Review Article - Interventional Cardiology (2024)
Patent foramen ovale and atrial septal defects: A focused overview
- Corresponding Author:
- Joe Aoun
Department of Cardiology, Houston Methodist DeBakey Heart & Vascular Centre, Houston, United States of America,
E-mail: jaoun@houstonmethodist.org
Received date: 08-Nov-2024, Manuscript No. FMIC-24-152046; Editor assigned: 11-Nov-2024, PreQC No. FMIC-24-152046 (PQ); Reviewed date: 26-Nov-2024, QC No. FMIC-24-152046; Revised date: 03-Dec-2024, Manuscript No. FMIC-24-152046 (R); Published date: 10-Dec-2024, DOI: 10.37532/1755- 5310.2024.16(S25).636
Abstract
The foramen ovale is a key fetal cardiac structure enabling oxygenated blood flow from the right to the left atrium, bypassing the non-functional fetal lungs. Postnatally, this opening typically closes due to increased left-sided atrial pressure. However, the closure remains incomplete in approximately 25%-30% of adults, resulting in a Patent Foramen Ovale (PFO), which can have significant clinical implications. PFO has been associated with cryptogenic strokes, migraines, Platypnea-Orthodeoxia Syndrome (POS) and decompression sickness. In cryptogenic strokes, PFO serves as a potential pathway for paradoxical embolism. Migraine, particularly with aura, has shown a correlation with PFO, although trials like MIST and PREMIUM did not support routine PFO closure for migraines.
Diagnosis of PFO includes Transthoracic (TTE) and Transesophageal Echocardiography (TEE), with TEE being the gold standard due to its high sensitivity and specificity. Management strategies for PFO-associated strokes involve medical therapy (antiplatelets or anticoagulants) and transcatheter closure. Trials such as CLOSURE, RESPECT and DEFENSE-PFO demonstrated variable outcomes, with newer guidelines advocating closure in high-risk cases.
Atrial Septal Defects (ASDs) manifest later in life with potential complications like atrial arrhythmias and Eisenmenger syndrome. Diagnosis primarily relies on echocardiography, with cardiac Magnetic Resonance Imaging (MRI) and Computed Tomography (CT) providing further assessment. Management depends on defect type and size, with percutaneous closure preferred for ostium secundum defects. Surgical repair is reserved for larger defects or associated structural abnormalities. Complications of percutaneous procedures include arrhythmias and device embolization. Overall, optimal patient selection and multidisciplinary collaboration are essential to achieving positive outcomes in managing PFO and ASD.
Keywords
Left Atrium • Antiplatelets • Transcatheter closure • Echocardiography • Atrial arrhythmias
Introduction
The foramen ovale is a normal interatrial prenatal communication that plays a key role in fetal blood circulation, allowing oxygenated blood to pass from the right atrium to the left atrium, avoiding the not-yet-functioning fetal lungs [1]. Normally, this opening closes shortly after birth due to changes in pressure that occur with breathing, driving the pulmonary resistance down, leading to an increase in pulmonary circulation blood flow and increased left-sided resistance, ultimately causing the septum primum to seal against the septum secundum, effectively closing the foramen ovale [2]. However, in about a third of adults, this process is incomplete, leading to a PFO [3]. The medical implications of PFO have been considered since the late 19th century, linking it to diverse conditions such as unexplained embolic strokes, migraines, platypnea-orthodeoxia syndrome and decompression sickness [4-6].
Literature Review
Anatomy and pathophysiology
During gestation, the human fetus grows in a relatively low-oxygen environment, necessitating a unique circulatory path. Blood rich in nutrients and oxygen from the placenta reaches the fetus via the umbilical vein, which is partly directed into the liver circulation as well as into the ductus venosus. This vessel transports the blood to the right atrium, from where it preferentially moves to the left atrium through the foramen ovale, ensuring essential organs like the brain and heart receive oxygenated blood necessary for their development [7]. The heart’s septal structures, particularly the interatrial septum, form in complex stages; the septum primum grows toward the endocardial cushions, resulting in an opening at its distal edge known as the ostium primum. As the muscular ostium primum grows downwards, its superior portion becomes fenestrated and forms the foramen secundum, which plays a crucial role in maintaining blood flow from the right to the left system during the rest of gestation. Once the foramen secundum is fully developed, the ostium primum closes and forms the septum primum, later becoming the valve structure of the foramen ovale [8]. Postnatally, decreased pulmonary vascular resistance and increased left atrial pressure result in the fusion of the septum primum to the septum secundum, closing the passage between the atria. A failure in this fusion process occurs in around 25% of the population, leaving a PFO, which in turn provides a potential route for emboli to cross between the right and left circulation [9].
