Review Article - Interventional Cardiology (2012) Volume 4, Issue 3
Patient selection for alcohol septal ablation for hypertrophic obstructive cardiomyopathy: clinical and echocardiographic evaluation
- Corresponding Author:
- Danita M Yoerger Sanborn
Massachusetts General Hospital, Yawkey 5B-5916, 55 Fruit Street, Boston, MA 02114–2696, USA
Tel: +1 617 726 1543
Fax: +1 617 726 7684
E-mail: dysanborn@partners.org
Abstract
Keywords
alcohol septal ablation,echocardiography,ethanol,hypertrophic cardiomyopathy,hypertrophic obstructive cardiomyopathy,myocardial contrast echocardiography
Introduction
Hypertrophic cardiomyopathy (HCM) is a primary disease of heart muscle that results in idiopathic hypertrophy of the left ventricle in the absence of abnormal loading conditions. The disease has a genetic basis and is often inherited in an autosomal dominant fashion. The frequency of the disease is approximately one in 500 adults [1]. A variety of HCM phenotypes exist, but the variant which has been the subject of the most interest and investigation is hypertrophic obstructive cardiomyopathy (HOCM), associated with a left ventricular outflow tract (LVOT) gradient. It has been estimated that approximately 25% of individuals with HCM have resting obstruction [2–4]; more recent prospective data, however, suggest that up to 70% of symptomatic HCM patients have obstruction with activity [5,6].
The cardinal features of HOCM include asymmetric septal hypertrophy, systolic anterior motion (SAM) of the mitral valve, LVOT obstruction and mitral regurgitation (MR). Treatment goals include relief of symptoms, prevention of sudden cardiac death and family screening and counseling. When symptoms persist despite adequate conservative therapy, consideration of septal reduction therapy for relief of LVOT obstruction should be considered.
Relief of obstruction can be achieved surgically via septal myectomy or by a catheterbased technique, alcohol septal ablation (ASA) [7]. Regardless of the technique employed to relieve obstruction, successful gradient reduction with resultant lowering of left ventricular (LV) systolic pressure is associated with relief of lifestyle-limiting symptoms [8–11]. As experience with ASA grows, it has become clear that appropriate patient selection enhances procedural success and reduces complication rates.
Clinical features of HOCM
The symptoms of HOCM include dyspnea on exertion, angina, lightheadedness, presyncope and syncope. Factors contributing to dyspnea on exertion include LVOT obstruction, diastolic dysfunction due to hypertrophy and fibrosis, myocardial ischemia and MR [12]. Angina, which is generally associated with effort and has the likelihood of occurring in the absence of epicardial coronary artery disease, may represent myocardial ischemia due to a demand/supply mismatch, since the metabolic needs of the hypertrophied myocardium cannot be met by the coronary microcirculation. The occurrence of lightheadedness, presyncope and syncope may be due to a confluence of abnormalities including LVOT obstruction, systemic vasodilation, diastolic dysfunction and ventricular tachyarrhythmias.
Echocardiography in HOCM
Echocardiography in HOCM usually reveals a triad of abnormalities:
▪ Asymmetric LV hypertrophy (ratio of interventricular septal [IVS] to posterior wall thickness ≥1.3);
▪ SAM of the mitral valve;
▪ A dynamic, late-peaking LVOT gradient by Doppler.
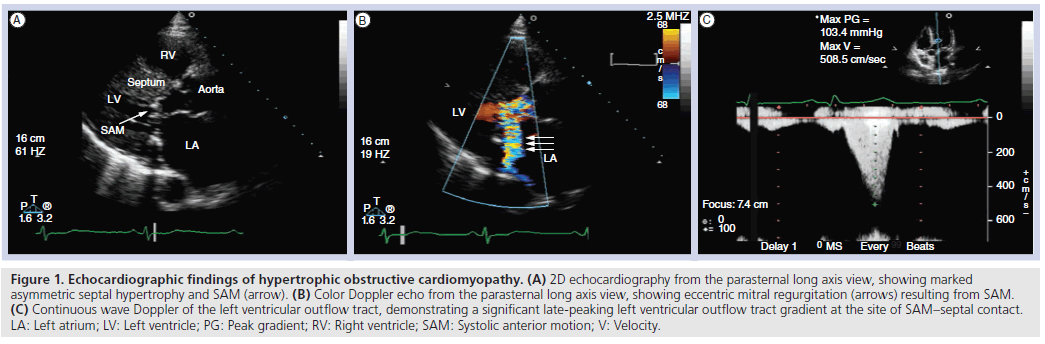
Obstruction to LV outflow occurs due to apposition of the thickened septum and the anterior leaflet of the mitral valve; there is anterior displacement of the coaptation point of the mitral leaflets due to hypertrophy and displacement of the papillary muscles. This in turn leads to residual anterior mitral leaflet length. The mitral leaflet tips move into the LVOT during systole (SAM) due to drag forces [13,14] and the Venturi effect [15] (Figure 1a). As the anterior mitral leaflet tip comes into contact with the IVS during the systolic ejection period, there is acceleration of blood flow in the LVOT and thus a late-peaking LVOT gradient detectable by spectral Doppler (Figure 1c). This obstruction is a dynamic process, and thus varies based on loading conditions and cycle length. Factors that increase contractility or decrease LV filling (exercise, Valsalva maneuver and postextrasystolic potentiation) lead to increased obstruction and an increased LVOT gradient. MR results from malcoaptation of the anterior and posterior mitral leaflets during SAM, is typically posteriorly directed and may be late-systolic. In patients with MR that is not posteriorly directed, careful assessment for structural abnormalities of the mitral valve is imperative. In the setting of significant LVOT obstruction, Doppler interrogation of aortic flow often reveals a biphasic flow pattern, with a decrease in the midsystolic ejection velocity. This pattern correlates with premature closure of the aortic valve demonstrated by 2D and M-mode imaging, as well as with the ‘spike and dome’ pattern in the aortic pulse tracing.
