Research Article - Interventional Cardiology (2023)
Partial anomalous pulmonary venous return, the neglected congenital malformation: Catheterization assisted echocardiography
- Corresponding Author:
- Sophie Pierard
Division of Cardiology, Saint-Luc University Clinics, Brussels, Belgium,
E-mail: sophie.f.pierard@saintluc.uclouvain.be
Received date: 02-Jun-2023, Manuscript No. FMIC-23-101068; Editor assigned: 05-Jun-2023, PreQC No. FMIC-23-101068 (PQ); Reviewed date: 19-Jun-2023, QC No. FMIC-23-101068; Revised date: 26-Jun-2023, Manuscript No. FMIC-23-101068 (R); Published date: 04-Jul-2023, DOI: 10.37532/1755- 5310.2023.15(S17).438
Abstract
Background: Partial Anomalous Pulmonary Venous Return (PAPVR) is a rare congenital heart disease that can lead to major complications in adulthood, such as Pulmonary Arterial Hypertension (PAH). Methods and Results: Fifty PAPVR patients aged ≥ 18 years were included. All underwent transthoracic echocardiography and multislice imaging. Of the 26 incidentally diagnosed patients, 14(54%) did not have a cardiac follow-up. Of the patients who had an echocardiography and were identified at high risk of PH (n=16), 5(31%) did not have Right Heart Catheterization (RHC) investigation. Moreover, the correlation between RHC-derived Qp/Qs and echocardiographic Qp/Qs was weak (r²=0.29, p=0.016), especially if there is an associated atrial septal defect (r=0.009, p=0.98). Conclusion: There is a lack of follow-up for patients newly diagnosed with PAPVR and the inadequate management of these patients in current clinical practice, both in terms of screening for PAH and identifying at-risk profiles, as well as the lack of recourse to Right Heart Catheterization (RHC) when this is recommended.
Keywords
Partial anomalous pulmonary venous return • Pulmonary hypertension • Congenital heart disease • Shunt lesion
Abbreviations
ASD: Atrial Septal Defect; CSA: Cross Sectional Area; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; PAH: Pulmonary Arterial Hypertension; PAPVR: Partial Anomalous Pulmonary Venous Return; PH: Pulmonary Hypertension; PVR: Pulmonary Vascular Resistance; Qp/Qs: Pulmonary- to-systemic flow ratio; RHC: Right Heart Catheterization; RVOT: Right Ventricular Outflow Tract; TRV: Tricuspid Regurgitation Velocity; WU: Wood UnitsUnits
Introduction
Partial Anomalous Pulmonary Venous Return (PAPVR) is a rare congenital heart disease with an estimated prevalence of approximately 0.1% to 0.2% in the recent literature [1-3]. This cardiac defect causes a left-to-right shunt at the pre-tricuspid level, resulting in volume overload in the right ventricle and pulmonary vasculature [4]. Over time, this can lead to endothelial remodeling of the pulmonary arteries and increased pulmonary vascular resistance, characteristic of Pulmonary Arterial Hypertension (PAH) [5]. If the defect is not corrected, the consequences can be dramatic, such as right heart failure, equalization of left and right pressures, or even reversal of the shunt characteristic of Eisenmenger syndrome. It is therefore essential, on the one hand, to screen patients diagnosed with PAPVR for PAH and, on the other hand, to identify patients who are at higher risk of developing PAH. However, despite the existing clear guidelines [6,7], both screening and identification of patients at risk of PAH is not as straightforward as recommended [3]. In this mini-review we will discuss these two points
Methods
Details of the methods used are given at length in our article entitled “Partial anomalous pulmonary venous return in adults: insight into pulmonary hypertension” [3]. Briefly, we enrolled 78 PAPVR patients in this clinical series. Of these 78 patients, patients aged ≥ 18 years who had not undergone operation or were operated on after the age of 18 years were retained. All the patients had at least one imaging test performed in our institution to confirm the PAPVR (Computed Tomography (CT), Magnetic Resonance Imaging (MRI), or cardiac catheterization). Exclusion criteria were among others presence of potential causes of PH other than PAPVR, including post-capillary PH, and associated congenital heart disease other than ASD.
