Contrast Agent Evaluation - Imaging in Medicine (2012) Volume 4, Issue 1
Overview on the efficacy and safety of gadobutrol: an MRI contrast agent for the CNS, body and vessels
Juan E Gutierrez*1, Sara Koenig1 & Josy Breuer21University of Texas at San Antonio, Health Science Center 7703 Floyd Curl Drive, San Antonio, TX 78229-3900, USA
2Bayer-Schering AG, Berlin, Germany
- Corresponding Author:
- Juan E Gutierrez
University of Texas at San Antonio
Health Science Center 7703 Floyd Curl Drive
San Antonio, TX 78229-3900, USA
Tel: +1 210 567 1978
Fax: +1 210 567 6464
E-mail: gutierrezje@uthscsa.edu
Abstract
Contrast-enhanced MRI has become a mainstay of clinical imaging with unlimited applications. However, a limitation of conventional contrast agents in MRI is the insufficient signal-to-noise ratios due to relaxivity. Gadobutrol (1.0 mmol/ml), an extracellular macrocyclic contrast agent of high stability, is indicated for contrast-enhanced MRI of adults, adolescents and children 2 years of age and older. Gadobutrol offers increased T1 relaxivity, which improves MR image quality and clinical use of the technique.
Keywords
contrast-enhanced MRA ▪ contrast-enhanced MRI ▪ CNS n Gadavist™ ▪ gadobutrol ▪ gadolinium-based contrast agent ▪ Gadovist™ ▪ kidney ▪ liver
The use of gadolinium-based contrast agents (GBCAs) to improve MRI has been well established for over 20 years [1,2]. Unenhanced MRI of the CNS, abdomen/pelvis, breast and vascular system has mainly been used to display/ demarcate focal pathologies or areas of stenosis; however, GBCAs have improved the detection, characterization and visualization of lesions involved in such pathologies, improving the diagnostic work-up, therapy planning and monitoring evolution after therapeutic interventions [3]. Thus, when any lesion located in this body area are clinically suspected, combined MRI with and without GBCAs is the best method for defining lesion characteristics.
Gadobutrol is a nonionic, macrocyclic, extracellular MRI contrast agent. Like gadopentetate dimeglumine (MAGNEVIST® Bayer-Schering, Berlin, Germany) and other extracellular MRI contrast agents, gadobutrol contains gadolinium ions (Gd; Gd3+). Gadolinium is a rare earth element that causes contrast enhancement in MRI scans. Gadobutrol is the active ingredient, it is a paramagnetic gadolinium chelate with low osmolarity and viscosity, and high paramagnetic effect (relaxivity) [4], the Gd is tightly bound in a macrocyclic complex with a high stability. The relevant stability parameter for macrocyclic contrast agents in contrast to open-chain GBCAs is kinetic stability instead of thermodynamics. A study in 2006 examined the stability of different compounds by using possible transmetallation by zinc ions and calculated a so-called ‘long time index’ and ‘ratio index’. The ratio index, for example, showed a decomplexation of less than 10% after 5000 min, compared with 70–600 min for the open-chain compounds [5].
As with other GBCAs, the paramagnetic qualities of the gadolinium ion shortens the T1 relaxation times of nearby water protons and results in increased signal on T1-weighted images improving the lesion detection (sensitivity), characterization (specificity) and staging. Besides the registered trade name (GADAVIST® Injection, USA and GADOVIST®, in the rest of the world, the generic name (gadobutrol) and the laboratory nomenclature (Gd-DO3Abutriol or ZK 135079) have been used in some publications. Due to the unique physicochemical qualities of gadobutrol, solutions of 1.0 M concentration of Gd can be prepared. This concentration is twice that of all other extracellular MRI contrast media currently marketed (0.5 M).
At the same dose as 0.5 M agents, injected intravenously at the same rate, the 1.0 M concentration results in higher first-pass concentrations than a 0.5 M formulation [6]. In brain perfusion MRI, these improved bolus characteristics of 1.0 M gadobutrol were studied to evaluate the differences in contrast between ischemic and nonischemic tissue as compared with the 0.5 M concentration at the same dose [7]. Furthermore, gadobutrol is known to provide similar imaging properties in conventional contrast-enhanced MRI (CE-MRI) of various body regions other than the CNS, as is the case with other approved contrast agents. Gadobutrol is suitable for all clinically used MRI sequences (e.g., imaging of focal disease in different body regions) without the need to modify the parameters used with other GBCAs, as the concentration of the contrast agent has no significant impact on the extracellular distribution including the distribution in lesions.
Thus, gadobutrol can provide diagnostic information to classify benign and malignant lesions independent of the body region under examination.
Overview of the market
MRI is currently used for a wide range of clinical applications and is not limited to specific body areas. In the majority of cases, extracellular contrast agents are used to improve the diagnostic information in terms of lesion detection, localization and differentiation between benign and malignant lesions. Some more targeted MR contrast agents exist that either are blood-pool agents with a strong protein binding affinity for MR angiography (MRA) [8], or hepatocyte-specific agents like gadoxetate (Eovist® Bayer-Schering, Berlin, Germany, synonym Primovist®) or MultiHance® (Bracco Diagnostics Inc., NJ, USA) for liver MRI [9]. In general, the clinical usefulness of extracellular contrast agents for various applications to detect or exclude a disease has been documented by their approval for imaging areas all over the body. The contribution of a CE‑MRI is commonly accepted in the medical–scientific community. It can lead to a change in the diagnostic work-up, differential diagnosis and patient management [6,7,10]. The first approval of a GBCA occured in 1988 and has since led to the performance of more than 160 million CE-MRI examinations worldwide.
Advances in hardware and software have created additional needs regarding high relaxivity and/or small volumes of MRI contrast media, especially in perfusion MRI and MRA with rapid image acquisition. Particularly in dynamic first-pass MRI studies, a tight bolus (i.e., a short-lasting bolus containing a high concentration of contrast agent) is a prerequisite for good visualization [11]. A highly concentrated contrast agent like gadobutrol is ideal for such applications, resulting in high transitory Gd concentrations in the area of interest, for example, in brain perfusion MRI and dynamic contrast-enhanced MR angiography (CE-MRA).
Introduction to the compound
Gadobutrol is an electrically neutral gadolinium- containing contrast agent from a family of macrocyclic compounds. Gadobutrol was designed to give radiologists an additional option of an MRI contrast agent with high relaxivity and a potential higher capacity to provide contrast in areas of interest.
Chemistry
The chemica l name for Gadovist is [10- [2,3-dihydrox y-1- (hydrox yme thyl ) propyl]-1,4,7,10-tetraazacyclododecane-1, 4,7-triacetato- (3-)-N1,N4,N7,N10,O1,O4,O7] gadolinium (Figure 1). It has a molecular weight of 604.72 g/mole. It has a pK value of less than 2.0 (conjugate acid) and a pH value of 6.0–8.0, and it is freely soluble in water. Gadobutrol is a nonionic injectable contrast medium for use in MRI. It is provided as a sterile, clear, colorless and pyrogen-free aqueous, 0.5 or 1.0 mol/l solution, which is ready for use.
Pharmacodynamics
The signal intensity and contrast on MR images is determined by the water content and the behavior of the water protons in the magnetic field applied by the scanner, and the characteristic parameters for this are: the (proton) relaxation times (T1 and T2); due to some inherent differences in these parameters between normal tissue and pathological states (e.g., lesions), water content is higher and relaxation times are shorter, these differences allow normal tissue and lesions to be differentiated. However, if the differences in these parameters are low or there is no difference, no delineation of organs, tissues and lesions is feasible.
The use of the contrast agent causes enhancement of organs, tissues and lesions on MR images by shortening the relaxation times T1 and T2. Since gadobutrol shortens the relaxation times of tissues, it enables enhanced delineation of organs, tissues and lesions. This characteristic parameter for the potential (strength) of an MR contrast agent is called relaxivity. The relaxivity values of gadobutrol under clinically relevant conditions (in plasma, 37°C and at 1.5 T) are high (i.e., the relaxivity [r1] is about 5.2 l/[mmol*s] and the relaxivity [r2] is about 6.1 l/[mmol*s]) [4]. In combination with the unique 1 M formulation, gadobutrol has the highest effect on relaxation times per milliliter of drug product formulation, compared with all other extracellular contrast agents. The effect on the relaxation times in tissues, and consequently the signal intensity and contrast on the MR images, is dependent on the relaxivity and the concentration of the contrast agent in the area of interest. Gadobutrol improves the detection and visualization of specific features of pathological states (e.g., lesions) by showing: abnormal vascularity (called first-pass imaging as the contrast agent is still in the vessel within the first seconds after administration); leaking through a disrupted blood barrier; or being distributed into the extracellular space, thereby increasing the contrast enhancement of a lesion or displaying vessel structures.