Figure 1: Echocardiographic findings of hypertrophic obstructive cardiomyopathy. (A) 2D echocardiography from the parasternal long axis view, showing marked asymmetric septal hypertrophy and SAM (arrow). (B) Color Doppler echo from the parasternal long axis view, showing eccentric mitral regurgitation (arrows) resulting from SAM. (C) Continuous wave Doppler of the left ventricular outflow tract, demonstrating a significant late-peaking left ventricular outflow tract gradient at the site of SAM–septal contact. LA: Left atrium; LV: Left ventricle; PG: Peak gradient; RV: Right ventricle; SAM: Systolic anterior motion; V: Velocity.
Conservative therapy for HOCM
First-line therapy for symptomatic HOCM consists of negative inotropic drugs, including b-adrenergic blockers, calcium channel blockers, and disopyramide. b-blockers are the drugs of choice. Dose titration is governed by symptoms, side effects and heart rate, with a heart rate goal of 50–60 for amelioration of the symptoms. In patients with contraindications to b-blockade, such as severe asthma, and in those requiring discontinuation of b-blockade because of side effects, verapamil is often substituted and titrated similarly to symptoms, side effects and heart rate. In patients with symptoms refractory to optimal dosages of these medications, disopyramide can be added in the absence of a contraindication to this medication. Disopyramide is a class IA antiarrhythmic drug that has the additional property of being a negative inotropic agent. It is necessary to monitor the corrected QT interval after initiation of disopyramide or an increase in dosage. Anticholinergic side effects such as dry mouth and constipation can be managed with pyridostigmine.
Patients with symptoms refractory to medical therapy who have a dual‑chamber pacemaker or implantable cardioverter defibrillator already in place may be managed with a trial of pacing with short atrioventricular delay to pre-excite the LV apex [16]. While this strategy is effective in only a minority of patients in whom it is attempted, it sometimes obviates the need to expose patients to the risks of septal reduction therapy.
Patient selection for ASA
The ideal setting for ASA is in a program that incorporates a multidisciplinary team of experienced clinicians, imagers and interventional cardiologists familiar with HCM and the procedure. Integration of clinical and echocardiographic data is required when deciding whether ASA is the relevant therapy for a patient with HOCM, since appropriate patient selection will enhance procedural success and limit complications. Indications for ASA are listed in Box 1. Since ASA has not been shown to prolong survival, the procedure should not be performed, irrespective of the magnitude of the LVOT gradient, in patients with no or mild symptoms.
▪ Clinical
Patients should be considered for ASA if they have symptoms, despite optimal medical therapy, that interfere sufficiently with their lifestyle, allowing them to be willing to assume the morbidity and mortality of the procedure. The symptoms that prompt ASA are dyspnea or chest pain in most instances, syncope and near syncope on occasion. Clinical assessment should include cardiac auscultation during the Valsalva maneuver (with the patient supine to avoid syncope) for detection of provocable obstruction. Some patients referred for ASA respond to intensification of conservative therapy, as described above, obviating the need for septal reduction therapy.
▪ Echocardiographic
Echocardiography is a critical tool for screening prior to consideration of ASA. The presence of asymmetric septal hypertrophy, SAM, LVOT obstruction and eccentric MR all confirm the diagnosis (Figure 1). Measurement of septal thickness ≥16 mm at the site of SAM–septal contact is important for avoiding creation of a ventricular septal defect [17]. Echocardiography also provides an important assessment of maximal LV wall thickness, which when ≥30 mm is a risk factor for sudden cardiac death and may be associated with a lower success rate of ASA [18]. Careful color and spectral Doppler interrogation of the LV cavity, LVOT and aortic valve exclude stenoses ‘in series’ (coexisting midcavitary or aortic valve obstruction). Stepwise pulsed wave Doppler interrogation of the LV cavity starting at the apex and moving toward the aortic valve is necessary to determine the site of maximal obstruction, as the goal of ASA is to relieve obstruction at the site of SAM–septal contact (or, on occasion, at the midventricular level [19]). If maximal obstruction lies at the aortic valve then ASA is unlikely to succeed in relieving symptoms.