Pulmonary pressures were estimated by cardiac catheterization, when available. According to the prevailing guidelines at the time of catheterization, PH was defined as a resting mPAP ≥ 25 mmHg and PAH as a mPAP ≥ 25 mmHg with a PAWP ≤ 15 mmHg and a PVR >3 Wood Units (WU) [8]. In the absence of invasive measurements, pulmonary pressures were estimated from echocardiographic data. PH was considered when systolic transtricuspid peak velocity was ≥ 3.4 m/s. This cut-off indicates a high probability of PH according to the European Society of Cardiology (ESC) guidelines [8,9].
Results
In total, 50 patients were included. Thirty five (70%) were non- operated. The mean age at inclusion was 50 ± 18 years and most of the patients were female (n=30) (60%).
Management of PAPVR
Twenty-six patients were incidentally diagnosed PAPVR (on the basis of chest CT-scan and cardiac MRI). Among them, more than half (54%) of the cases did not undergo at least echocardiographic medical follow-up after diagnosis. Furthermore, of the patients who had an echocardiography and were identified at high risk of PH based on echocardiography criteria (n=16), 5(31%) did not have RHC investigation, and another patient with an intermediate probability of PH and a Qp/Qs of 1.8 also did not undergo RHC. In addition, of the 14 patients with a surgical indication (12 patients with a class I indication and 2 patients with a class IIa indication), 4(28%) did not undergo surgery (including 1 patient with co-morbidities).
