Research Article - Clinical Practice (2018) Volume 15, Issue 1
Parvovirus B19 changes iron homeostasis during pathological pregnancy
- Corresponding Author:
- Manolov V
Department of Clinical laboratory and clinical immunology
Medical University, Bulgaria
E-mail: victhedoc4@abv.bg
Abstract
Objective: Viral infections during pregnancies are one of the main reasons for mother and fetus severe complications and mortality. Infections are associated with significant changes in iron homeostasis. The post-infectious anemic syndrome is characterized by low serum iron and increased hepcidin, which accumulates iron in endothelial macrophage system. The study aims to find the involvement of parvovirus B19 in the anemic syndrome development during pathological pregnancy. Methods: 52 pregnant women with anemia, diagnosed and hospitalized in University Obstetrics and Gynaecology “Maichin Dom” hospital were enrolled. The serological (indirect Enzyme-Linked Immunosorbent Assay (ELISA) tests) and molecular (B19V- Polymerase Chain Reaction (PCR) test) methods were used. In anemic pregnant women were evaluated iron homeostasis parameters with CLIA, ELISA and Ferrozine methods. For statistical evaluation of results the researchers used SPSS 13.0 (IBM). Correlations and significance were rated by Student’s paired t-test and Pearson’s correlation. Results: 8/52 (15.4%) of patients showed presence of B19V-IgM antibodies. B19-IgG antibodies were detected in 21/52 (40.4%) women. A positive PCR signal was determined in all patients with positive B19V-IgM, and 1 patient with anemia and positive B19V-IgG result. In two pregnant and positive women for acute viral B19V infection, anemia was classified as iron-deficiency, based on low serum hepcidin concentration 2.19 ± 0.5 µg/l, compared to 20.4 ± 2.9 µg/l pregnant women without B19V infection (ÃÂ <0.001). In other acute B19V infection women we found statistically increased serum hepcidin concentrations (61.9 ± 6.2 µg/l), ÃÂ <0.001. Conclusion: Because of transplanted pathway of parvovirus B19 infection transmission, in combination to B19V affinity for hematopoietic cell systems quantification of serum hepcidin levels would contribute for etiological clarification of anemia and prevention from inadequate iron supplementation in pregnant women.
Keywords
parvovirus B19, pregnancy, anemia, hepcidin, iron, inflammation
Review
A pregnancy is pathological when mother, fetus or appendages (placenta and amniotic fluid) diseases occur. Viral infections during pregnancy, along with some forms of pregnancyrelated illnesses such as diabetes, anemia, cardiovascular, gastrointestinal, kidney disease, etc., are one of the main reasons for mother and fetus severe complications and mortality [1]. Pregnancy cannot affect the course of the infection, but the infection can affect the fetus [2]. The clinical manifestations of neonatal infections vary depending on the viral agent and gestational age. The infections reach the fetus perinatally (from vaginal secret or blood) or after birth (with mother’s milk).
Parvovirus B19 is a single-chained DNA virus and is responsible for the development of erythema infectiosum or “fifth childhood disease”. Infection with this pathogen in the past has often been diagnosed as “rubella”. Although in most cases by adults, the infection passes asymptomatically or with an atypical clinical picture, including influenza-similar illnesses, arthralgia and arthropathy, toxoinfectious syndrome, etc., the effects on the fetus are serious and include miscarriage, fetal anemia, myocarditis and/or intrauterine fetal death. Starting from the B19V tropism, the fetus and the placenta are its target due to the presence of specific P antigen, situated on their surface. The frequency of vertical transmission by maternal infection is estimated by different authors between 17 and 33% [3]. 30 to 40% of pregnant women are seronegative for B19V and thus are sensible to infection, with the expected incidence of primary B19V infection during pregnancy varies from 1 to 5%. Several studies showed long-term effects on the fetuses of seronegative mothers infected with B19V [4-6]. A number of authors report neonatal complications after maternal B19V infection, including transfusion-dependent anemia [7,8], liver failure, myocarditis [9,10]. According to different information, from 3 to 14.8% of B19V seronegative pregnant women caused by viral infection, intrauterine fetal death will happen before 19 to 20 gestational weeks [11]. However, intrauterine fetal death as a result of B19V infection may occur in any pregnancy trimester. The reason is still unclear, but it is probably caused by multi-organ damage.