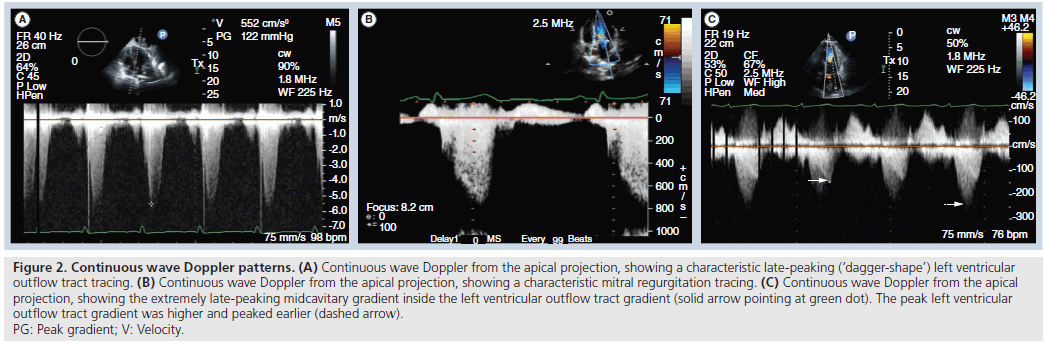
A pitfall of Doppler interrogation in HOCM is that it can sometimes be difficult to separate the peak MR velocity from the LVOT velocity, which can lead to overestimation of the LVOT gradient. This may be due to the position of the heart in the chest, which can vary with respiration, as well as unusual orientation of the MR jet. For accurate determination of the LVOT gradient, it is thus imperative to obtain separate continuous wave Doppler tracings of the MR jet and of the LVOT gradient. In general, the MR jet should start earlier, have a higher peak velocity and appear more symmetric than the LVOT jet, which is typically late-peaking or ‘dagger-shaped’. Some patients with HOCM also have midcavitary gradients, which occur as the opposing walls of the ventricle come into contact in late systole. The typical midcavitary gradient is extremely latepeaking. Figure 2 shows examples of continuous wave Doppler tracings of a typical LVOT gradient, a typical MR jet, and a midcavitary gradient. In cases where it is difficult to distinguish the MR and LVOT jets, the presence of premature closure of the aortic valve by 2D or M-mode imaging suggests the presence of a hemodynamically significant LVOT gradient.
Figure 2: Continuous wave Doppler patterns. (A) Continuous wave Doppler from the apical projection, showing a characteristic late‑peaking (‘dagger-shape’) left ventricular outflow tract tracing. (B) Continuous wave Doppler from the apical projection, showing a characteristic mitral regurgitation tracing. (C) Continuous wave Doppler from the apical projection, showing the extremely late‑peaking midcavitary gradient inside the left ventricular outflow tract gradient (solid arrow pointing at green dot). The peak left ventricular outflow tract gradient was higher and peaked earlier (dashed arrow). PG: Peak gradient; V: Velocity.
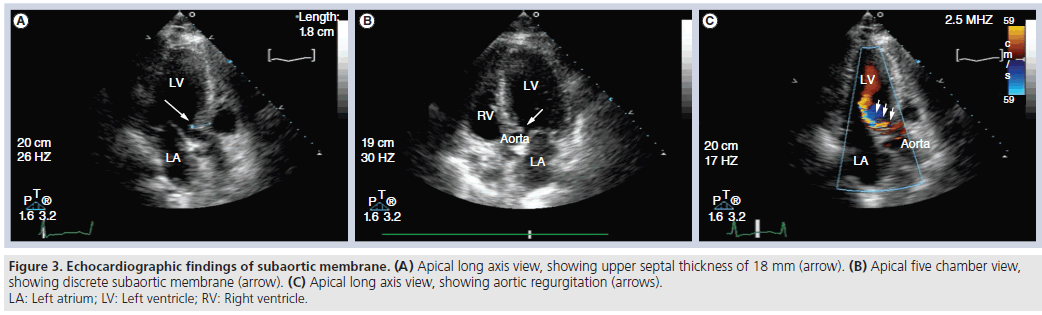
It is also important to exclude other pathologic conditions or anatomic variants such as mitral valve prolapse, excessive elongation of the mitral leaf lets, anomalous papillary muscle insertion, discrete subaortic membrane and extensive mitral annular calcification, which might make ASA unlikely to succeed in relieving symptoms. It is possible for significant septal hypertrophy to develop just under a subaortic membrane, which can make visualization of the membrane difficult. Clues to the presence of a subaortic membrane include aortic regurgitation and an LVOT gradient that is not late-peaking (Figure 3).
Figure 3: Echocardiographic findings of subaortic membrane. (A) Apical long axis view, showing upper septal thickness of 18 mm (arrow). (B) Apical five chamber view, showing discrete subaortic membrane (arrow). (C) Apical long axis view, showing aortic regurgitation (arrows). LA: Left atrium; LV: Left ventricle; RV: Right ventricle.
While patients with HOCM typically have asymmetric septal hypertrophy, some patients have symmetric LVH and some of these patients have a history of hypertension. Patients who have refractory symptoms, SAM, and LVOT obstruction may be candidates for septal reduction therapy independent of whether or not they have genetic HCM [20].