Symptomatology and clinical manifestations
Stroke: PFOs are typically clinically silent, but they can exhibit certain clinical manifestations concerning. Paradoxical embolism is of primary concern, where emboli from the venous system bypass the pulmonary filtration and enter the systemic arterial circulation, potentially causing an embolic ischemic stroke. In fact, almost one-third of ischemic strokes have unknown causes and are hence termed cryptogenic strokes and patients who experienced a cryptogenic stroke have a higher prevalence of PFOs compared to the rest of the population [10]. Moreover, up to 50% of young individuals who experience a cryptogenic embolic stroke will be diagnosed with a PFO [11].
Interestingly, a patient-level meta-analysis of 12 cryptogenic stroke cohorts conducted by Kent et al., known as the Risk of Paradoxical Embolism (RoPE) study, showed a correlation between the prevalence of PFO and the likelihood of it being the cause of cryptogenic stroke in individuals who lacked classical cerebrovascular risk factors, were under the age of 65 and had evidence of a cortical brain infarct on presentation [12]. Furthermore, the presence of an Atrial Septal Aneurysm (ASA) along with a PFO was associated with higher rates of recurrent embolic strokes in these groups [13].
Migraine
Migraine, especially those presenting with aura, have been correlated with PFO. While the underlying pathophysiological explanation remains ambiguous, the hypothesis suggests that microembolic phenomena or unfiltered biologically active compounds might trigger migraines. Despite the association, current clinical guidelines do not support routine PFO screening in migraine patients and randomized controlled clinical trials exploring PFO closure for migraine management, such as MIST (Migraine Intervention with STARFlex Technology) and PREMIUM (Prospective, Randomized Investigation to Evaluate Incidence of Headache Reduction in Subjects with Migraine and PFO using the Amplatzer PFO Occluder to Medical Management), found no difference between PFO device closure and sham procedure regarding headache cure or responder rate at 6-month [15,16]. However, other studies have shown that PFO closure in individuals with large shunts detected by Transcranial Doppler (TCD) and subclinical brain Magnetic Resonance Imaging (MRI) lesions or in individuals with high-risk PFO characteristics seen on TCD and TEE, resulted in a significant reduction in both severity and frequency of migraines [17,18]. Future trials are therefore needed to involve these patients with high PFO risk features and subclinical brain MRI lesions and determine whether PFO closure proves beneficial.
Rare manifestations
Decompression sickness, particularly affecting divers, involves nitrogen bubbles forming during rapid ascents that can enter the systemic circulation through a PFO, potentially causing ischemic embolic events. Current guidelines do not recommend routine closure of the PFO in individuals with prior decompression sickness unless they have experienced PFO-associated strokes [19- 21].
Finally, POS is another rare condition linked to PFO, characterized by dyspnea and hypoxia that worsen when upright and improve when supine. This is attributed to a significant right-to-left shunt through a PFO, as seen on echocardiography, triggered by positional shifts and/or changes in right heart dynamics, particularly conditions that increase the pressures in the right heart [22,23]. PFO closure could improve symptoms in patients with POS [24].
Diagnosis
Multiple ultrasound methods, such as TTE, TEE and TCD have been involved in visualizing, assessing and diagnosing PFOs. Agitated saline is the main method used to identify the presence of a right-to-left shunt during TTE or TEE: The appearance of at least 3 microbubbles in the left atrium, spontaneously or after provocative maneuvers such as coughing or Valsalva, within 3 cycles after opacification of the right atrium is considered diagnostic of an intracardiac shunt. TEE is considered the gold standard test in diagnosing PFOs, given its near 100% sensitivity and specificity, as shown in autopsy studies with near-perfect correlation. TEE also allows for the detailed characterization of the PFO and other interatrial structures, given its superior image resolution.
The use of Three-Dimensional Echocardiography (3DE) during TTE has become more prevalent and has been shown to increase the sensitivity in detecting PFOs compared to the TTE contrast study [25]. Finally, contrast-enhanced cardiac MRI and cardiac CT have shown good agreement with TEE in PFO diagnosis. However, TEE remains the gold standard given superior sensitivity, ability to characterize the PFO, its lesser cost and higher availability [26].