Pharmacokinetics & metabolism
Gadobutrol has first-order pharmacokinetics in the body. After single intravenous administration, gadobutrol rapidly distributes into the extracellular space followed by fast renal elimination of the unchanged drug. The concentrations in plasma decrease in a biphasic manner characterized by an early distribution phase during the first minutes, followed by a second phase governed by elimination from plasma. Protein binding of gadobutrol is negligible [12].
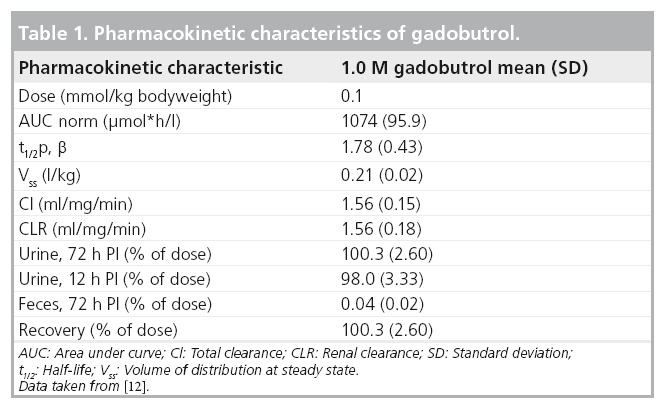
The renal clearance (CLR) is almost identical to the total clearance and mainly attributed to glomerular filtration, since the CLR is similar to the clearance of creatinine. Gadobutrol is excreted from the kidneys unchanged, and eliminated from plasma with a mean half-life of 1.81 h (1.33–2.13 h). In healthy subjects, CLR of gadobutrol is 1.1–1.7 ml/min/kg, which is almost identical to the total clearance and thus comparable to the CLR of inulin, confirming that gadobutrol is eliminated by glomerular filtration. Within 2 and 12 h after intravenous administration, more than 50 and 90% of the given dose is eliminated via the urine. Only a small amount (2.9%) of the dose was excreted after 12 h. Gadolinium concentrations were measurable in urine up to 72 h following administration (Table 1) [13].
Fecal excretion was only measured in one study and varied between 0.03 and 0.06% (standard deviation 0.02–0.04) of the injected dose [12].
As with other strongly extracellular GBCAs, there are no ethnic differences in the pharmacokinetics of gadobutrol across Caucasian, Japanese, black or Hispanic populations. The pharmacokinetics of gadobutrol in a pediatric population aged 2 to 17 years old is similar to those in adults [14]. Age has a moderate effect on the pharmacokinetics of gadobutrol in plasma, showing a reduced plasma clearance, leading to an increase in systemic exposure and to the half-life. Gender has no effect on the pharmacokinetics of gadobutrol, except in elderly women, where a slightly higher area under the plasma concentration versus time curve (area under curve) and a lower clearance may be observed.
No formal drug–drug interaction studies have been performed with gadobutrol and thus the potential is unknown. It is known that gadobutrol has no effect on zinc or iron metabolism. Since gadobutrol is not metabolized, a metabolic drug interaction with a coadministered drug is unlikely.
Clinical efficacy
Gadobutrol was developed worldwide, for different indications. Globally, gadobutrol has been administered to 313 subjects in Phase I studies and to 4705 patients in Phase II–IV studies including 138 pediatric patients from 2 to 17 years of age (Phase I/III study). Data for pediatric patients are presented in the sections of Phase II–IV. All studies were performed under Good Clinical Practice. Early clinical development in Europe and in Japan focused on a broad range of indications, especially those indications for which other extracellular agents are currently approved.
■ Phase I
In nine Phase I studies, a total of 313 subjects received 0.5 M gadobutrol or 1.0 M gadobutrol at doses between ≤0.11 and >1.51 mmol/kg bodyweight (BW).
The trials originated in Europe (n = 196), Japan (n = 56) and the USA (n = 61).
■P hase II–IV
In 35 Phase II–IV studies, gadobutrol has been administered to 4705 patients overall, including 138 pediatric patients.
The trials were performed in the EU (n = 2901), Asia (n = 1223), South/Central America (n = 308), USA/Canada (n = 264) and Australia (n = 9).
A total of 1002 patients received gadobutrol in the concentration of 0.5 M and 3703 patients in the concentration of 1.0 M, with a minimum dose applied of 0.01 mmol/kg BW, and a maximum dose of 0.5 mmol/kg BW. For the distribution of patients by indication, see Table 2.
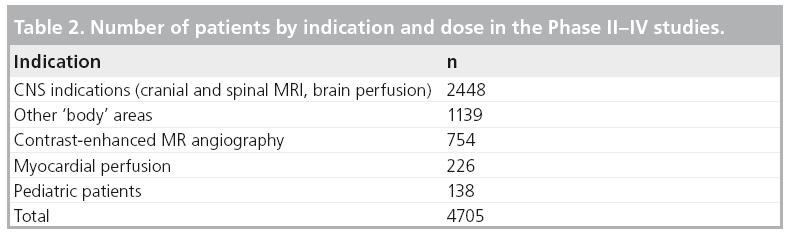
Special populations
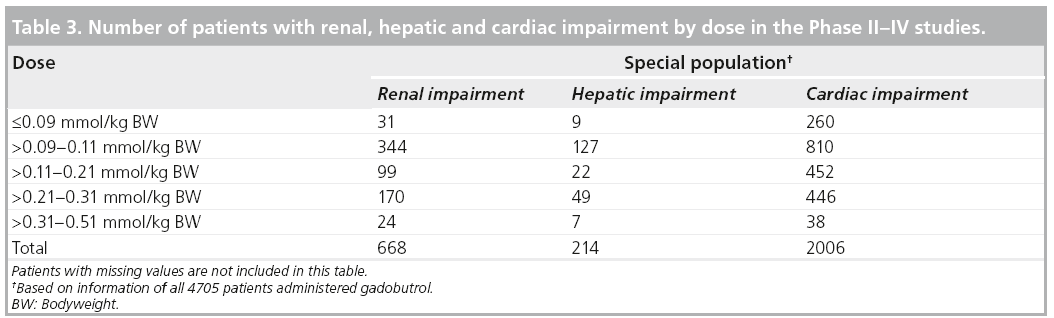
Three patient subgroups were identified: patients with impaired renal function, patients with impaired hepatic function and patients with impaired cardiac function. The percentages are related to the respective total number of patients (n = 4705) who received any dose of gadobutrol. Some studies did not include laboratory measurements.
A total of 668 of 4705 (14.2%) patients of the safety population had any degree of renal impairment with an estimated glomerular filtration rate of less than 90 ml/min.
A total of 214 patients were included with hepatic impairment defined as maximum (AST or ALT) over more than the upper limit of normal value (ULN) or between 1.8 ULN and less than maximum (ALT or AST), less than or equal to 3.0 ULN.
There were 2006 (42.6%) patients with cardiac impairment.
Table 3 shows the number of patients with renal, hepatic, and cardiac impairment by dose. There were no clinically significant safety effects revealed during exposure of gadobutrol in the special populations.
Ongoing Phase III studies
Further developmental activities are currently ongoing for gadobutrol. Two studies with identical study design will evaluate the efficacy and safety of gadobutrol for enhanced breast MRI.
A further study is being conducted in the Asia–Pacific region evaluating the value of gadobutrol in MRI for different body regions (e.g., breast, heart, abdomen, kidney, pelvis or extremities).
Two studies are planned in the field of contrast-enhanced MRA in renal arteries and supra-aortic arteries for the USA. More detailed information can be found at [101].