Some researchers indicate a favorable outcome for the fetus at infecting a seronegative mother in 85% of cases [11]. Parvovirus B19 is not considered as teratogenic agent, affecting embryogenesis (8-10 weeks), therefore there is no indication for the interruption of pregnancy [2].
Parvovirus B19 has affinity to the hematopoietic system, including erythroid progenitor cells and, to a lesser extent, leukocytes and megakaryocytic cell lines [12]. The virus attacks the cell of the red blood cell line in the bone marrow, which results into intravascular hemolysis and erythrocyte aplasia [12].
Viral infections are connected to changed iron homeostasis. Postifectious anemic syndrome is characterized with low serum iron and increased hepcidin, which causes iron accumulation in endothelial macrophageal system and lack of iron for erythropoiesis, which is very important for pathology pregnancy monitoring.
Systematic iron homeostasis includes control of absorption in duodenal enterocytes, use for erythropoiesis, effective re-use from sequestrated erythrocytes, deposition in hepatocytes and macrophages. The use of iron from erythrone depends primarily on the efficiency of element absorption through transferring cycle in erythroid precursors. The other three important aspects of homeostasis (absorption, recycling, and storage) are controlled systemically and coordinated by hepcidin, a peptide synthesized in the liver, which has the main responsibility to modulate the availability of iron according to the needs of the body.
Hepcidin is synthesized from hepatocytes as 25-aminoacid peptide with molecular weight from 2789.4 Da [13]. Following transformations form two smaller isoforms with 22- and 20 amino acid residues [14] with four intermolecular disulfide bridges.
Hepcidin-25 is a key regulator of iron absorption and its release from cells. Hepcidin blocks ferroportin, the only known iron intracellular exporter [13], thus leading to iron deposition in cells, decreased element absorption and low blood stream concentrations.
Increased hepcidin expression during inflammation is mainly mediated by interleukin-6 (IL-6). Liver interaction between IL-6 and its receptors activates Janus Kinase (JAK) and signal transducer and activator of transcription (STAT-3) [15-17]. Hepcidin expression increases due to oxidative stress and stress of endoplasmic reticulum [18,19].
Infections are connected to changed iron homeostasis. Anemia during inflammation is characterized with low serum iron and increased hepcidin [20-23]. Probably increased hepcidin plays protective role against microorganism growth, decreasing extracellular iron. On the other hand increased hepcidin leads to iron deficiency and incomplete oral supplementation, caused by suppressed intestinal absorption. In cases of iron supplementation without iron deficiency may occur side effects due to stimulated proliferation of latent pathogens [24,25].
The study aims to determine the involvement of parvovirus B19 in the anemic syndrome development in the course of / during pathological pregnancy. The researchers aimed to enrich the laboratory diagnostic approach in anemia during pathological pregnancy, and improvement of treatment and prognostic nature of pregnancy outcome.
Methodology applied
Clinical samples
52 pregnant women with anemia, diagnosed and hospitalized in University Obstetrics and Gynaecology “Maichin Dom” hospital were enrolled.
Signed informed consent was obtained from all included patients and controls according to the Declaration of Helsinki (Directive 2001/20/ EO). This study is part of Grant 2015, sponsored by Medical University, Sofia, Bulgaria and was approved by its Ethics Committee.
Serological analysis
An ELISA is used to demonstrate the presence of specific Parvovirus B19 IgM/ IgG antibodies (commercial test for indirect immunoassay analysis Euroimmun Parvovirus B19 IgM/IgG) The assays were performed as recommended by the manufacturer and the results were interpreted qualitatively as positive (Ratio R) ≥ 1,1), negative (Ratio<0.8) or equivocal (Ratio R ≥ 0.8 - <1). ELISA method was used for serum hepcidin quantification. Established results were compared to age matched healthy controls – females with normal pregnancy, with no Parvovirus B19 infection and with no anemia.
Molecular biological analysis
From source material (serum samples) using a commercial test (Invitrogen). Viral DNA extraction was attempted from all serum samples using the PureLink®Viral RNA/DNA test kits. To demonstrate the presence of parvovirus B19 DNA, PCR analysis was performed using a commercial kit (KAPA Taq PCR Kits) and the following consensus primers e1905f e1987r (20 p/mol) restrict the conserved NS1 region (NS1- PCR) [26] from the parvovirus genome and the KAPA Taq PCR kit.