Management
In patients with a history of cryptogenic stroke and a PFO, management options to prevent the recurrence of these strokes include medical therapy like antiplatelet or anticoagulant medication and procedural closure using transcatheter devices. Surgical closure can be considered concomitantly when a patient is undergoing a cardiovascular surgery for another indication. The key to minimizing procedural risks and maximizing its benefits is appropriate patient selection. Only patients with cryptogenic strokes and high RoPE scores (age less than 65, no traditional cerebrovascular risk factors and cortical embolic strokes on presentation) should be considered for PFO closure. Those are the patients in whom the PFO is least likely to be an “innocent” bystander. Emerging guidelines from the Society of Cardiovascular Angiography and Interventions (SCAI) provide valuable insights for PFO management based on an expert panel review of the available literature.
Medical therapy
Choosing between antiplatelet and anticoagulant therapy for patients with cryptogenic stroke and PFO remains uncertain. The Warfarin-Aspirin Recurrent Stroke Study (WARSS) found no significant difference in preventing recurrent strokes or death between warfarin and aspirin in cryptogenic stroke patients [27]. Similarly, the PICSS (Patent Foramen Ovale in Cryptogenic Stroke Study), a subset of WARSS focused on patients who underwent TEE, showed that warfarin-treated patients had similar stroke and death rates over two years compared to those on antiplatelet treatment. In contrast, the PFO-ASA (Patent Foramen Ovale- Atrial Septal Aneurysm) study noted more frequent strokes with aspirin in patients with PFO and ASA, indicating the need for additional prevention strategies for high-risk PFOs. A meta analysis of retrospective studies suggests anticoagulation might be more effective than aspirin in reducing recurrent neurological events for these patients.
In light of these studies and the limited potential benefits of medical therapy compared to transcatheter interventions, the recent SCAI guidelines recommend transcatheter PFO closure over medical therapy alone for patients with cryptogenic stroke attributed to PFO [28].
PFO closure trials
Several major trials on PFO closure and medical therapy, highlighted in Table 1, have influenced current management practices. The CLOSURE trial assessed the STARFlex device’s ability to lower recurrent stroke or (transient ischemic attack) TIA risk compared to medical therapy alone in patients with cryptogenic stroke or TIA but found no significant reduction in outcomes, including recurrent stroke/TIA at 30 days, overall mortality, or neurological death over two years, involving 909 patients [29]. Complications such as post-operative atrial fibrillation and peri-operative vascular bleeding were more frequent in the device cohort. The main limitations of the CLOSURE trial are the inclusion of TIA patients, the withdrawal of the STARFlex device from use and inadequate statistical power to identify significant differences in the primary outcome [30].
The RESPECT (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment) trial compared the Amplatzer device to medical therapy alone and showed no significant difference in nonfatal ischemic strokes, stroke mortality, or all-cause mortality over a follow-up of 2.6 years in an intention-to-treat analysis. However, a subgroup analysis hinted at benefits from closure for patients with large shunts, ASAs, or cortical strokes. Moreover, long-term follow-up over a median of 5.9 years revealed a relative reduction of 62% in the risk of stroke in the device cohort [31].
The Gore REDUCE (Gore Helex Septal Occluder/Gore Cardioform Septal Occluder and Antiplatelet Medical Management for Reduction of Recurrent Stroke or Imaging-Confirmed TIA in Patients with Patent Foramen Ovale (PFO); NCT00738894) trial compared septal occluders against medical therapy in patients aged 18 to 60 with a recent cryptogenic stroke. Results showed lower recurrent ischemic stroke rates in the device group (1.4%) than the medical group (5.4%) over 3.2 years, despite a higher atrial fibrillation incidence [32].
Similarly, the DEFENSE-PFO (Device Closure Versus Medical Therapy for Cryptogenic Stroke Patients with HighâRisk Patent Foramen Ovale) trial studied the Amplatzer PFO closure device. However, it focused on 120 patients with high-risk PFO and revealed no strokes, vascular deaths, or major bleeding in the device group versus a 12.9% event rate in the medical group after a two-year follow-up. This trial defined high-risk PFOs as those associated with hypermobile ASA (excursion ≥ 1 cm), or size exceeding 2 mm [33].