Clinical indications
■ CNS indication
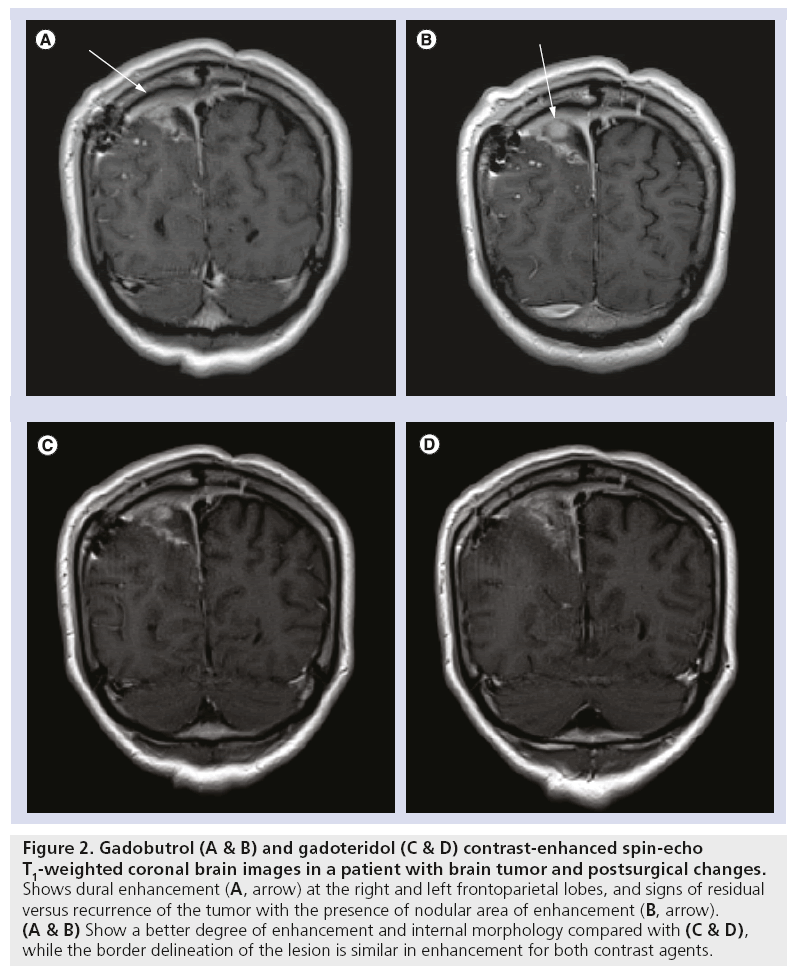
In all trials with CNS indication the common objectives regarding efficacy were to demonstrate an adequate signal increase in diseased areas of brain and spine on T1-weighted MRI images, assessment of visualization parameters like border delineation, contrast enhancement, lesion size, internal morphology and adequate signal loss in perfusion imaging of T2*-weighted sequences after intravenous injection of gadobutrol. The majority of trials used the unenhanced (no contrast medium) scans and also an approved extracellular MR contrast agent as the comparator (Figures 2 & 3).
Figure 2: Gadobutrol (A & B) and gadoteridol (C & D) contrast-enhanced spin-echo T1-weighted coronal brain images in a patient with brain tumor and postsurgical changes. Shows dural enhancement (A, arrow) at the right and left frontoparietal lobes, and signs of residual versus recurrence of the tumor with the presence of nodular area of enhancement (B, arrow). (A & B) Show a better degree of enhancement and internal morphology compared with (C & D), while the border delineation of the lesion is similar in enhancement for both contrast agents.
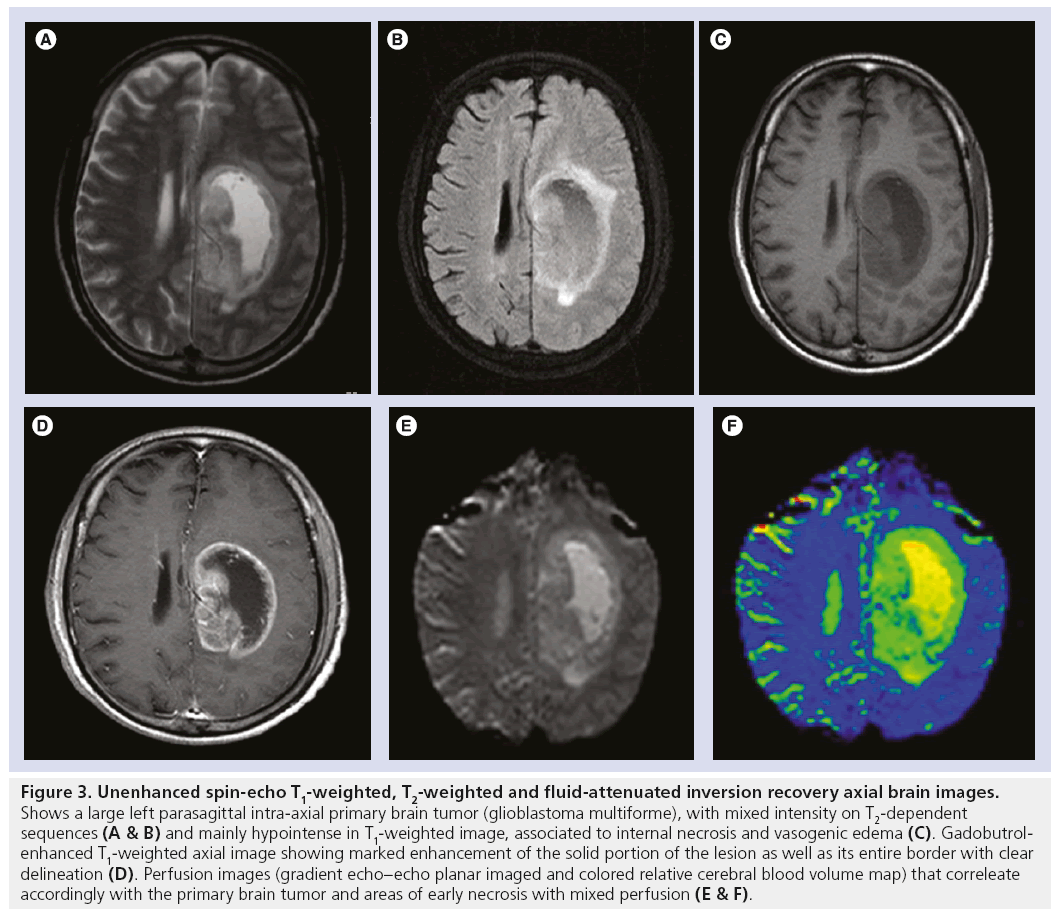
Figure 3: Unenhanced spin-echo T1-weighted, T2-weighted and fluid-attenuated inversion recovery axial brain images. Shows a large left parasagittal intra-axial primary brain tumor (glioblastoma multiforme), with mixed intensity on T2-dependent sequences (A & B) and mainly hypointense in T1-weighted image, associated to internal necrosis and vasogenic edema (C). Gadobutrolenhanced T1-weighted axial image showing marked enhancement of the solid portion of the lesion as well as its entire border with clear delineation (D). Perfusion images (gradient echo–echo planar imaged and colored relative cerebral blood volume map) that correleate accordingly with the primary brain tumor and areas of early necrosis with mixed perfusion (E & F).
Regardless of the gadobutrol concentration, brain and spine lesions were enhanced in the majority of patients on T1-weighted images after administration of the contrast media. In perfusion studies at different doses and using T2* fast gradient echo sequences, a signal loss was observed, as expected, demonstrating a clear dose dependency, and with echo-planar image sequences, it was shown that the dose can be reduced to 0.1 mmol/kg. Based on prior publications, the possible advantage in perfusion imaging is the better bolus characteristics and high relaxivity as well as the higher concentration of gadolinium in the lesion or region of interest. This has been postulated to open the way for improved diagnostic efficacy not only for the brain but also for CE-MRA [15]. Following the same line of publications, in other comparison studies, it was concluded that the higher concentration 1.0 M gadobutrol can offer advantages over standard 0.5 M gadopentetate dimeglumine, particularly with respect to delineation between gray and white matter and for the demarcation of highly vascularized tumor tissue on brain PWI performed at 3 T [16].
A dose-related improvement for all parameters evaluated could be shown after administration of gadobutrol compared with the unenhanced scans in all studies performed on conventional T1-weighted imaging. With regard to lesion detection and visualization of the lesion (border delination, contrast enhancement, internal morphology and lesion size evaluated in some studies), the dose of 0.1 mmol/kg proved efficacious in the majority of the subjects, including patients with brain metastases. In comparison to approved extracellular MR contrast agents, the visualization of lesions was proven to be noninferior.
Sensitivity, specificity and accuracy in determining malignant lesions, as evaluated in some studies, increased from unenhanced MRI compared with combined (unenhanced plus gadobutrol enhanced) MRI [17,18].
Based on a majority assessment of blinded readers, sensitivity increased significantly from 51.3 to 71.2%, accuracy increased significantly from 81.7 to 87.5% and specificity increased slightly from 93.7 to 94.0%.
■ Diagnostic confidence
Diagnostic confidence was assessed in some studies, and showed that after an initial dose of 0.1 mmol/kg gadobutrol, there was an increase in confidence by 85 to 90 compared with the unenhanced MRI.