Forward Primer (e1905f): 5’
TGCAGATGCCCTCCACCCA 3’
Reverse Primer (e1987r): 5’
GCTGCTTTCACTGAGTTCTTC 3’
Cycling parameters of NS1-ℜ19 PCR were according to Servant, A., et al. [26]
1 cycle 94°C for 6 min; 5 cycles 94°C for 30 sec, 55°C for 1 min, and 72°C for 1 min; 45 cycles 94°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec; final elongation 72°C for 7 min. Final: hold ∫ 4°C
Agarose gel electrophoresis
To visualize the obtained PCR products, electrophoresis is performed on a 2% agarose gel. Positive controls and a molecular weight marker are used thought 100 base pairs.
Evaluated iron homeostasis parameters. In anaemic pregnant women were evaluated iron homeostasis parameters with CLIA, ELISA and Ferrozine methods.
Statistical processing of the received results
For statistical evaluation of results the researchers used SPSS 13.0 (IBM). Correlations and significance were rated by Student’s paired t-test and Pearson’s correlation.
Results and Discussion
The average age of included 52 pregnant women was 20 - 36 years. Specific primaryreactive B19-IgM were proved in 8/52 (15.4%) of all analyzed samples (TABLE 1). Protective B19-IgG antibodies, evidences of past infection, are found in 21/52 (40.4%) of women (TABLE 1).
| Patients | B19V ELISA IgM n (%) |
B19V ELISA IgG n (%) |
B19V DNA n (%) |
|---|---|---|---|
| Pregnant women with anemia (n=52) | 8/52 (15.4 %) | 21/52 (40.4 %) | 9/52 (17.3 %) |
*B19V ELISA IgM – parvovirus B19 enzyme linked immunoassay immunoglobulin M antibodies; B19V ELISA IgG – parvovirus B19 enzyme linked immunoassay immunoglobulin G antibodies; B19V DNA – parvovirus B19 deoxyribonucleic acid
Table 1: Included pregnant women in this study.
All serum samples were molecularly tested for the detection of B19-viral DNA. A positive PCR signal was showed in all patients with positive B19V-IgM, and one patient with anemia and positive B19V-IgG result.
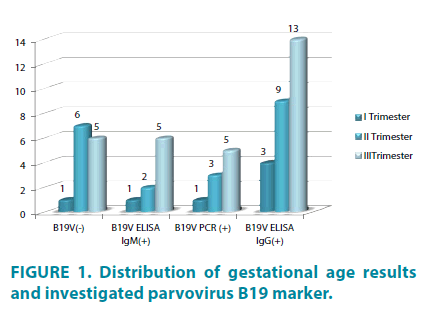
The study included 7 pregnant women in first, 15 in second and 30 in third trimester (FIGURE 1). The higher percent of included patients was third 30/52 (57.7%), as a result from intensive medical care during this period, although during first and second trimesters occur specific fetus damages, like fetal loss (miscarriage up to 30 weeks), congestive anemia, hydrops fetalis and dead fetus, caused by pathogenetic mechanisms of B19 V. B19V ELISA IgM was negative in one of the patients in second trimester, although viral DNA was isolated. Five of women in third trimester were positive for tested markers (B19V IgM and B19V DNA) (FIGURE 1).
Patients with presence of a marker for acute viral infection (IgM and / or pathogenic DNA) are recommended monitoring the pregnancy (FV monitoring of the fetal and Doppler screening) and laboratory testing with traceable serum samples (1-3-6 months).
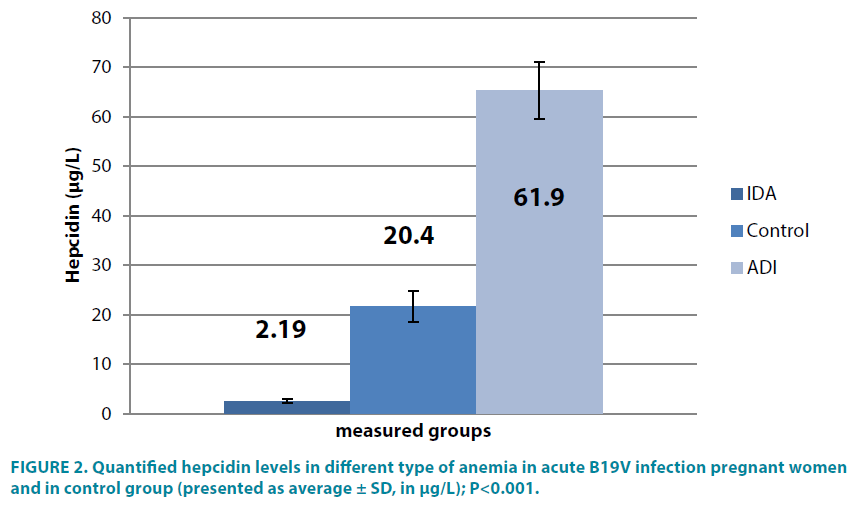
In two pregnant and positive women for acute viral B19V infection, anemia was classified as iron-deficiency, because of low serum hepcidin levels 2.19 ± 0.5 μg/l, compared to 20.4 ± 2.9 μg/l pregnant females without B19V infection (∠<0.001). In other acute B19V infection women the study found statistically increased serum hepcidin concentrations (61.9 ± 6.2 μg/l), ∠<0.001 (TABLE 2 and FIGURE 2).