A meta-analysis conducted by Turc et al., included major PFO closure trials, confirmed fewer recurrent neurological events with PFO closure versus medical therapy, especially in patients with elevated RoPe scores (>7), or PFO with high-risk features. These findings underscore again the advantage of PFO closure in patients whose initial strokes were most likely PFO-related, suggesting personalized treatment pathways for those with cryptogenic strokes and PFO [34].
For migraines, a meta-analysis incorporated two trials, PRIMA (Percutaneous Closure of PFO in Migraine with Aura) and PREMIUM, accounting for 337 patients, 176 of whom underwent PFO closure and 161 received only medical therapy. After one year, the analysis showed that PFO closure significantly reduced monthly migraine days (3.1 vs. 1.9 days; p=.02), decreased the frequency of migraine attacks (2.0 vs. 1.4; p=.01) and resulted in more patients experiencing total migraine cessation (9% vs. 0.7%; p<.001).
Limitations of PFO clinical trials
Clinical trials examining PFO closure face several challenges, including difficulties in patient recruitment, accurately defining study populations and addressing the complex nature of cryptogenic strokes and recurrent embolic events (Table 1). Understanding whether the initial neurological events are linked with a PFO is key, as diagnostic variations and alternative embolic sources could dilute closure effects. For instance, in the CLOSURE trial, most neurological events in either group were found to be unrelated to PFO and caused by other conditions such as atrial fibrillation, aortic arch atheroma and vasculitis. Finally, variability in adherence rates, patient retention and follow-up practices on hidden conditions, like occult atrial fibrillation, also affected the results of some of those trials. This again stresses the importance of having better-designed RCTs, focusing on patients with no traditional cerebrovascular risk factors, younger in age and cortical/ischemic embolic infarcts on presentation.
| Trial name | Year published | Patients | Age (Years) | Device used | Follow-Up (Years) | Primary outcome | Outcome |
|---|---|---|---|---|---|---|---|
| CLOSURE | 2012 | 909 | 18-60 | StarFlex | 2 | Composite of stroke and death | No significant benefit (HR=0.78, p=0.37) |
| PC | 2013 | 414 | 18-60 | Amplatzer PFO Occluder | 4.1 | Composite of death, nonfatal stroke, TIA, or peripheral embolism | No significant benefit (HR=0.63, p=0.34) |
| RESPECT | 2017 | 980 | 18-60 | Amplatzer PFO Occluder | 5.9 | Recurrent stroke or death | Significant benefit (HR=0.55, p=0.04) |
| CLOSE | 2017 | 663 | 18-60 | Amplatzer PFO Occluder and others | 5.3 | Recurrent stroke or silent brain infarction | Strong benefit (HR=0.03, p<0.001) |
| Gore REDUCE | 2017 | 664 | 18-59 | GSO and HELEX | 3.2 | Recurrent stroke | Significant benefit (HR=0.23, p=0.002) |
| DEFENSE-PFO | 2018 | 120 | 18-80 | Amplatzer PFO Occluder | 2.8 | Composite of stroke, vascular death, or major bleeding | Significant benefit (0% vs.12.9%,p=0.013) |
Table 1: PFO closure trials for cryptogenic stroke/tia: Outcomes and devices.
Indications
Following the PC (Comparing Percutaneous closure of PFO using the Amplatzer PFO Occluder with medical treatment in patients with cryptogenic embolism) and RESPECT trials, the Amplatzer PFO Occluder was FDA-approved in October, 2016 for treating PFO-related stroke in patients aged 18 to 60. The 2021 AHA/ ASA (American Heart Association/American Stroke Association) guidelines recommend: a) A team approach with a cardiologist and neurologist for managing non-lacunar ischemic stroke, b) Preferring PFO closure over medical therapy in patients with high-risk PFO anatomy, like ASA or a large shunt, is a reasonable approach. However, it’s unclear if transcatheter PFO closure offers more benefits than medical therapy for those with low-risk PFO anatomy [35].
Devices and potential associated complications
The GORE CARDIOFORM Septal Occluder, FDA-approved for PFO closure on March 30, 2018, features a platinum-filled Nitinol wire frame covered with expanded PTFE (Polytetrafluoroethylene). It includes a 10 Fr. 75 cm braided, pre-curved delivery catheter with a distal radiopaque marker. The device has three eyelets with five petal structures each, designed to cover the septal wall. Importantly, this device is repositionable and retrievable, available in 20 mm, 25 mm and 30 mm diameters.