An assessment of the degree of improvement in diagnostic confidence was measured in two studies, with a rating of 'excellent' or 'good' given to 61 and 43% of patients, respectively, with no difference between gadobutrol and gadodiamide [18].
Overall, 1.0 M gadobutrol for CNS imaging is advantageous due to its tight bolus administration and high relaxivity. Gadobutrol 1.0 M has been shown to be noninferior to other approved contrast agents for CNS imaging and has been found by some to have a higher sensitivity and accuracy as well as noninferior diagnostic confidence.
■ CE-MRA indication
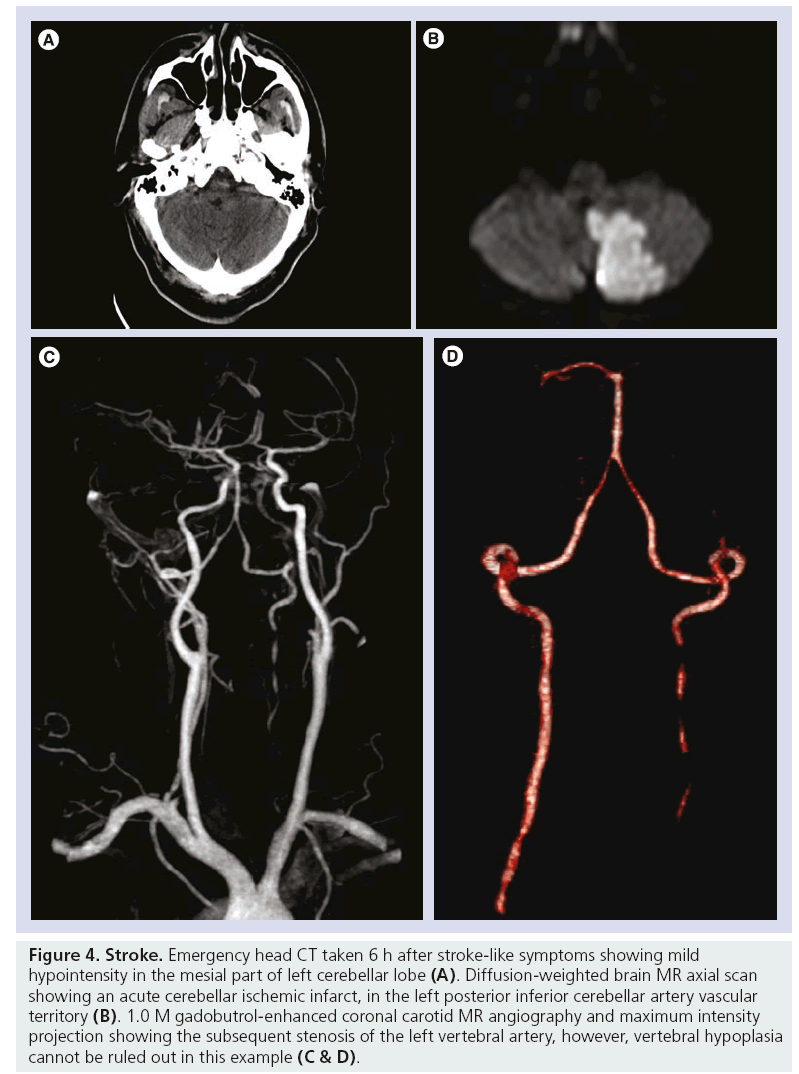
In MRA studies, the primary objective was to show diagnostic agreement between gadobutrol- enhanced MRA and intra-arterial digital subtraction angiography (IADSA) for clinically relevant diagnostic patient groups and for prospectively defined vessel segments. Among the studies, vessel segments evaluated were the aortic arch, common carotid arteries, vertebral arteries, subclavian arteries, common or external iliac artery, femoral artery, abdominal aorta, mesenteric arteries, renal artery and peripheral arteries (Figures 4 & 5). In one study, the results showed that gadobutrol-enhanced MRA gave comparable results to IADSA for the larger arteries of the pelvis and thigh [19], and another study showed that 1.0 M gadobutrol-enhanced MRA provided an excellent diagnosis or exclusion of pathology in the aortoiliac vessels [20,21]. Additionally, it was found that contrast-enhanced MRA has very similar outcomes to IADSA for detecting stenosis of the left and right internal carotid arteries and external and common iliac arteries [22], and it has also been shown to be efficacious in imaging of the arteries supplying the spinal cord [23]. Pulmonary studies have indicated that in gadobutrol 1.0 M enhanced-MRA, there was a higher signal-to-noise ratio and contrastto- noise ratio with noninferior image quality when compared with 0.5 M gadopentetate dimeglumine [24].
Figure 4: Stroke. Emergency head CT taken 6 h after stroke-like symptoms showing mild hypointensity in the mesial part of left cerebellar lobe (A). Diffusion-weighted brain MR axial scan showing an acute cerebellar ischemic infarct, in the left posterior inferior cerebellar artery vascular territory (B). 1.0 M gadobutrol-enhanced coronal carotid MR angiography and maximum intensity projection showing the subsequent stenosis of the left vertebral artery, however, vertebral hypoplasia cannot be ruled out in this example (C & D).
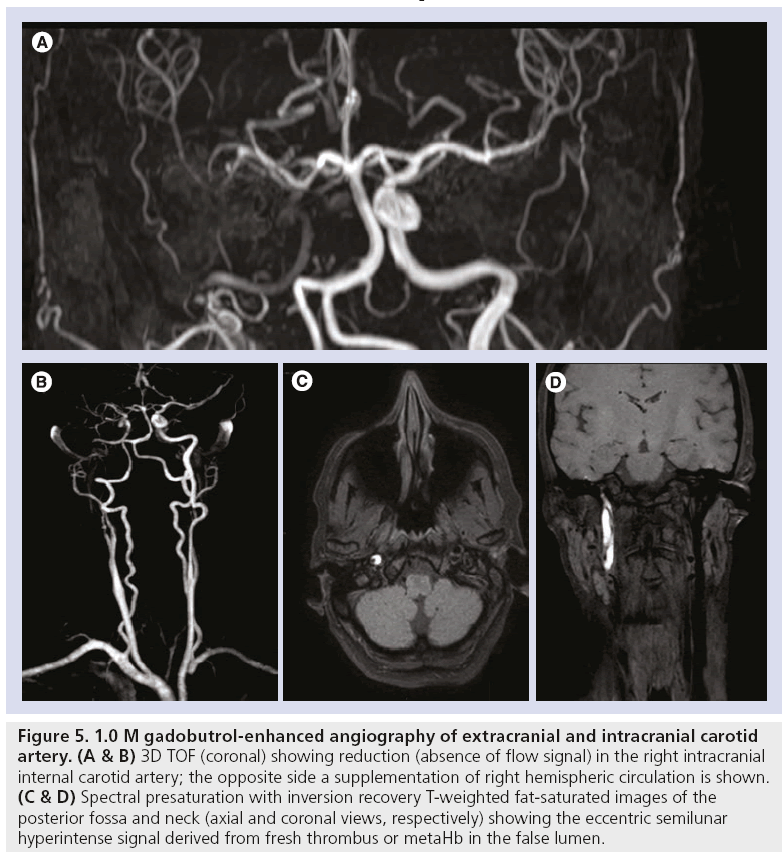
Figure 5: 1.0 M gadobutrol-enhanced angiography of extracranial and intracranial carotid artery. (A & B) 3D TOF (coronal) showing reduction (absence of flow signal) in the right intracranial internal carotid artery; the opposite side a supplementation of right hemispheric circulation is shown. (C & D) Spectral presaturation with inversion recovery T-weighted fat-saturated images of the posterior fossa and neck (axial and coronal views, respectively) showing the eccentric semilunar hyperintense signal derived from fresh thrombus or metaHb in the false lumen.
In other clinical studies it has been shown that gadobutrol is noninferior to MAGNEVIST for CE-MRA.
Overall, gadobutrol-enhanced MRA has been found to be equally as efficacious as IADSA and MAGNEVIST-enhanced MRA for imaging large to medium-sized vessel segments (Figures 6–8).