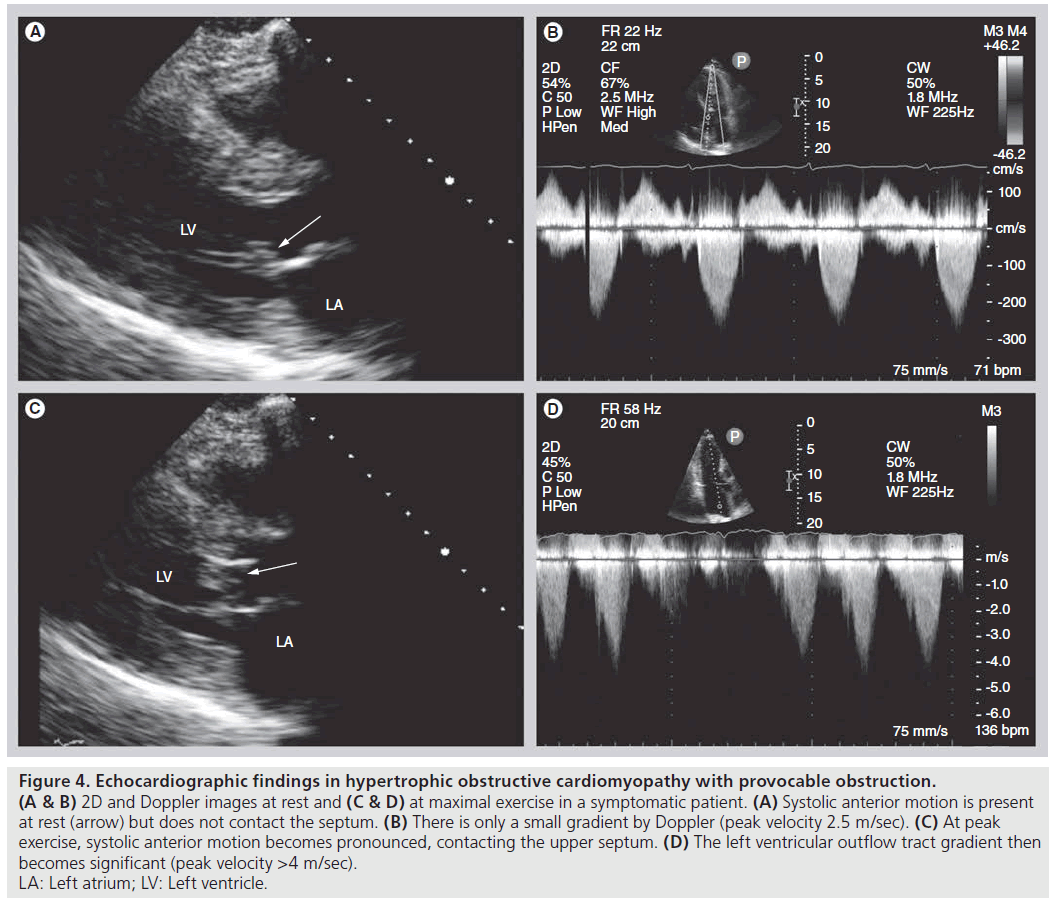
Provocation of LVOT obstruction with Valsalva maneuver and/or exercise echocardiography is a useful tool in selecting patients for mechanical therapy. Exercise increases heart rate, increases contractility and shortens the diastolic period, leading to decreased LV filling, and thus may provoke LVOT obstruction. Echocardiography can be performed during or immediately following exercise (bicycle or treadmill), and can detect increases in degree and duration of SAM, LVOT gradient and severity of MR (Figure 4). Dobutamine stress echo is an alternative to exercise echo for the diagnosis of ischemia in individuals who cannot exercise. It should be noted, however, that this modality is not an ideal substitute for exercise echocardiography to provoke gradients in HCM, since dobutamine infusion has the potential to induce intracavitary gradients of no clinical importance, even in individuals without HCM [21].
Figure 4: Echocardiographic findings in hypertrophic obstructive cardiomyopathy with provocable obstruction.
(A & B) 2D and Doppler images at rest and (C & D) at maximal exercise in a symptomatic patient. (A) Systolic anterior motion is present
at rest (arrow) but does not contact the septum. (B) There is only a small gradient by Doppler (peak velocity 2.5 m/sec). (C) At peak
exercise, systolic anterior motion becomes pronounced, contacting the upper septum. (D) The left ventricular outflow tract gradient then
becomes significant (peak velocity >4 m/sec).
LA: Left atrium; LV: Left ventricle.
Outcome of ASA
ASA is successful in improving symptoms and lowering the LVOT gradient in ≥80% of appropriately selected patients [22,23]. The clinical benefit of ASA in patients with provocable LVOT obstruction is similar to that in patients with resting obstruction [24,25]. Results in older patients are comparable with those in younger patients [26,27]. With the lower doses of ethanol used in current practice, the most common complication, complete heart block necessitating permanent pacemaker insertion, has been reduced to approximately 10% [28]. Procedural mortality is <2%, similar to that of septal myectomy [29].
ASA versus septal myectomy
With surgical myectomy, the resected segment of myocardium is under the direct control of the surgeon, whereas the affected area in ASA may be constrained by septal anatomy [30]. Nevertheless, nonrandomized comparisons and meta-analysis of ASA and septal myectomy have not demonstrated the superiority of either procedure. In practice, many patients express a strong preference for nonsurgical therapy when feasible [9]. Triage of patients to ASA or septal myectomy requires careful assessment of the benefits and risks for the individual patient, a balanced presentation of those considerations and, in the end, clinical experience and judgment and patient preference. Younger patients with marked septal hypertrophy (e.g., ≥30 mm) are generally offered septal myectomy, while older patients with comorbidities are usually offered ASA.