Evaluation of Qp/Qs ratio
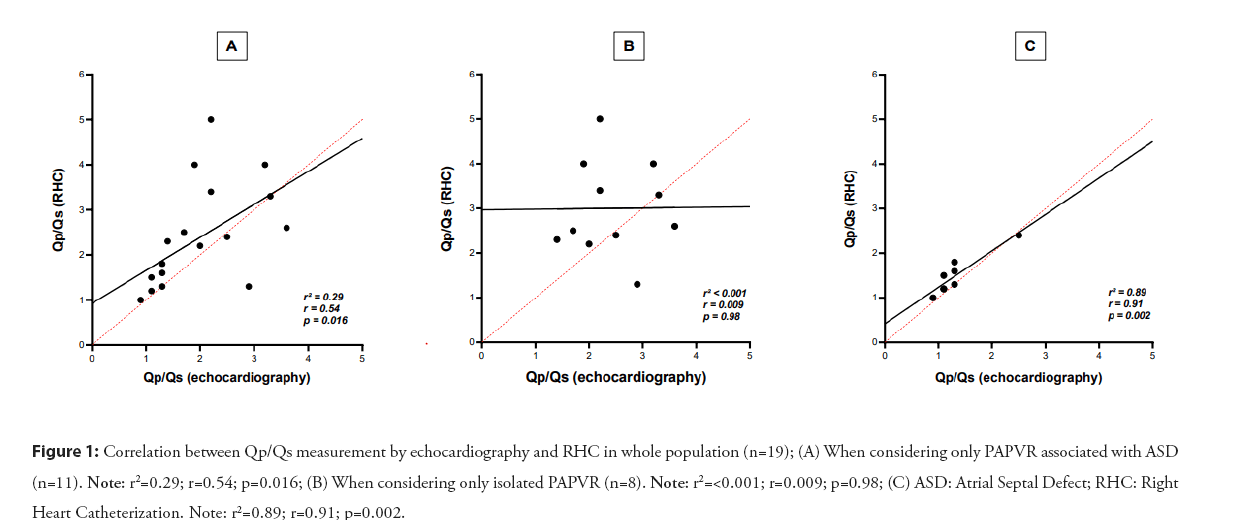
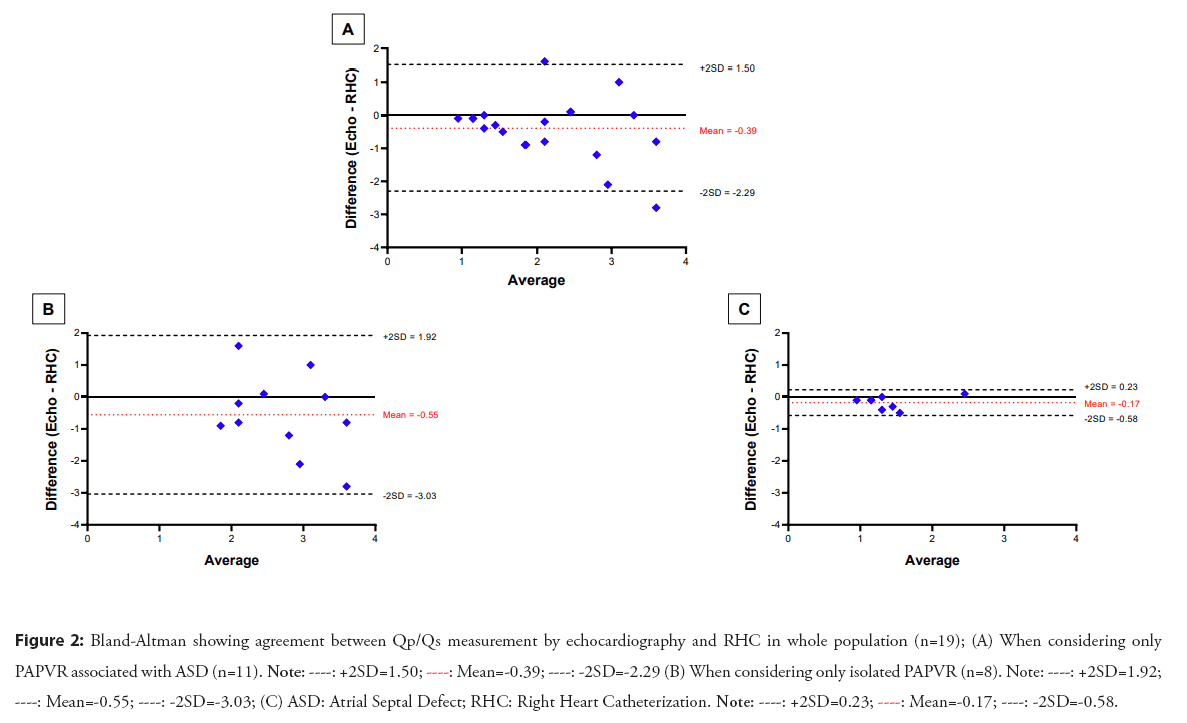
In our series, the mean Qp/Qs measured by RHC were 2.4 ± 1.1. When comparing patients with PAPVR associated with an ASD to patients with isolated PAPVR, their RHC-derived Qp/Qs measurement was significantly higher than patients with isolated PAPVR (2.56[2.34-3.70] vs 1.38 [1.19-1.61], p=0.002) as expected. However, in this group of patients, there was no correlation between the two methods of Qp/Qs measurement, echocardiography and catheterization (r=0.009, p=0.98- Figure 1), and the agreement of these methods according to the Bland-Altman plot was poor, whereas the correlation (r=0.91, p=0.002) and the agreement between the methods was excellent when only patients with isolated PAPVR were consider as shown in the Figure 2.
Figure 1: Correlation between Qp/Qs measurement by echocardiography and RHC in whole population (n=19); (A) When considering only PAPVR associated with ASD (n=11). Note: r2=0.29; r=0.54; p=0.016; (B) When considering only isolated PAPVR (n=8). Note: r2=<0.001; r=0.009; p=0.98; (C) ASD: Atrial Septal Defect; RHC: Right Heart Catheterization. Note: r2=0.89; r=0.91; p=0.002.