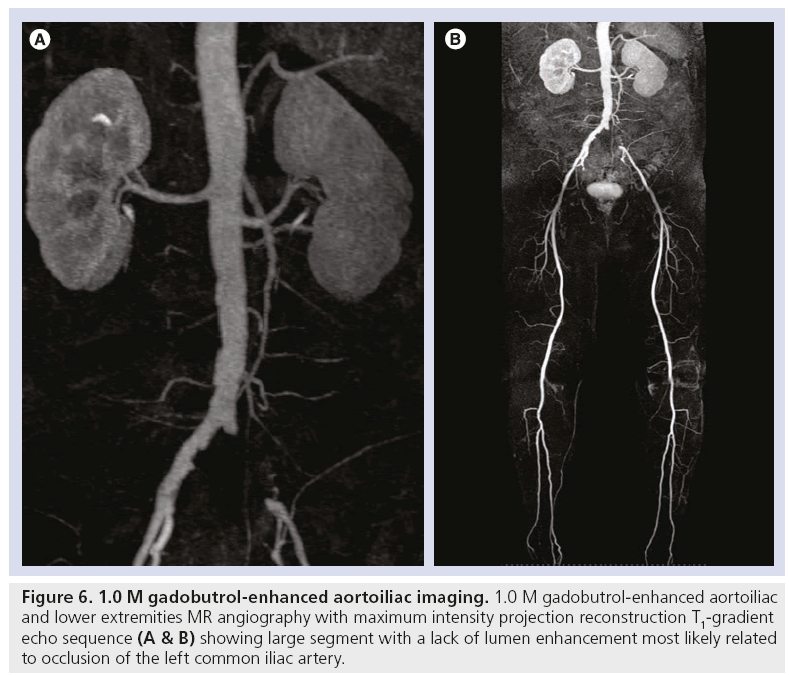
Figure 6: 1.0 M gadobutrol-enhanced aortoiliac imaging. 1.0 M gadobutrol-enhanced aortoiliac and lower extremities MR angiography with maximum intensity projection reconstruction T1-gradient echo sequence (A & B) showing large segment with a lack of lumen enhancement most likely related to occlusion of the left common iliac artery.
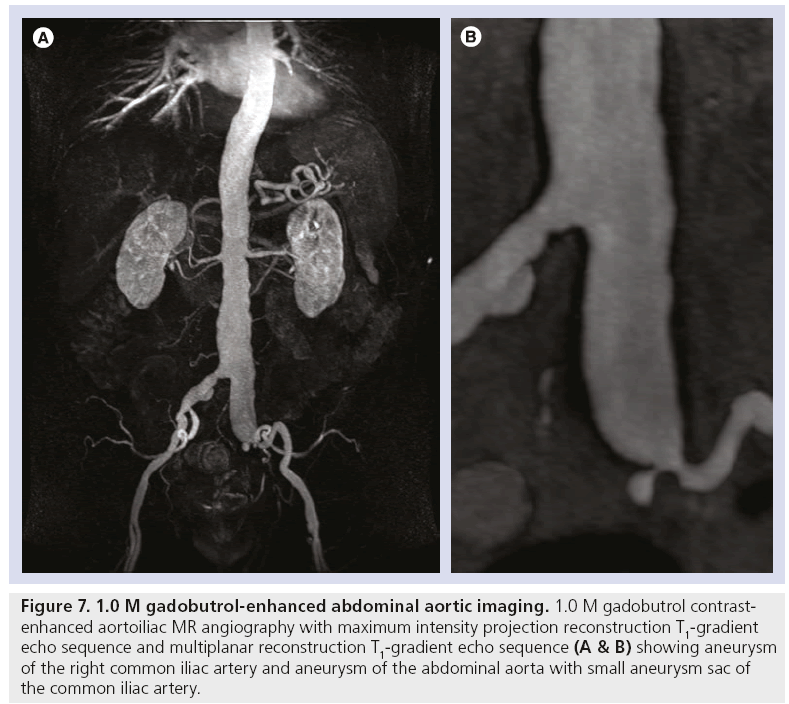
Figure 7: 1.0 M gadobutrol-enhanced abdominal aortic imaging. 1.0 M gadobutrol contrastenhanced aortoiliac MR angiography with maximum intensity projection reconstruction T1-gradient echo sequence and multiplanar reconstruction T1-gradient echo sequence (A & B) showing aneurysm of the right common iliac artery and aneurysm of the abdominal aorta with small aneurysm sac of the common iliac artery.
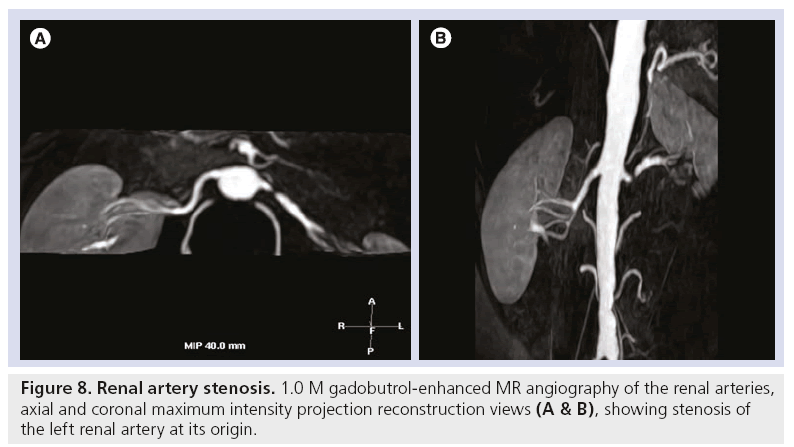
Figure 8: Renal artery stenosis. 1.0 M gadobutrol-enhanced MR angiography of the renal arteries, axial and coronal maximum intensity projection reconstruction views (A & B), showing stenosis of the left renal artery at its origin.
■ CE-MRI of other body regions: liver & kidneys
In several studies for visceral applications of gadobutrol, the primary efficacy variables for gadobutrol at a dose of 0.1 mmol/kg BW proved an increase in diagnostic accuracy from pre- to combined pre- and post-contrast MRI scans, thereby a noninferiorty to MAGNEVIST was elucidated. Efficacy was determined by clinical investigators and blinded readers. The standard of reference for each study was assessment by an independent truth panel or against a predefined and independent standard of truth. The results showed that the performance of gadobutrol is comparable to MAGNEVIST for both studies and noninferiority was proven for kidney and liver studies [25,26]. Another study indicated that 1.0 M gadobutrol-enhanced MRI of the upper abdominal organs, especially the spleen, pancreas, and kidney, is better due to the higher gadolinium concentration of gadobutrol than in gadoxetic acid [9,27]. In summary, 1.0 M gadobutrol has been shown to be an efficacious agent for imaging of the liver, kidneys, and upper abdominal organs (Figures 9–11).