The Amplatzer PFO Occluder is another available device, approved by the FDA on October 28, 2016, for PFO closure. It is a selfexpanding, double-disk device made from 0.005 inch Nitinol wire with polyester patches in each disk to prevent blood flow. It features a thin, mobile waist, with the right atrial disk larger than the left, differing from the Amplatzer ASO device. It comes in four sizes (18, 25, 30 and 35 mm), with sheath sizes of 8 or 9 Fr. depending on the device size.
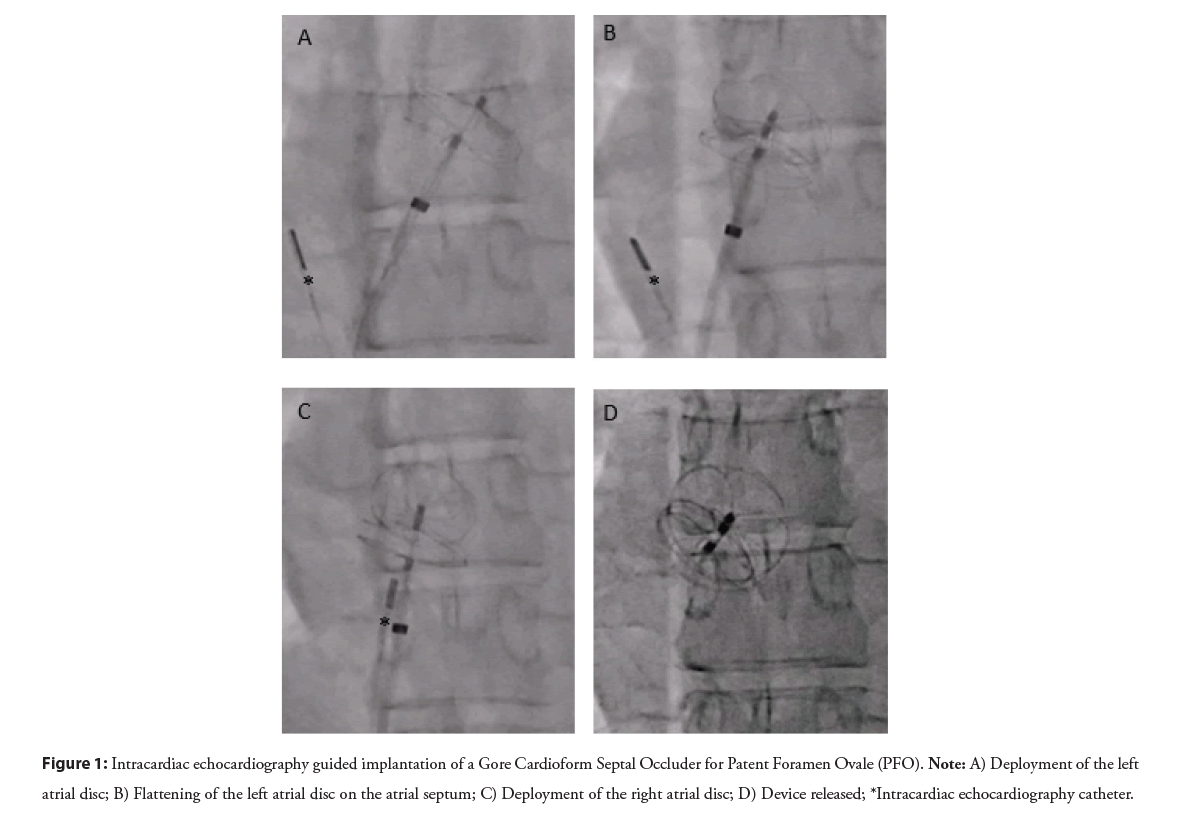
Although generally safe, transcatheter PFO closure can lead to complications, the most common being atrial arrhythmias such as atrial fibrillation and flutter (Figure 1). Rarely, issues like device thrombosis or migration may arise. More serious complications such as major bleeding, pericardial effusion and tamponade are rare, with an incidence of <0.5% as reported in the PC and RESPECT trials. Finally, air embolism is another severe complication that can be avoided with proper flushing and careful fluoroscopy during device insertion into the delivery sheath.
Figure 1: Intracardiac echocardiography guided implantation of a Gore Cardioform Septal Occluder for Patent Foramen Ovale (PFO). Note: A) Deployment of the left atrial disc; B) Flattening of the left atrial disc on the atrial septum; C) Deployment of the right atrial disc; D) Device released; *Intracardiac echocardiography catheter.
