosis and restoration of flow into the apical LAD which now appeared as a small diameter vessel. SCAD was felt to be the diagnosis based on the appearance of the apical LAD even though no distal reconstitution was seen (Figure 3B).
The patient was evaluated in SCAD clinic four months later and reported ongoing low threshold and rest angina. A third coronary angiogram was performed that showed persistent small caliber of the apical LAD consistent with normal anatomical variation. The lesion in the mid LAD has progressed to severe (Figure 3C). OCT confirmed this to be an atherosclerotic lesion and PCI of mid LAD was performed with implantation of one DES (Figures 3D and 3E).
Figure 3: Left coronary angiogram. (3A) Caudal view showed a moderate lesion of the mid LAD (white line) and apical occlusion (dotted line). (3B) After 3 months, cranial view showed same appearance with absence of apical reconstitution. (3C) Cranial view after 4 months showed persistent small caliber of the apical LAD consistent with normal anatomical variation. The lesion in the mid LAD has progressed to severe (white line). (3D) Cranial view post PCI. (3E) Optical coherence tomography, Cross section showed intimal thickening consistent with fibro-fatty plaque with MLA of 1.46 mm2 at the culprit lesion site.
Results and Discussion
Typical angina is not usually a presentation of SCAD. Among the 750 patients in the Canadian SCAD registry, 100% presented with ACS and 99.6% were troponin positive. The focal stenosis may represent SCAD (type 3) or CAD and intravascular imaging is indicated in these cases to clarify the etiology. In addition, caution is warranted in diagnosing SCAD of the apical segment with a type 2b pattern. Although there is apparent narrowing of this segment, there is no distal reconstitution of the vessel, and the diagnosis can only be confidently made with repeat angiography showing interval change in vessel caliber. The absence of any change in this segment after two months rules out the apical LAD as a SCAD involved segment as more than 95% of lesions are healed at 30 days [4]. This patient presents with Non-ST Segment Elevation Myocardial Infarction (NSTEMI) and SCAD should be strongly considered. However, the culprit stenosis is focal (<20 mm) and located at the ostium of the LAD which is an unusual segment to be affected by SCAD representing only 3% of cases in the Canadian SCAD registry [5]. Most LAD SCAD is detected in the apical LAD and is type 2a pattern. Angiographic ambiguity should be acknowledged and either upfront intravascular imaging or planned repeat angiography should be arranged to resolve the uncertainty and avoid the potential for ongoing misdiagnosis. Type 4 SCAD is a non-specific pattern. While it may represent SCAD, it may also represent abrupt occlusion caused by embolization from a more proximal coronary or non-coronary source. At the second angiogram, the apical LAD segment had restored flow, but small diameter and this appearance persists on the third angiogram. The stability of the appearance of the apical LAD between these two studies suggests this segment was not intrinsically abnormal. Furthermore, persistence of the angiographic stenosis in the mid LAD is not typical of SCAD and should have suggested an alternative diagnosis at the time of the second angiogram. The progression of this lesion observed on the third angiogram is consistent with an untreated culprit stenosis for ACS, highlighting the morbidity that can potentially result from overdiagnosis of SCAD and inappropriate medical treatment. Spontaneous coronary artery dissection is an increasingly recognised cause of acute coronary syndromes, especially in young and middle-age women. Recognising its particularities and differences with atherosclerotic disease is central for appropriately identifying and approaching these patients [6].
Conclusion
Virtually all patients with SCAD present with troponin positive ACS. Many SCAD patients can be diagnosed by coronary angiography alone, but in some the angiographic pattern of disease is ambiguous. Repeat angiography and/or intracoronary imaging should be considered where there is diagnostic uncertainty. Persistence of angiographic findings at repeat angiography makes SCAD less likely and may increase the safety of performing intravascular diagnosis and where needed, PCI.
Limitations of the Study
In light of the greater attention applied to the angiographic diagnosis of SCAD, interventional cardiologists should identify clinical and angiographic scenarios in which the diagnosis of SCAD remains uncertain. Plans should be made to resolve the uncertainty by selective use of repeat angiography and/or intravascular imaging. Although there are appropriate concerns of instrumenting coronary arteries during an acute SCAD episode, persistence of angiographic findings at repeat angiography makes SCAD less likely and increases the safety of performing intravascular diagnosis and where needed, PCI. All three patients presented here suffered ongoing symptoms due to undiagnosed and untreated atherosclerotic CAD. The burden of overdiagnosis of SCAD can be high and interventional cardiologists must be mindful to avoid it.
Consent
The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from patients.
Authors Contribution
All authors have contributed to the patient care, manuscript preparation and final approval.
Conflicts of Interest
Dr Sibbald, Dutra, Pinilla, and Sheth are consultants for Abbott Vascular Inc. None of the other authors have conflicts of interest.
Funding
The authors have no funding.
References
- Saw J. Spontaneous coronary artery dissection. Can J Cardiol. 29: 1027-1033 (2013).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 84(7): 1115-22 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Saw J, Mancini GB, Humphries K, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 87(2): E54-61 (2016).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Saber H, Prakash R, Starovoytov A, et al. Natural history of spontaneous coronary artery dissection with spontaneous angiographic healing. J Am Coll Cardiol Intv. 12(6): 518-527 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Saw J, Starovoytov A, Humphries K, et al. Canadian spontaneous coronary artery dissection cohort study: In-hospital and 30-day outcomes. Eur Heart J. 40(15): 1188-1197 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Macaya F, Salinas P, Gonzalo N, et al. Spontaneous coronary artery dissection: Contemporary aspects of diagnosis and patient management. Open Heart. 5(2): e000884 (2018).
[CrossRef] [Google Scholar] [PubMed]