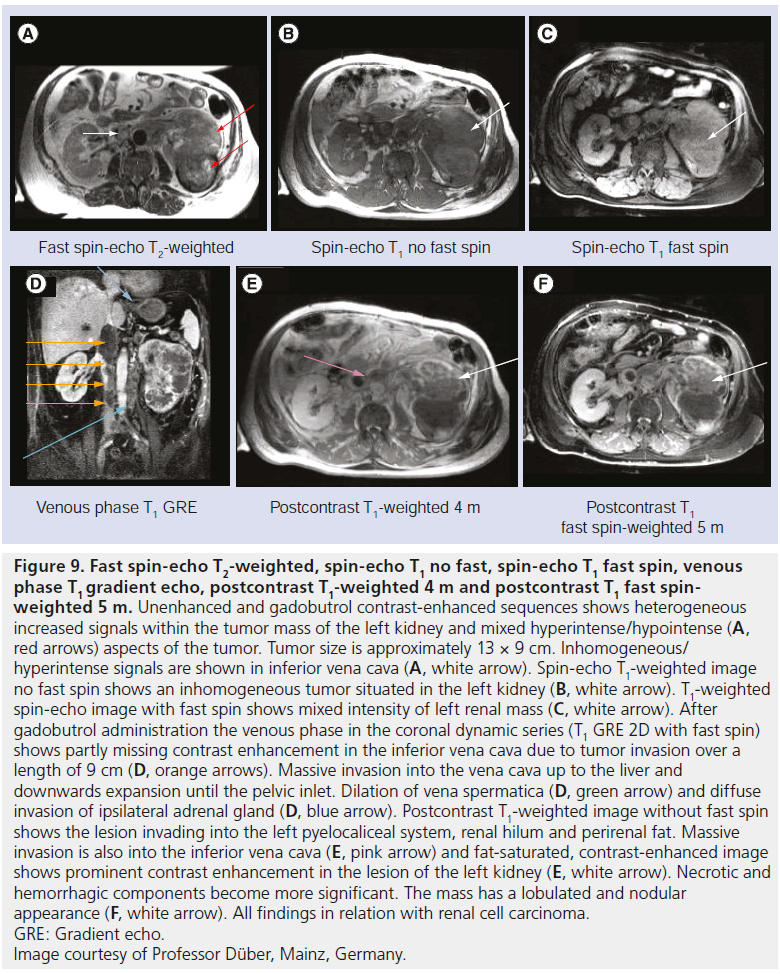
Figure 9: Fast spin-echo T2-weighted, spin-echo T1 no fast, spin-echo T1 fast spin, venous phase T1 gradient echo, postcontrast T1-weighted 4 m and postcontrast T1 fast spinweighted 5 m. Unenhanced and gadobutrol contrast-enhanced sequences shows heterogeneous increased signals within the tumor mass of the left kidney and mixed hyperintense/hypointense (A, red arrows) aspects of the tumor. Tumor size is approximately 13 × 9 cm. Inhomogeneous/ hyperintense signals are shown in inferior vena cava (A, white arrow). Spin-echo T1-weighted image no fast spin shows an inhomogeneous tumor situated in the left kidney (B, white arrow). T1-weighted spin-echo image with fast spin shows mixed intensity of left renal mass (C, white arrow). After gadobutrol administration the venous phase in the coronal dynamic series (T1 GRE 2D with fast spin) shows partly missing contrast enhancement in the inferior vena cava due to tumor invasion over a length of 9 cm (D, orange arrows). Massive invasion into the vena cava up to the liver and downwards expansion until the pelvic inlet. Dilation of vena spermatica (D, green arrow) and diffuse invasion of ipsilateral adrenal gland (D, blue arrow). Postcontrast T1-weighted image without fast spin shows the lesion invading into the left pyelocaliceal system, renal hilum and perirenal fat. Massive invasion is also into the inferior vena cava (E, pink arrow) and fat-saturated, contrast-enhanced image shows prominent contrast enhancement in the lesion of the left kidney (E, white arrow). Necrotic and hemorrhagic components become more significant. The mass has a lobulated and nodular appearance (F, white arrow). All findings in relation with renal cell carcinoma. GRE: Gradient echo. Image courtesy of Professor Düber, Mainz, Germany.
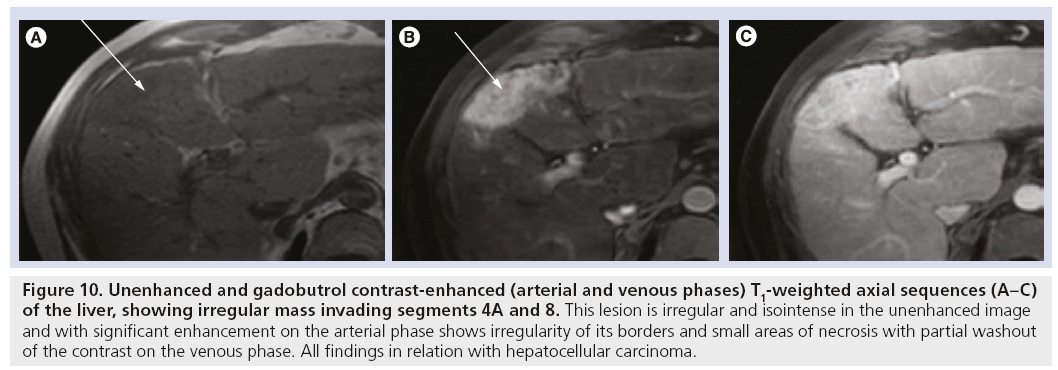
Figure 10: Unenhanced and gadobutrol contrast-enhanced (arterial and venous phases) T1-weighted axial sequences (A–C) of the liver, showing irregular mass invading segments 4A and 8. This lesion is irregular and isointense in the unenhanced image and with significant enhancement on the arterial phase shows irregularity of its borders and small areas of necrosis with partial washout of the contrast on the venous phase. All findings in relation with hepatocellular carcinoma.
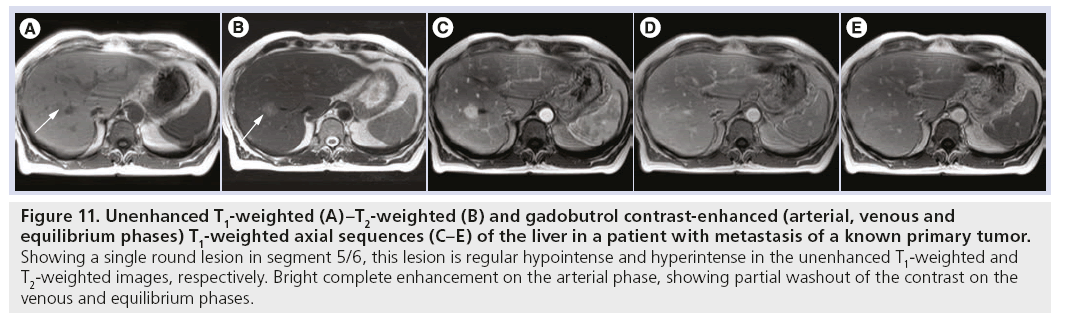
Figure 11: Unenhanced T1-weighted (A)–T2-weighted (B) and gadobutrol contrast-enhanced (arterial, venous and equilibrium phases) T1-weighted axial sequences (C–E) of the liver in a patient with metastasis of a known primary tumor. Showing a single round lesion in segment 5/6, this lesion is regular hypointense and hyperintense in the unenhanced T1-weighted and T2-weighted images, respectively. Bright complete enhancement on the arterial phase, showing partial washout of the contrast on the venous and equilibrium phases.
■ Efficacy data for pediatric population
In pediatric patients (aged 2–17 years old) scheduled for CE‑MRI of CNS, liver and kidneys or CE-MRA diagnostic efficacy evaluated by means of contrast quality, number of lesions, border delineation, internal morphology and MRI diagnosis, an increase in diagnostic confidence was demonstrated for all parameters evaluated in the study and there was no difference among the age groups.
Safety & tolerability
■ Postmarketing surveillance
Gadobutrol is approved in 73 countries and has been marketed in 63 countries worldwide as of February 2011. According to the accumulated global sales and marketing data, it is estimated that Gadovist was administered to approximately 6.8 million patients between its introduction to the Swiss market in 1999 and February 2011. This number includes approximately 180,000 pediatric patients. During the worldwide postmarketing use, there was a total of 1343 suspected adverse drug reactions reported involving gadobutrol. Of those, 942 cases were classified as nonserious and 401 cases were classified as serious case reports. The most serious adverse reactions reported with gadobutrol were cardiac arrest, respiratory arrest, anaphylactoid shock and nephrogenic systemic fibrosis (NSF)- like symptoms. Other individual events were loss of consciousness, convulsion, conjunctivitis, eyelid edema, tachycardia, circulatory collapse, flushing, bronchospasm, cyanosis, oropharyngeal swelling, cough, sneezing, face edema, hyperhidrosis, pruritus, erythema, feeling hot and malaise, which occurred rarely. No episodes of prolonged QT/QTc, malignant arrythmias or torsades de pointes have been reported. Also, no reports of adverse events have been received in connection with inadvertant double dosing.
Six prospective observation studies were conducted during the period from 2000 to 2007 [11]. In these studies, gadobutrol was administered to a total of 14,299 patients. Examination of the brain and, head and neck was most frequent (54.4%), followed by vessels/MRA (14.4%) and spinal examinations (7.1%). At least one adverse reaction event was observed in 78 of 14,299 patients (0.55%), and two serious adverse reaction events (anaphylactic symptoms and itching and swelling of throat) requiring hospitalization were reported. None of these AEs exceeded a 1% incidence rate.
The most frequently reported adverse reaction was nausea, which was observed in 36 patients (0.25%). Safety data obtained from postmarketing studies suggest high tolerance for gadobutrol, and the safety profile of gadobutrol is consistent with the published data of other extracellular GBCAs including MAGNEVIST. The postmarketing data show that the incidence of adverse reactions is low and with events recognized as adverse drug reactions of Gd-containing contrast agents [28]. The general safety profile was found to correspond well to the data published for other extracellular GBCAs like gadopentetate dimeglumine [102], gadoversetamide [29], gadodiamide [25] or gadoteridol [30].