Atrial septal defects
ASDs are among the common congenital heart defects in adults, with an estimated occurrence of 0.88 in 1000 individuals [36]. ASDs are often diagnosed late as most cases are asymptomatic until the second to fourth decades of life. During this period, increased blood flow through the pulmonary arterial system caused by the left to right shunt causes remodeling of the pulmonary arteries. In extreme cases, this remodeling can be severe and irreversible, affecting the direction of the shunt, causing bidirectional flow and cyanosis, known as Eisenmenger syndrome.
ASDs are categorized into four main types, each with its specific anatomical defect. The most common ASD is the ostium secundum type, resulting from the absence of tissue in the fossa ovalis. Ostium secundum accounts for about 80% of ASDs. The second most common type is the ostium primum ASD, often referred to as partial atrioventricular septal defect, given the lack of tissue near the atrioventricular valves and is often associated with a cleft in the mitral valve. Sinus venosus defects are associated with partial anomalous pulmonary venous return. Finally, the least common type of ASDs is coronary sinus defects, also known as unroofed coronary sinus and is often associated with persistent left superior vena cava. Those are often missed on conventional cardiac imaging.
ASDs typically exhibit no symptoms initially but can manifest through gradually diminishing exercise tolerance, dyspnea on exertion, or notable atrial arrhythmias such as atrial fibrillation or atrial flutter. Paradoxical embolisms causing peripheral or cerebral ischemic conditions may also be the initial clinical manifestation of ASD.
Discussion
Physical exam features that should raise a suspicion of an ASD include a systolic flow murmur across the pulmonic valve and a fixed splitting of S2 caused by the increased flow through the pulmonic valve secondary to the left or right shunt. Moreover, in cases of ostium primum, one could also notice a tricuspid or a mitral regurgitant murmur. On electrocardiography, the presence of right atrial enlargement, right axis deviation, right ventricular hypertrophy, an incomplete right bundle branch block and a firstdegree AV block should also raise suspicion of an ASD.
ASD diagnosis remains primarily echocardiographic, using agitated saline to enhance the echocardiographic diagnostic accuracy. Findings on TTE include an enlarged right atrium and right ventricle, elevated Pulmonary Artery Systolic Pressure (PASP) and increased pulmonary flow compared to flow across the Left Ventricular Outflow Tract (LVOT), with the ability to assess Qp:Qs ratio [36]. TTE will also be able to identify certain associated conditions like tricuspid or mitral valve regurgitation (ostium primum). More advanced imaging modalities also play a role (cardiac MRI and CT) in the diagnosis and evaluation of ASD, primarily in assessing for anomalous pulmonary venous return, providing visualization of the defect, as well as accurately measuring the degree of shunt MRI.
Finally, Right Heart Catheterization (RHC) is one of the last diagnostic tools used in assessing ASD, especially when considering defect repair. This procedure can quantify shunt degree and direction by calculating Qp:Qs. This is crucial to determine indications and contraindications for defect repair.
Management
While small ASDs might close spontaneously, larger defects will cause progressive pulmonary vascular remodeling, leading to clinical deterioration and will need to be repaired before the development of Eisenmenger syndrome. The current guidelines from. The current ACC/AHA (American College of Cardiology/ American Heart Association) guidelines recommend closure of ASD in the presence of a significant shunt (Qp:Qs>1.5) and right-sided volume overload (class I) [37]. This includes both symptomatic and asymptomatic individuals, as long as the PASP is under 50% of the systemic pressure and the Pulmonary Vascular Resistance (PVR) remains lower than one-third of the systemic vascular resistance. The repair is aimed at preventing further pulmonary vascular remodeling and restoring normal dimensions on the right side. Contraindications to repair include severe PVR>8; or substantial right to left shunt.
Repair of other than secundum ASDs is mainly surgical. This necessitates an open sternotomy approach and the use of a cardiopulmonary bypass to allow direct visualization of the defect. Associated defects such as mitral or tricuspid valve regurgitation and anomalous pulmonary venous return are also repaired surgically as part of the same procedure [38]. Surgical repair done before the age of 25 offers a 30-year survival benefit compared to matched cohorts. However, this does not affect the risk of present or future atrial arrhythmias, which remain elevated despite the repair [39].