Contrast echocardiographic guidance during ASA
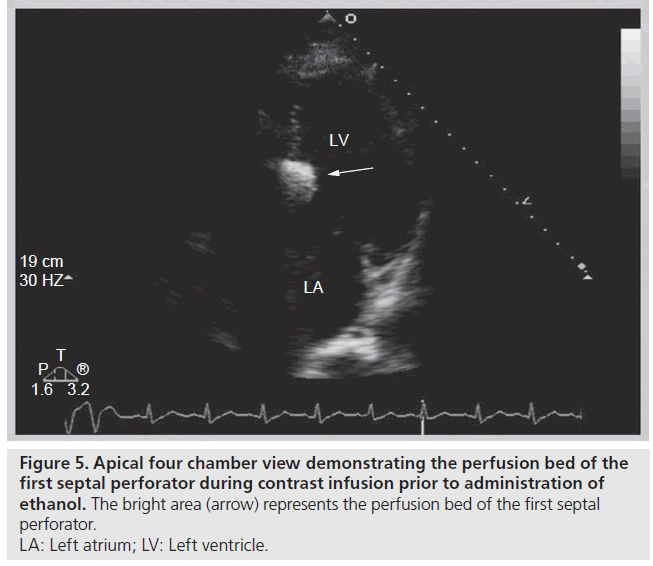
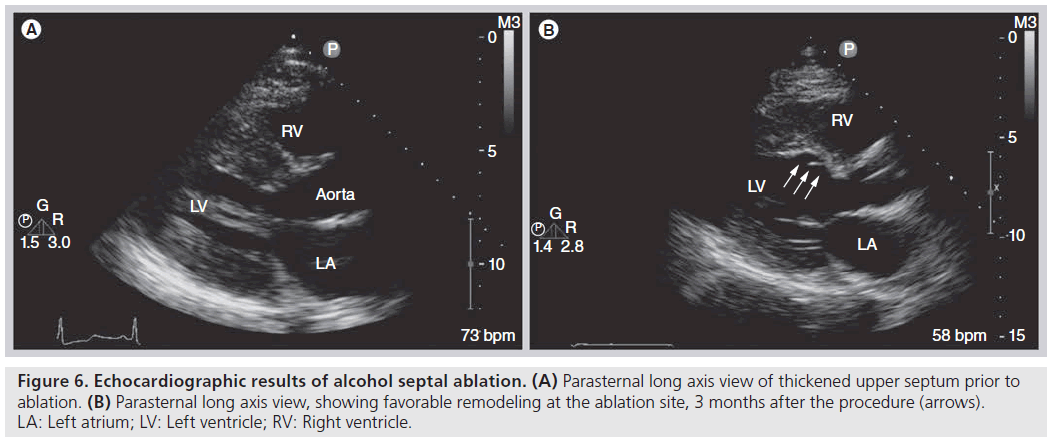
The use of contrast echocardiography to guide ASA was introduced not long after the procedure was first described [31–33]. The addition of myocardial contrast agents to the ASA procedure allows for visualization of the infarct area prior to administration of ethanol, which leads to improved procedural success. In one series, use of contrast impacted the choice of septal perforator in 11% of cases and informed the decision to abandon the procedure in 8% of cases [34]. In addition, contrast use has been shown to reduce infarct size [35], while lowering the incidence of permanent pacemaker requirement after ASA from 17 to 7% [31]. Diluted echocardiographic contrast or agitated radiographic contrast is injected through the inflated balloon catheter into the target septal perforator, and simultaneous 2D echocardiography is performed to determine the perfusion bed prior to administration of ethanol. During infusion, a highly reflective area appears and thus the predicted infarct area of the proposed target is demarcated (Figure 5). Following ethanol administration, echocardiography again demonstrates a more intense and longer‑lasting reflective area in the septum supplied by the perforator. The target area immediately exhibits decreased contractility following ethanol infusion, thus providing immediate gradient relief and eventually will thin out and scar [36], leading to favorable remodeling and sustained gradient relief over the following months [37–39] (Figure 6).
Figure 5: Apical four chamber view demonstrating the perfusion bed of the
first septal perforator during contrast infusion prior to administration of
ethanol. The bright area (arrow) represents the perfusion bed of the first septal
perforator.
LA: Left atrium; LV: Left ventricle.
Figure 6: Echocardiographic results of alcohol septal ablation. (A) Parasternal long axis view of thickened upper septum prior to ablation. (B) Parasternal long axis view, showing favorable remodeling at the ablation site, 3 months after the procedure (arrows). LA: Left atrium; LV: Left ventricle; RV: Right ventricle.