NSF is a rare, but serious, disease first described in the medical literature in 2000, with the first reported case going back to 1997. NSF has predominantly been reported in patients with severe or end-stage kidney insufficiency or acute kidney injury. Although the precise cause of the disease has not been identified, it is thought to be due to a combination of multiple factors, including the use of GBCAs. One of the prevailing theories as to how GBCAs may be related to NSF is through the dissociation of the gadolinium ion from the underlying chelate. Therefore, based on this theory, the different complex stability of GBCAs may have an impact on the likelihood of triggering NSF [31–35]. Regulators have classified GBCAs (EMA; ACR) based mainly on the NSF reporting frequency. The European Medicines Agency – Committee for Medicinal Products for Human Use (EMACHMP) assessment, has grouped marketed GBCAs into three different NSF risk categories depending on their conclusions regarding the potential for different GBCAs to trigger NSF [103]. Applying this approach, macrocyclic compounds, including gadobutrol, have been assigned to the low risk group (EMA). Similarly, the ACR also assigned gadobutrol in the low risk class in their guidelines (ACR).
During the entire reporting period through July 2011. Bayer Healthcare has received ten reports suggesting the development of NSF after a reported administration of gadobutrol, including three already published reports [36,37]. An update on NSF can be found at [104]. Based on all available but sometimes incomplete data, an association of NSF-like symptoms to the reported administration of gadobutrol cannot be excluded in three of the overall ten cases. However, in no case was a definite causal role of gadobutrol determined. None of the patients from any clinical trial administering gadobutrol even at doses higher than 0.1 mmol/kg BW, including 194 patients with impaired renal function, have been reported to have developed NSF [38].
■ Safety & tolerability from clinical trials
After the completion of gadobutrol clinical trials, the drug was found to be well tolerated with a comparable safety profile to other extracellular MR contrast agents. Evaluation of special populations like pediatric patients, renal impairment, hepatic impairment and patients with cardiovascular disorders did not reveal any specific risk in these populations.
Overall, 4.0% of 4705 subjects reported one or more adverse drug reactions during a follow-up period from 24 h to 7 days after gadobutrol administration. The most frequent (≥0.5%) adverse drug reactions associated with the use of gadobutrol are headache, nausea, injection-site reaction, dysgeusia and feeling hot. Adverse reactions following gadobutrol administration are usually mild to moderate in severity and transient in nature. No obvious relationship was noted between the incidence of drug-related adverse events and the dose of gadobutrol (Table 4).
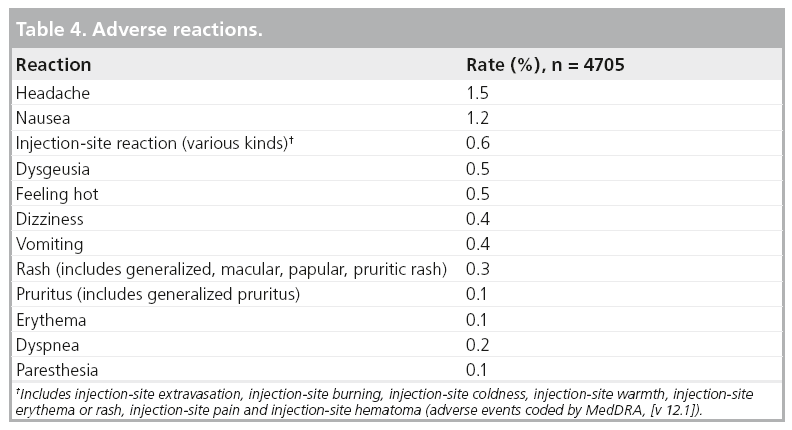
Adverse reactions that occurred with a frequency of less than 0.1% in subjects in clinical trials and treated with gadobutrol include: hypersensitivity/anaphylactoid reactions (hypotension, urticaria, flushing, pallor), loss of consciousness, convulsion, parosmia, tachycardia, palpitation, dry mouth, malaise and feeling cold.
From more than 6.8 million administrations after the first launch of gadobutrol in Switzerland in 1999, the following adverse drug reactions have been reported during postmarketing use. As these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
▪ Cardiac arrest
▪ NSF
Hypersensitivity/anaphylactoid reactions reported from postmarketing use include the following: anaphylactoid shock, circulatory collapse, blood pressure increase, chest pain, respiratory arrest, bronchospasm, cyanosis, oropharyngeal swelling, laryngeal edema, face edema, angioedema, conjunctivitis, eyelid edema, hyperhidrosis, cough, sneezing and burning sensation.
To minimize the risk of NSF, health authorities worldwide have implemented respective warnings for the use of GBCAs. This also applies to gadobutrol. The warning describes the risk for this disease in patients receiving these agents and who suffer from renal dysfunction meeting one of the following conditions:
▪ Chronic, severe kidney disease (GFR <30 ml/min/1.73 m2)
▪ Acute kidney injury
▪ Acute renal insufficiency of any severity due to hepatorenal syndrome or in the perioperative liver transplantation period.
Regulatory affairs
Gadobutrol is currently approved for CNS indication in the USA, and for multiple other indications in the rest of the world. So far it is the only macrocyclic, highly concentrated (1.0 M) form of gadolinium available in the market worldwide.
Outside of the USA, 0.5 M and 1.0 M gadobutrol was first approved for CNS indication in Switzerland in 1998. Whereas in the EU, Australia and Canada approval took place between 1999 and 2000.
Discontinuation of development and marketing for the 0.5 M formulation occurred in 1997.
Approval for MRA indication 1.0 M Gadobutrol occurred in the EU during 2003. Gadobutrol 1.0 M received EU approval for body indications during 2007.
Approval for pediatric use aged 2 years and above in Switzerland, Canada, China, Korea and aged 7 years and above in EU 2010.
In the USA regulatory overview includes:
▪ Original investigational new drug, July 1988, then inactivated in 1999
▪ Investigational new drug reactivated in 2004
▪ US FDA approval for CNS in adults and pediatric patients 2 years and above 2011
Conclusion
It is well known that combined MRI with and without GBCA is the one of the best imaging methods to define normal anatomy and characteristics of lesions (location, size, number, border delineation, degree of edema, contrast enhancement). Gadobutrol is a macrocyclic nonionic, extracellular gadoliniumbased contrast agent with high paramagnetic effect (relaxivity). Its concentration is higher compared with other similar approved products (1.0 vs 0.5 M). Studies have shown that gadobutrol provides higher image quality in body, CNS and MRA contrast imaging. Its high concentration may have potential advantages, especially for fast dynamic images, which may need to be elaborated in further clinical studies.
Gadobutrol should be administered for MR contrast enhancement at a dose of 0.1 mmol/kg (0.1 ml/kg). It is as safe and effective as other approved gadolinium-based contrast agents based on the results of the different clinical trials worldwide. Furthermore, with the known NSF risk related to GBCAs, it is important to know that macrocyclic molecules have a high kinetic and thermodynamic stability.
Future perspective
1.0 M gadobutrol, the first GBCA approved for use at a concentration of greater than 0.5 M, it has the potential to increase the robustness of CE‑MRI of all types by providing better visibility of tissues with its high relaxivity. Since it was first approved in 1998, gadobutrol has continually demonstrated its broad range of applications, which are continually expanding and advancing the diagnostic and clinical application of CE‑MRI and MRA.
Financial & competing interests disclosure
J Breuer is a Bayer HealthCare employee, JE Gutierrez is a research consultant at Bayer HealthCare Gadovist Speaker Bureau. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
Papers of special note have been highlighted as:
• of interest
- Runge VM, Muroff LR, Jinkins JR. Central nervous system: review of clinical use of contrast media. Top. Magn. Reson. Imaging 12, 231–263 (2001).
- Runge VM, Muroff LR, Wells JW. Principles of contrast enhancement in the evaluation of brain diseases: an overview. J. Magn. Reson. Imaging 7(1), 5–13 (1997).
- Giesel FL, Mehndiratta A, Essig M. High-relaxivity contrast-enhanced magnetic resonance neuroimaging: a review. Eur. Radiol. 20, 2461–2474 (2010).
- Rohrer M, Bauer H, Mintorovitch J et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest. Radiol. 40, 715–724 (2005).
- Laurent S, Elst LV, Muller RN. Comparative study of the physiochemical properties of six clinical low molecular weight gadolinium contrast agents. Constrast Media Mol. Imaging 1(3), 128–137 (2006).
- Semelka R, Helmberger KG. Contrast agents for MR imaging of the liver. Radiology 218(1), 27–38 (2001).
- Siegel MJ. Pediatric liver magnetic resonance imaging. Magn. Reson. Imaging Clin. N. Am. 10(2), 253–273 (2002).
- Chiribiri A, Morton G, Nagel E. Gadofosvset injection for magnetic resonance angiography. Imaging Med. 2(4), 383–393 (2010).