Transcatheter closure is a recognized alternative to surgery for secundum atrial septal defects. Widely used devices in the U.S. include the Amplatzer Septal Occluder, Gore Cardioform ASD Occluder and Gore Cardioform Septal Occluder. The Amplatzer is favored for its simple deployment and suitability for larger defects. It features a self-expanding, double-disk design from nitinol wire. he Gore devices use a nitinol wire frame covered with PTFE and are available in sizes that can treat defects from 8 to 35 mm. Relative contra-indications to percutaneous repair include large defects >36 mm, insufficient margins for device anchoring and device interference with AV valves or venous drainage [40]. The Amplatzer Septal Occluder can treat defects as large as 38 mm.
Potential complications linked with percutaneous ASD closure include arrhythmias, AV block, embolism, rarely device erosion and thromboembolism. Matching appropriate device choice to defect size helps reduce the risks of device embolization [41,42].
Conclusion
PFO is found in around 1/3 of adults and holds clinical importance, particularly pertaining to embolic strokes. Transcatheter closure demonstrated efficacy in high-risk PFO and those associated with ASAs. The role of PFO closure in other conditions, such as migraines and decompression sickness, remains the focus of ongoing research. Appropriate patient selection through an effective multidisciplinary team, including interventional cardiologists and neurologists, is key to maximizing benefits from PFO closure while minimizing unnecessary risks. ASDs include various types, with ostium secundum being the most common. Repair decisions are based on clinical and anatomical considerations, with guidelines recommending closure for significant shunts and right-sided volume overload. The method of repair-surgical or percutaneousdepends on the patient’s characteristics and the specific defect’s type and size, with percutaneous closure being used exclusively for secundum ASDs. Each repair option has its own benefits and considerations.
References
- Dattilo PB, Kim MS, Carroll JD, et al. Patent foramen ovale. Cardiol Clin. 31(3):401-415 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation.112(7):1063-1072 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Di Tullio MR. Patent foramen ovale: Echocardiographic detection and clinical relevance in stroke. J Am Soc Echocardiogr. 23(2):144-155 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Handke M, Harloff A, Olschewski M, et al. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 357(22):2262-2268 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Lechat PH, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 318(18):1148-1152 (1988).
[CrossRef] [Google Scholar] [PubMed]
- Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med.345(24):1740-1746 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Morton SU, Brodsky D. Fetal physiology and the transition to extrauterine life. Clin Perinatol. 43(3):395-407 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Anderson RH, Webb S, Brown NA, et al. Clinical anatomy of the atrial septum with reference to its developmental components. Clin Ana.12(5):362-374 (1999).
[CrossRef] [Google Scholar] [PubMed]
- Hara H, Virmani R, Ladich E, et al. Patent foramen ovale: Current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. 46(9):1768-1776 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Meacham RR, Headley AS, Bronze MS, et al. Impending paradoxical embolism. Arch Intern Med. 158(5):438-448 (1998).
[CrossRef] [Google Scholar] [PubMed]
- Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: The PFO-ASA Study. Stroke. 33(3):706-711 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs. incidental patent foramen ovale in cryptogenic stroke. Neurology. 81(7):619-625 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Homma S, Sacco RL, Di Tullio MR, et al. Effect of medical treatment in stroke patients with patent foramen ovale: Patent foramen ovale in cryptogenic stroke study. Circulation. 105(22):2625-2631 (2002).
[CrossRef] [Google Scholar] [PubMed]
- Serena J, Marti-Fabregas J, Santamarina E, et al. Recurrent stroke and massive right-to-left shunt: results from the prospective Spanish multicenter (CODICIA) study. Stroke. 39(12):3131-3136 (2008).
[CrossRef] [Google Scholar] [PubMed]
- Dowson A, Mullen MJ, Peatfield R, et al. Migraine intervention with STARFlex Technology (MIST) trial: A prospective, multicenter, double-blind, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation. 117(11):1397-1404 (2008).
[CrossRef] [Google Scholar] [PubMed]
- Tobis JM, Charles A, Silberstein SD, et al. Percutaneous closure of patent foramen ovale in patients with migraine: The PREMIUM trial. J Am Coll Cardiol. 70(22):2766-2774 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Vigna C, Marchese N, Inchingolo V, et al. Improvement of migraine after patent foramen ovale percutaneous closure in patients with subclinical brain lesions: A case-control study. JACC Cardiovasc Interv. 2(2):107-113 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Rigatelli G, Dell'Avvocata F, Ronco F, et al. Primary transcatheter patent foramen ovale closure is effective in improving migraine in patients with high-risk anatomic and functional characteristics for paradoxical embolism. JACC: Cardiovascular Interventions. 3(3):282-287 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Agostoni P, Gasparini G, Destro G, et al. Acute myocardial infarction probably caused by paradoxical embolus in a pregnant woman. Heart. 90(3):e12 (2004).