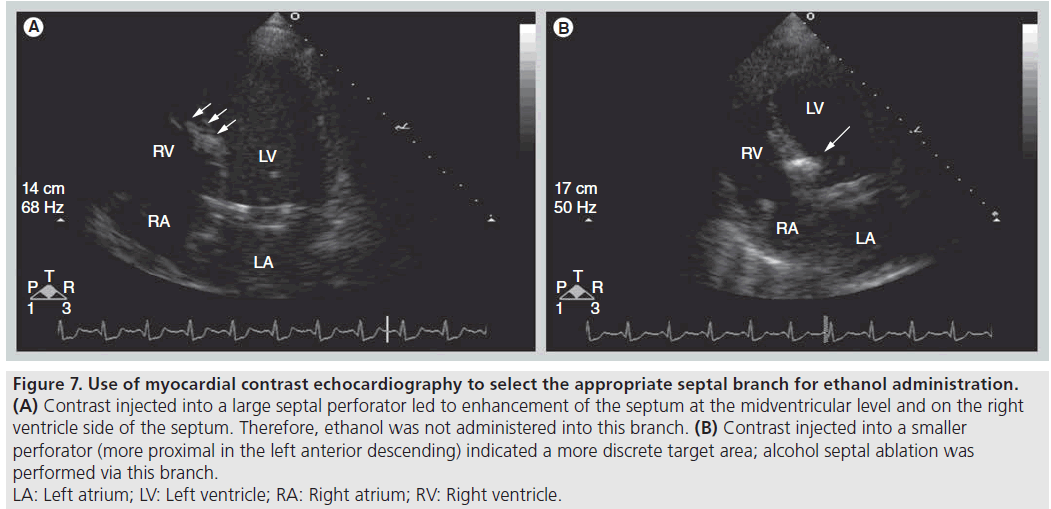
An ideal perfusion bed includes the most basal segment of the IVS and covers the area of SAM–septal contact. The advantage of real-time echocardiography is the ability to immediately determine the target perfusion bed and to detect unexpected areas of perfusion, which if injected with ethanol could lead to incorrect infarct location (Figure 7) or larger than desired infarct area (Figure 8) and to an increase in complication rates [35]. Integration of angiographic, hemodynamic and echocardiographic data is important to achieve the goal of administering the smallest amount of ethanol needed to cover the area of the IVS where SAM contact occurs, abolishing or lowering the LVOT gradient, while minimizing the risk of inducing heart block. Alternatively, some operators perform ASA without echocardiography, with visualization of the target area by cineangiography and selection of septal branches guided by the reduction in LVOT gradient during transient balloon occlusion.
Figure 7: Use of myocardial contrast echocardiography to select the appropriate septal branch for ethanol administration.
(A) Contrast injected into a large septal perforator led to enhancement of the septum at the midventricular level and on the right
ventricle side of the septum. Therefore, ethanol was not administered into this branch. (B) Contrast injected into a smaller
perforator (more proximal in the left anterior descending) indicated a more discrete target area; alcohol septal ablation was
performed via this branch.
LA: Left atrium; LV: Left ventricle; RA: Right atrium; RV: Right ventricle.
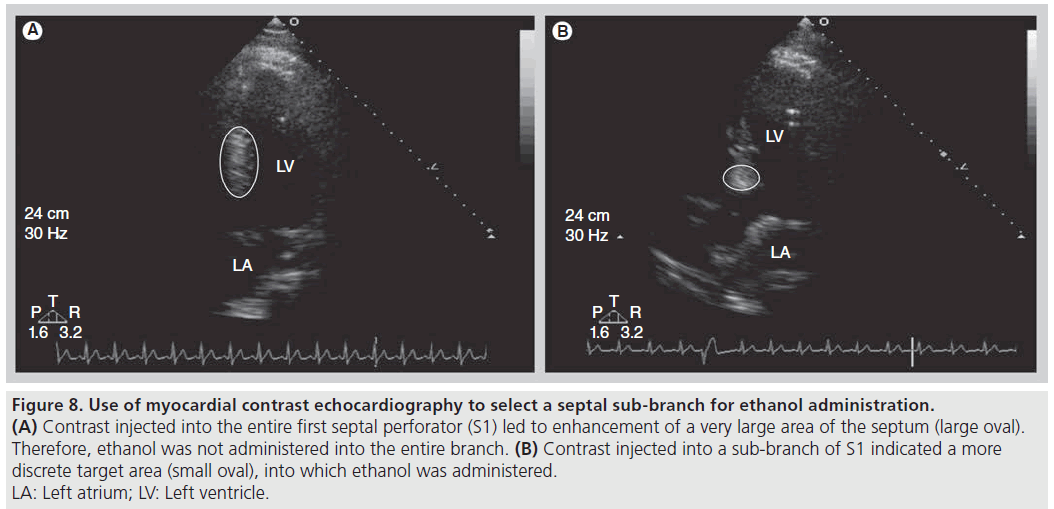
Figure 8: Use of myocardial contrast echocardiography to select a septal sub-branch for ethanol administration. (A) Contrast injected into the entire first septal perforator (S1) led to enhancement of a very large area of the septum (large oval). Therefore, ethanol was not administered into the entire branch. (B) Contrast injected into a sub-branch of S1 indicated a more discrete target area (small oval), into which ethanol was administered. LA: Left atrium; LV: Left ventricle.