| Group/Result | IDA | Controls | ADI |
| 2.54 ± 0.4 | 21.7 ± 3.1 | 65.3 ± 5.7 |
*IDA – iron-defficiency anemia; ADI – anemia during inflammation (infection)
Table 2: Measured hepcidin concentrations in acute B19V infection pregnant women (presented as average ± SD, in μg/L).
Serum hepcidin quantification in Bulgarian population, especially during pregnancy is still novelty [25].
Among the examined patients with anemia, although there was no clear clinical picture for this virus, including fever-rashes syndrome, the affinity of B19V appears to erythroid progenitor cells, leading to anemia. This might presents another marker in differential diagnosis in pregnant women with severe, undiagnosed type of anemia and a possible parvovirus B19 involved in the development of pathological pregnancy. The small number of included pregnant women is a limitation of our study. More cases need to be evaluated for more precise conclusion about Parvovirus B19 role in anemic syndrome.
Testing for the specific IgM antibodies in combination with presence of viral DNA is evidence of fresh infection, which is of particular importance in the monitoring of cases of pathological pregnancy. To this criterion in this study reply 15.5% of the patients surveyed. A positive PCR signal was showed in one patient with anemia and positive B19V-IgG result. This result demonstrates possible virus carrying (reactivation of latent infection or persistent one) [12].
In relation to clinical implications seven of included pregnant women gave birth to healthy newborns, one developed complication (hydrops fetalis) and the fetus was born premature.
Regarding the protection of the examined group of pregnant women and newborns towards B19V (presence of specific B19VIgG antibodies), a percentage ratio of 40.4% is calculated. This indicates that despite the widespread diffusion of the antibody in the population, viremia and the detection of viral DNA is rare. These data correspond to a small number of studies in other countries [5,27,28] and Bulgaria [29,30]. High attention should be exercised in seronegative pregnant women, which were 40% of the examined group of patients. Among the investigated clinical cases of women with anemia, the presence of acute B19 infection does not dominate, provided that the number of tested patients was small (n=52) and low number of pregnant women in first and second trimester.
In view of the various transmission of parvovirus B19, the transplanted pathway of the infection’s transmission as well as affinity for hematopoietic cell systems, including elytroid progenitor cells for presence of this viral agent and determining the frequency and extent of its involvement in the development of anemic syndrome during pregnancy and determining the serum level of hepcidin would contribute to the etiological clarification of the anemia and prevent the inadequate iron supplementation in pregnant women.
Acknowledgement
This project is implemented with the financial support of Medical University – Sofia, “Grant 2017”, Contract Д-124/2017.
Funding statement
This project is implemented with the financial support of the Medical University - Sofia, as a part of GRANT programs - “Grant 2017”, Contract Д-124/2017.
References
- Marinov B, Pramatarova T, Andreeva A, et al. Congenital thrombophilias and complications in pregnant women and newborns (pre-release). Obstet. Gynecol. XLII (2), 3-7 (2011).
- Crane J. Parvovirus B19 infection in pregnancy. J. Obstet. Gynaecol. Can. 24(9), 727-734 (2002).
- Lemont R, Sobel J, Vaisbuch E, et al. Parvovirus B19 infection in human pregnancy. BJOG. 118(2), 175-185 (2011).
- Haan TR, Jong EP, Oepkes D, et al. Infection with human parvovirus B19 (ʻfifth diseaseʼ) during pregnancy: potential life-threatening implications for the foetus. Ned. Tijdschr. Geneeskd. 152(21), 1185-1190 (2008).