[CrossRef] [Google Scholar] [PubMed]
- Iwasaki M, Joki N, Tanaka Y, et al. A suspected case of paradoxical renal embolism through the patent foramen ovale. Clin Exp Nephrol. 15:147-150 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Kavinsky CJ, Szerlip M, Goldsweig AM, et al. SCAI guidelines for the management of patent foramen ovale. J Soc Cardiovasc Angiogr Interv. 1(4):100039 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Agrawal A, Palkar A, Talwar A, et al. The multiple dimensions of Platypnea-Orthodeoxia syndrome: A review. Respir Med. 129:31-38 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Babu S, Zhu Z, Chavez M, et al. PFO closure after pulmonary valve intervention in patients with hypoxemia due to right-to-left intracardiac shunt. Methodist Debakey Cardiovasc J. 18(1):90 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Guerin P, Lambert V, Godart F, et al. Transcatheter closure of patent foramen ovale in patients with platypnea-orthodeoxia: Results of a multicentric French registry. Cardiovasc Intervent Radiol. 28:164-168 (2005).
[CrossRef] [Google Scholar] [PubMed]
- Monte I, Grasso S, Licciardi S, et al. Head-to-head comparison of real-time three-dimensional transthoracic echocardiography with transthoracic and transesophageal two-dimensional contrast echocardiography for the detection of patent foramen ovale. Eur J Echocardiogr. 11(3):245-249 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Soliman OI, Geleijnse ML, Meijboom FJ, et al. The use of contrast echocardiography for the detection of cardiac shunts. Eur J Echocardiogr. 8(3):s2-s12 (2007).
[CrossRef] [Google Scholar] [PubMed]
- Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 345(20):1444-1451 (2001).
[CrossRef] [Google Scholar] [PubMed]
- Alsheikh-Ali AA, Thaler DE, Kent DM, et al. Patent foramen ovale in cryptogenic stroke: Incidental or pathogenic?. stroke. 40(7):2349-2355 (2009).
[CrossRef] [Google Scholar] [PubMed]
- Aoun J, Hatab T, Volpi J, et al. Patent foramen ovale and atrial septal defect. Cardiol Clin 42(3):417-431 (2024).
[CrossRef] [Google Scholar] [PubMed]
- Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 366(11):991-999 (2012).
- Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 377(11):1022-1032 (2017).
- Søndergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 377(11):1033-1042 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Lee PH, Song JK, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: The DEFENSE-PFO trial. J Am Coll Cardiol. 71(20):2335-2342 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Turc G, Calvet D, Guerin P, et al. Closure, anticoagulation, or antiplatelet therapy for cryptogenic stroke with patent foramen ovale: Systematic review of randomized trials, sequential metaâanalysis, and new insights from the CLOSE study. J Am Heart Assoc. 7(12):e008356 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline from the American Heart Association/American Stroke Association. Stroke. (2021).
[CrossRef] [Google Scholar] [PubMed]
- Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 130(9):749-756 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Apr 2;73(12):e81-192 (2019).
- Geva T, Martins JD, Wald RM, et al. Atrial septal defects. The Lancet. 2383(9932):1921-1932 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Murphy JG, Gersh BJ, McGoon MD, et al. Long-term outcome after surgical repair of isolated atrial septal defect: Follow-up at 27 to 32 years. N Engl J Med. 323(24):1645-1650 (1990).
[CrossRef] [Google Scholar] [PubMed]
- Fraisse A, Latchman M, Sharma SR, et al. Atrial septal defect closure: Indications and contra-indications. J Thorac Dis. 10(24):S2874 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Yang MC, Wu JR. Recent review of transcatheter closure of atrial septal defect. Kaohsiung J Med Sci. 34(7):363-369 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Levi DS, Moore JW. Embolization and retrieval of the Amplatzer septal occluder. Catheter Cardiovasc Interv. 61(4):543-547 (2004).
[CrossRef] [Google Scholar] [PubMed]