Follow‑up echocardiography
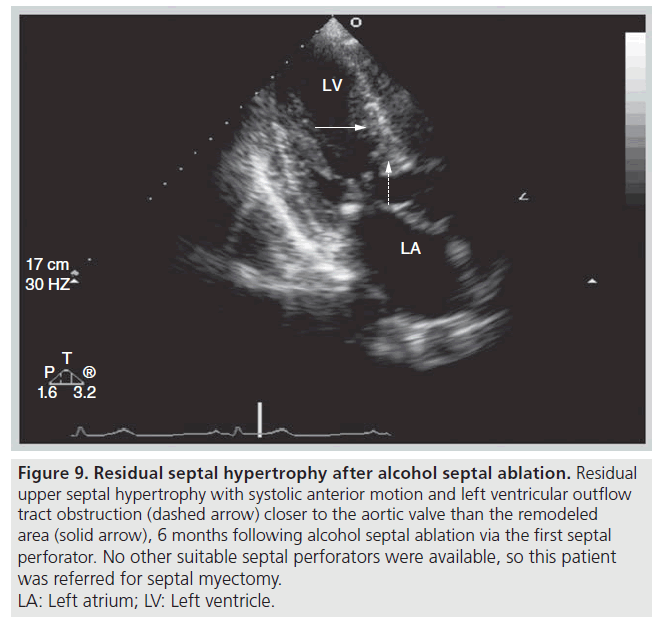
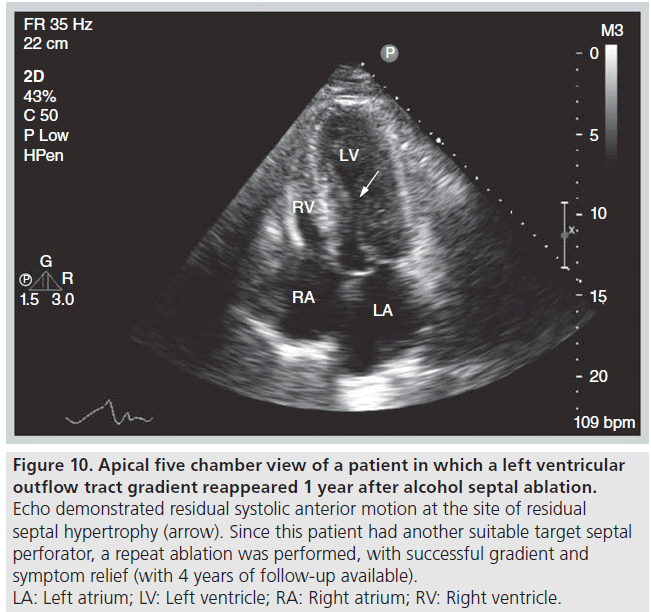
Echocardiography is useful for the follow‑up of ASA for assessment of procedural success, detecting complications and guiding further therapy, if indicated. We define success of ASA as improvement by at least one New York Heart Association heart failure or Canadian Cardiovascular Society angina class, accompanied by a sustained reduction in the resting or provocable gradient by ≥50% [39]. If symptoms and evidence of LVOT obstruction persist 3–6 months after ASA, patients can be considered for repeat ASA or surgical myectomy. The choice of appropriate follow-up therapy is guided by echocardiographic and angiographic appearance. There needs to be sufficient residual septal thickness at the site of obstruction (SAM–septal contact) and this segment needs to be supplied by an additional accessible septal perforator branch. If there is no target septal perforator supplying the offending area of septum, then septal myectomy should be considered (Figure 9). If obstruction is occurring more apical to the site of prior ASA, repeat ASA can be considered (Figure 10).
Figure 9: Residual septal hypertrophy after alcohol septal ablation. Residual
upper septal hypertrophy with systolic anterior motion and left ventricular outflow
tract obstruction (dashed arrow) closer to the aortic valve than the remodeled
area (solid arrow), 6 months following alcohol septal ablation via the first septal
perforator. No other suitable septal perforators were available, so this patient
was referred for septal myectomy.
LA: Left atrium; LV: Left ventricle.
Figure 10: Apical five chamber view of a patient in which a left ventricular
outflow tract gradient reappeared 1 year after alcohol septal ablation. Echo demonstrated residual systolic anterior motion at the site of residual
septal hypertrophy (arrow). Since this patient had another suitable target septal
perforator, a repeat ablation was performed, with successful gradient and
symptom relief (with 4 years of follow-up available).
LA: Left atrium; LV: Left ventricle; RA: Right atrium; RV: Right ventricle.
Future perspective
In the decade and a half since ASA was introduced, our understanding of the risks and benefits of the procedure has grown. Integration of clinical and anatomic data has been shown to be imperative for appropriate patient selection. Echocardiography has emerged as a critical tool in the screening process and for follow-up after ASA. Moreover, the addition of contrast echocardiography during ASA has led to significant reduction in complications, as well as increased procedural success.
As novel techniques in echocardiography become more broadly disseminated, real-time 3D echocardiography may allow for a better understanding of the dynamic 3D structure of the LVOT during SAM, which may help echocardiographers select a more discrete target area prior to ASA. Additionally, 3D echocardiography may play a role in following patients after ASA, to assess infarct size and remodeling of the LVOT. This technique may be particularly useful in individuals with residual gradients after ASA in deciding whether repeat ASA or myectomy would be preferable. Lastly, there have been reports of the use of echocardiographic contrast during real-time 3D imaging to assess myocardial perfusion [40,41]. As the field advances, the use of this technique to assess target area during ASA may become feasible.