- Zajkowska A, Garkowski A, Czupryna P, et al. Seroprevalence of parvovirus B19 antibodies among young pregnant women or planning pregnancy, tested for toxoplasmosis. Przegl. Epidemiol. 69(3), 479-482, 597-600 (2015).
- Sarfraz A, Samuelsen SO, Bruu AL, Jenum PA, Eskild A. Maternal human parvovirus B19 infection and the risk of fetal death and low birthweight: a case–control study within 35 940 pregnant women. BJOG. 116(11), 1492-1498 (2009).
- Jong EP, Haan TR, Kroes AC, et al. Parvovirus B19 infection in pregnancy. J. Clin. Virol. 36(1), 1-7 (2006).
- Tolfvenstam T, Broliden K. Parvovirus B19 infection. Semin. Fetal Neonatal Med. 14(4), 218-221 (2009).
- Bock CT, Klingel K, Kandolf R. Human parvovirus B19-associated myocarditis. N. Engl. J. Med. 1248-1249 (2010).
- Bock CT, Klingel K, Kandolf R. Human parvovirus B19-associated myocarditis. N. Engl. J. Med. 1248-1249 (2010).
- Crane J, Mundle W, Boucoiran I, et al. Parvovirus B19 infection in pregnancy. J. Obstet. Gynaecol. Can. 36(12), 1107-1116 (2014).
- Servant-Delmas A, Lefrère JJ, Morinet F, Pillet S. Advances in human B19 erythrovirus biology. J. Virol. 84(19), 9658-9665 (2010).
- Andrews NC. Forging a field: the golden age off iron biology. Blood 112(2), 219-230 (2008).
- Jordan JB, Poppe L, Haniu M, et al. Hepcidin revisited, disulfide connectivity, dynamics, and structure. J. Biol. Chem. 284(36), 24155-24167. (2009).
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 306(5704), 2090-2093 (2004).
- Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 108(9), 3204-329 (2006).
- Verga MV, Vujic SM, Kessler R, et al. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 109(1), 353-358 (2007).
- Vecchi C, Montosi G, Zhang K, et al. ER stress controls iron metabolism through induction of hepcidin. Science. 325(5942), 877-880 (2009).
- Oliveira SJ, Pinto JP, Picarote G, et al. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPalpha activity. PLoS One. 4(8), e6618 (2009).
- Cherian S, Forbes DA, Cook AG, et al. An insight into the relationships between hepcidin, anemia, infections and inflammatory cytokines in pediatric refugees: a cross-sectional study. Plos One. 3(12), e4030 (2008).
- Mast Q, Nadjm B, Reyburn H, et al. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J. Infect. Dis. 199(2), 253-262 (2009).
- Mast Q, Syafruddin D, Keijmel S, et al. Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica 95(7), 1068-1074 (2010).
- Nweneka CV, Doherty CP, Cox S, Prentice A. Iron delocalisation in the pathogenesis of malarial anaemia. Trans. R. Soc. Trop. Med. Hyg. 104(3), 175-184 (2010).
- Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectro-metry-based hepcidin measurements in serum and urine: analytical aspects and clinical implications. Clin. Chem. 53(4), 620-628 (2007).
- Manolov V, Marinov B, Velizarova M, et al. Anemia in pregnancy and serum hepcidin levels. Int. J. Adv. Res. 3(1) 758-761 (2015).
- Servant A, Laperche S, Lallemand F, et al. Genetic diversity within human erythroviruses: identification of three genotypes. J. Virol. 76(18), 9124-9134 (2002).
- Kelly HA, Siebert D, Hammond R, et al. The age-specific prevalence of human parvovirus immunity in Victoria, Australia compared with other parts of the world. Epidemiol. Infect. 124(3), 449-457 (2000).
- Mossong J, Hens N, Friederi V, et al. Parvovirus B19 infection in five European countries: seroepidemiology, force of infection and maternal risk of infection. Epidemiol. Infect. 136(8), 1059-1068 (2008).
- Ivanova SK, Mihneva ZG, Toshev AK. Insights into epidemiology of human parvovirus B19 and detection of an unusual genotype 2 variant, Bulgaria, 2004 to 2013. Euro. Surveill. 21(4), doi: 10.2807/1560-7917.ES.2016.21.4.30116. (2016).
- Ivanova St, Toshev A, Mihneva M. Seroprevalence of parvovirus B19 infection among women of childbearing age and pregnancy. Sci. Infectol. Parasitol. 2, 37-40 (2014).