Figure 2: Bland-Altman showing agreement between Qp/Qs measurement by echocardiography and RHC in whole population (n=19); (A) When considering only PAPVR associated with ASD (n=11). 

Discussion
Screening for PAH, not so easy as it should be
Currently, European guidelines [6], recommend screening for Pulmonary Hypertension (PH) by echocardiography. Patients are classified into 3 categories according to their echocardiographic probability of PH: high probability (peak Tricuspid Regurgitation Velocity (TRV) >3.4 m/s or between 2.9 and 3.4 m/s with indirect signs of PH), intermediate probability (peak TRV between 2.9 and 3.4 m/s without any other echocardiographic signs of PH or ≤ 2.8 m/s with indirect signs), and low probability (peak TRV ≤ 2.8 m/s without any other signs of PH) [7]. These guidelines also recommend to confirm the existence of elevated pulmonary pressures by right heart catheterization (RHC) in patients with a high probability of PH or an intermediate probability with risk factors for PAH.
However, despite the potentially dramatic complications (cf. Eisenmenger) and the clear guidelines, our study has highlighted that PAPVR unfortunately remains a neglected and poorly managed condition. Indeed, more than half of the newly diagnosed cases did not undergo any follow-up after diagnosis, and in particular, were not screened for PAH.
PAPVR is therefore a disease that seems to be poorly known by the medical professionals even though its prevalence is similar to the atrial septal defects (0.2%) and the consequences can be dramatic, particularly if patients develop PAH, or even its extreme form: Eisemenmenger syndrome. Lewis et al. in fact showed that out of 90 PAPVR patients with PAH, the investigation of PAPVR had not been carried out in 71 of them even though it was the cause of their PAH and despite 69% of these having previously undergone CT scan [10]. There is therefore a real need to raise awareness of this congenital malformation and its consequences.
Predicting the risk of developing PAH: Right heart catheterization to help echocardiography
In our recent study [3], we showed that the Qp/Qs ratio was the only predictor of PAH in PAPVR population. On the other hand, Qp/Qs is also relevant for surgical decision making in patients with pre-tricuspid shunts according to the ESC 2020 guidelines, with a Qp/Qs>1.5 being a surgical indication in patients with PVR between 3-5 WU and with right ventricular dilatation (class IIa) [6]. Therefore, an assessment of the shunt by an accurate measurement of the Qp/Qs ratio is essential.
Currently, the gold standard method for PAH diagnosis and Qp/Qs measurement is RHC [7]. However, in clinical practice, echocardiography is used as the first-line of screening for PH but also for the assessment of the Qp/Qs ratio. However, the measurement of the shunt ratio by echocardiography is highly inaccurate [11]. Indeed, Faherty et al. recently showed that in patients with ASD, the correlation between echocardiographic Qp/Qs and RHC-derived Qp/Qs was very weak (r²=0.32, P<0.0001), whatever the age of the patients. The reasons for these errors are essentially anatomical. The measurement of Qp/Qs on echocardiography uses the following formula: SV=CSA × VTI, where SV is the stroke volume, CSA is the cross-sectional area of the valve which depends on the square of the diameter (R²), and VTI is the velocity-time integral. In practice, the measurement of pulmonary CSA is often more subject to error than aortic CSA due to [1], poor visualisation of the Right Ventricular Outflow Tract (RVOT) and pulmonary valve [12,13], but also [2], the variation in the diameter of the RVOT during the cardiac cycle, as the aortic CSA increases by 5-10% during systole compared to 18% for the pulmonary valve [14]. Therefore, even the smallest error in the measurement of the valve diameter will have a large impact on the final Qp/Qs calculation and, in the best of hands; these calculations may have up to a 20% error [15]. This was also observed in our cohort where almost half of the patients (44%) underwent RHC: The correlation between RHC-derived Qp/Qs and echocardiographic Qp/Qs was weak (r²=0.29, p=0.016) (Figure 1). Agreement between methods was particularly poor when the shunt was larger (Figure 2-Bland-Altman plot), as previously shown in the work of Dittman et al. and Faherty et al. where agreement between the two methods becomes particularly poor when the Qp/Qs exceeded 1.5 [11,16].