- Fidler J, Hough D. Heaptocyte-specific magnetic resonance imaging contrast agents. Hepatology 53(2), 678–682 (2011).
- Westphal M, Heese O, de Wit M. Intracranial metastases: therapeutic options. Ann. Oncol. 14(Suppl. 3), iii4–iii10 (2003).
- Forsting M, Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol – a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur. J. Radiol. 74(3), e186–e192 (2010).
- Staks T, Schuhmann-Giampieri G, Frenzel T, Weinmann HJ, Lange L, Platzek J. Pharmacokinetics, dose proportionality, and tolerability of gadobutrol after single i.v. injection in healthy volunteers. Invest. Radiol. 29(7), 709–715 (1994).
- Janus N, Launay-Vacher V, Karie S et al. Prevalence of nephrogenic systemic fibrosis in renal insufficiency patients: results of the FINEST study. Eur. J. Radiol. 73, 357–359 (2010).
- Gutierrez J, Koenig S, Brant-Zawadzki M et al. Multicenter, crossover, open label, Phase 3 study to determine the safety and efficacy of gadobutrol 1.0 molar in patients referred for contrast-enhanced MRI of the central nervous system. Presented at: 96th Radiological Society of North America Meeting. Chicago, IL, USA, 27 November–3 December 2010.
- Hentsch A, Aschauer M, Balzer J et al. Gadobutrol-enhanced moving-table magnetic resonance angiography in patients with peripheral vascular disease: a prospective, multi-centre blinded comparison with digital substraction angiography. Eur. Radiol. 13(9), 2103–2114 (2003).
- Huppertz A, Rohrer M. Gadobutrol, a highly concentrated MR-imaging contrast agent: its physicochemical characteristics and the basis for its use in contrast enhanced MR angiography and perfusion imaging. Eur. Radiol. 14(Suppl. 5), 12–18 (2004).
- Giesel FL, Mehndiratta A, Risse F et al. Intraindividual comparison between gadopentetate dimeglumine and gadobutrol for magnetic resonance perfusion in normal brain and intracranial tumors at 3 Tesla. Acta Radiol. 50(5), 521–530 (2009).
- Gutierrez J, Koenig S, Rossi S et al. Results from a multicenter, open-label, Phase 3 study to determine the safety and efficacy of gadobutrol a macrocylic 1.0 molar GBCA in patients referred for contrast-enhanced MRI of the central nervous system (CNS). Presented at: 48th American Society of Neuroradiology Meeting. Boston, MA, USA, 15–20 May 2010.
- Mohrs OK, Oberholzer K, Krummenauer F et al. Comparison of contrast-enhanced MR angiography of the aortoiliac vessels using a 1.0 molar contrast agent at 1.0 T with intra-arterial digital subtraction angiography. ROFO 176(7), 985–991 (2004).
- Achenbach M, Figiel JH, Burbelko M, Heverhagen JT. Prospective comparison of image quality and diagnostic accuracy of 0.5 molar gadobenate dimeglumine and 1.0 molar gadobutrol in contrast-enhanced run-off magnetic resonance angiography of the lower extremities. J. Magn. Reson. Imaging 32(5), 1166–1171 (2010).
- Schaefer FW, Schaefer PJ, Altjohann C, Bourne M, Decobelli F, Goyen M. A multicenter, site-independent, blinded study to compare the diagnostic accuracy of contrast-enhanced magnetic resonance angiography using 1.0M gadobutrol (Gadovist) to intraarterial digital subtraction angiography in body arteries. Eur. J. Radiol. 61(2), 315–323 (2007).
- Tombach B, Benner T, Reimer P et al. Do highly concentrated gadolinium chelates improve MR brain perfusion imaging? Radiology 226(3), 880–888 (2003).
- Nijenhuis R, Gerretsen S, Leiner T, Jacobs M, Engelshoven J, Backes W. Comparison of 0.5M Gd-DTPA with 1.0M gadobutrol for magnetic resonance angiography of the supplying arteries of the spinal cord in thoracoabdominal aortic aneurysm patients. J. Magn. Reson. Imaging 22, 136–144 (2005).
- Fink C, Boch M, Kiessling F et al. Time-resolved contrast-enhanced threedimensional pulmonary MR-angiography: 1.0 Gadobutrol vs. 0.5 M gadopentetate dimeglumine. J. Magn. Reson. Imaging 19(2), 202–208 (2004).
- Brown J, Kristy R, Stevens G et al. The Optimark clinical development program: summary of safety data. J. Magn. Reson. Imaging 15, 446–455 (2002).
- Hammerstingl R, Adam G, Ayuso JR et al. Comparison of 1.0M gadobutrol and 0.5M gadopentetate demeglumine-enhanced magnetic resonance imaging in five hunder seventy-two patients with known or suspected liver lesions: results of a multicenter, double-blind, interindividual, randomized clinical Phase-III trial. Invest. Radiol. 44, 168–176 (2009).
- Kuhn J, Hegenscheid K, Siegmund W, Foriehlich C, Hosten N, Puls R. Normal Dynamic MRI enhancement patterns of the upper abdominal organs: gadoxetic acid compared with gadobutrol. Am. J. Roentgenol. 193(5), 1318–1323 (2009).
- Hahn G, Sorge I, Gruhn B et al. Pharmacokinetics and safety of gadobutrolenahnced magnetic resonance imaging in pediatric patients. Invest. Radiol. 44(12), 776–783 (2009).
- Niendorf H, Alhassan A, Balzer T et al. Safety and risk of gadolinium-DTPA: extended clinical experience after more than 20 million applications. In: Magnevist Monograph (3rd Edition). Felix R, Hosten N, Hricak H (Eds). Blackwell Science (1998).
- Aslanian V, Lemaignen H, Bunouf P et al. Evaluation of the clinical safety of gadodiamide injection, a new nonionic MRI contrast medium for the central nervous system: a European perspective. Neuroradiology 38, 537–541 (1996).
- Marckmann P, Skov L, Dupont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J. Am. Soc. Nephrol. 17, 2359–2362 (2006).
- Becker S, Walter S, Witzke O et al. The German registry for nephrogenic systemic fibrosis: findings from 23 patients. Clin. Nephrol. 73(6) 426–430 (2010).
- Thomsen H. Nephrogenic systemic fibrosis: history and epidemiology. Radiol. Clin. North Am. 47, 827–831 (2009).
- Ledneva E, Karie S, Launay-Vacher V et al. Renal safety of gadolinium-based contrast media in patients with chronic renal insufficiency. Radiology 250, 618–628 (2009).
- Olukotun AY, Parker JR, Meeks MJ et al. Safety of gadoteridol injection: U.S. clinical trial experience. J. Magn. Reson. Imaging 5, 17–25 (1995).
- Wollanka H, Weidenmaier W, Giersig C. NSF after Gadovist exposure: a case report and hypothesis of NSF development. Nephrol. Dial. Transplant 24, 3882–3884 (2009).
- Elmholdt TR, Jorgensen B, Ramsing M et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gabobutrol. NDT Plus 3(3), 285–287 (2010).
- Voth M, Rosenberg M, Breuer J. Safety of gadobutrol, a new generation of contrast agents: experience from clinical trials and postmarketing surveillance. Invest. Radiol. 46(11), 663–671 (2011).
- US NIH clinical trials. www.clinicaltrials.gov
- ACR manual on contrast media. Version 7, 2010. www.acr.org/secondarymainmenucategories/ quality_safety/contrast_manual.aspx
- EMEA. Questions and answers on the review of gadolinium-containing contrast agents. www.ema.europa.eu/docs/en_gb/document_ library/referrals_document/gadolinium_31/ wc500015635.pdf (Accessed 14 October 2010)
- Bayer. www.diagnostic-imaging. bayerscheringpharma.de/scripts/pages/en/ auth/healthcare_professionals_area/services/ nsf_safety_update/index.php
• Reviews uses of contrast media in the clinical setting.
• Provides background information on gadolinium-based contrast agents.
• Provides an overview of relaxivity.
• Basic valuable pharmacokinetic information.
• Comapares gadobutrol to other gadolinium-containing contrast agents.
• Discusses the impact of contrast-enhanced imaging on therapeutic course in cancer.
• Valuable safety information.
• Development program in the USA.
• Clinical application.
• Development program in the USA.
• Clinical application.
• Clinical application.
• Clinical application.
• Clinical application.
• Background information on nephrogenic systemic fibrosis.
• Presents valuable safety information.
• Reviews important regulatory information.
■ Websites
• Safety guidelines.
• Safety guidelines.