Moreover, in contrast to the study by Faherty, et al. [11], echocardiography seemed to rather underestimate the shunt ratio in our series. The underestimation of the Qp/Qs ratio by echocardiography and the lack of correlation and concordance with the reference method, especially for the largest shunts, limit the clinician in his therapeutic decision making, i.e. the surgical correction of the patient. It is therefore essential to complete the work-up of a PAPVR with a RHC, in particular if it is associated with an ASD, in order to get an accurate measurement of pulmonary pressures and resistances but also of the shunt ratio, and therefore to better classify patients at risk of developing elevated pulmonary pressures and to manage them in accordance with the current guidelines.
Conclusion
PAPVR is a rare congenital heart disease that unfortunately remains neglected and poorly managed to date, both in terms of medical follow-up after diagnosis and screening for complications Pulmonary Arterial Hypertension (PAH) and risk factors for developing PAH. More extensive use of current guidelines is essential in the assessment of these patients and referral to RHC should be systematic.
References
- Haramati LB, Moche IE, Rivera VT, et al. Computed tomography of partial anomalous pulmonary venous connection in adults. J Comput Assist Tomogr. 27(5):743-749 (2003).
- Ho ML, Bhalla S, Bierhals A, et al. MDCT of Partial Anomalous Pulmonary Venous Return (PAPVR) in adults. J Thorac Imaging. 24(2):89-95 (2009).
- Rahnama N, Kubangumusu L, Pasquet A, et al. Partial anomalous pulmonary venous return in adults: Insight into pulmonary hypertension. Int J Car Congenit Heart Dis. 11:100426 (2023).
- Sears EH, Aliotta JM, Klinger JR, et al. Partial anomalous pulmonary venous return presenting with adult‐onset pulmonary hypertension. Pulm Circ. 2(2):250-255 (2012).
- Babb JD, McGlynn TJ, Pierce WS, et al. Isolated partial anomalous venous connection: A congenital defect with late and serious complications. Ann Thorac Surg. 31(6):540-543 (1981).
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease: The task force for the management of adult congenital heart disease of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Adult Congenital Heart Disease (ISACHD). Eur Heart J. 42(6):563-645 (2021).
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur Heart J. 43(38):3618-3731 (2022).
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37:67-119 (2016).
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur Heart J. 2022.
- Lewis RA, Billings CG, Bolger A, et al. Partial anomalous pulmonary venous drainage in patients presenting with suspected pulmonary hypertension: A series of 90 patients from the ASPIRE registry. Respirology. (10):1066-1072 (2020).
- Faherty E, Rajagopal H, Lee S, et al. Correlation of transthoracic echocardiography-derived pulmonary to systemic flow ratio with hemodynamically estimated left to right shunt in atrial septal defects. Ann Pediatr Cardiol. 15(1):20 (2022).
- Colan SD. Quantitative applications of Doppler cardiography in congenital heart disease. Cardiovasc Intervent Radiol. 10:332-347 (1987).
- Lai WW, Mertens LL, Cohen MS, et al. Echocardiography in pediatric and congenital heart disease: From fetus to adult. (2015).
- Sanders SP, Yeager S, Williams RG, et al. Measurement of systemic and pulmonary blood flow and QP/QS ratio using Doppler and two-dimensional echocardiography. Am J Cardiol. 51(6):952-956 (1983).
- Quiñones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of doppler echocardiography: A report from the Doppler quantification task force of the nomenclature and standards committee of the American society of echocardiography. J Am Soc Echocardiogr. 15(2):167-184 (2002).
- Dittmann H, Jacksch R, Voelker W, et al. Accuracy of Doppler echocardiography in quantification of left to right shunts in adult patients with atrial septal defect. J Am Coll Cardiol. 11(2):338-342 (1988).