Cardiac computed tomography has also been described as a potential tool for screening patients prior to ASA, as well as in evaluating the LVOT geometry after the procedure. A recent case report described the ability of electrocardiography-gated multidetector computed tomography to detect the degree and extent of septal hypertrophy, identify the point of SAM–septal contact and define septal perforator anatomy noninvasively prior to ablation [42]. Further investigation is warranted to determine whether this technique will be feasible as a noninvasive screening tool in a larger number of patients with HOCM.
Cardiac MRI is also a useful tool in assessing LVOT geometry and the precise location and extent of hypertrophy prior to consideration of ASA. An additional advantage of cardiac MRI is its ability to accurately assess infarct size and extent of fibrosis after the procedure [37,43].
While the roles of multidetector computed tomography and cardiac MRI may expand in the future, the portability, cost and widespread availability of echocardiography will ensure its continued importance for both screening and procedural guidance for ASA.
Recently, a new approach to reducing septal thickness in HOCM has been described in a small number of patients using endocardial radiofrequency ablation of septal hypertrophy [44,45]. As experience with this technique grows, it may become an acceptable treatment option for the small subset of symptomatic patients in whom neither ASA nor myectomy are feasible.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪▪ Optimal patient selection for alcohol septal ablation (ASA) requires careful clinical and echocardiographic assessment.
▪▪ Patients considered for ASA should have symptoms that interfere substantially with lifestyle despite optimal, often combination, medical therapy.
▪▪ Echocardiography is critical for identifying a resting or provocable gradient and sufficient septal thickness at the point of systolic anterior motion–septal contract.
▪▪ Echocardiography may also identify conditions such as extreme septal hypertrophy, predominantly midcavity gradient, structural abnormality of the mitral valve, subaortic membrane or valvular aortic stenosis, which constitute absolute or relative contraindications to ASA.
▪▪ Intraprocedural transthoracic echocardiography allows for targeted administration of the smallest dosage of ethanol that ‘covers’ the area of systolic anterior motion–septal contact and brings about an acute reduction in left ventricular outflow tract gradient.
▪▪ Echocardiography identifies perfusion of myocardial segments into which injection of ethanol would be inappropriate.
▪▪ Follow-up echocardiography is integral to the assessment of the success of ASA and to the selection of further therapy if required.
References
Papers of special note have been highlighted as:
▪ of interest
- Spirito P, Seidman CE, Mckenna WJ, Maron BJ. The management of hypertrophic cardiomyopathy. N. Engl. J. Med. 336(11), 775–785 (1997).
- Wigle ED, Sasson Z, Henderson MA et al. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Prog. Cardiovasc. Dis. 28(1), 1–83 (1985).
- Wigle ED, Rakowski H, Kimball BPP, Williams WG. Hypertrophic cardiomyopathy. Clinical spectrum and treatment. Circulation 92(7), 1680–1692 (1995).
- Minami Y, Kajimoto K, Terajima Y et al. Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 57(23), 2346–2355(2011).
- Maron MS, Olivotto I, Zenovich AG et al. Hypertrophic cardiomyopathyis predominantly a disease of left ventricular outflow tract obstruction. Circulation 114(21), 2232–2239(2006).
- Shah JS, Esteban MT, Thaman R et al. Prevalence of exercise-induced left ventricular outflow tract obstruction in symptomatic patients with non-obstructive hypertrophic cardiomyopathy. Heart 94(10), 1288–1294 (2008).
- Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 346(8969), 211–214 (1995).
- Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation 116(2), 196–206; discussion 206 (2007).
- Fifer MA. Controversies in cardiovascular medicine. Most fully informed patients choose septal ablation over septal myectomy. Circulation 116(2), 207–216; discussion 216(2007).
- Ommen SR, Maron BJ, Olivotto I et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy.J. Am. Coll. Cardiol. 46(3), 470–476(2005).
- Fifer MA, Sigwart U. Controversies in cardiovascular medicine. Hypertrophic obstructive cardiomyopathy: alcohol septal ablation. Eur. Heart J. 32(9), 1059–1064 (2011).
- Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation 117(3), 429–439 (2008).
- Sherrid MV, Gunsburg DZ, Pearle G. Mid-systolic drop in left ventricular ejection velocity in obstructive hypertrophic cardiomyopathy – the lobster claw abnormality. J. Am. Soc. Echocardiogr. 10(7), 707–712 (1997).
- Sherrid MV, Chu CK, Delia E, Mogtader A, Dwyer EM Jr. An echocardiographic study of the fluid mechanics of obstruction in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 22(3), 816–825 (1993).
- Pollick C, Rakowski H, Wigle ED. Muscular subaortic stenosis: the quantitative relationship between systolic anterior motion and the pressure gradient. Circulation 69(1), 43–49 (1984).
- Ommen SR, Nishimura RA, Squires RW, Schaff HV, Danielson GK, Tajik AJ. Comparison of dual-chamber pacing versus septal myectomy for the treatment of patients with hypertrophic obstructive cardiomyopathy: a comparison of objective hemodynamic and exercise end points. Am. Coll. Cardiol. 34(1), 191–196 (1999).
- Seggewiss H. Current status of alcohol septal ablation for patients with hypertrophic cardiomyopathy. Curr. Cardiol. Rep. 3(2), 160–166 (2001).
- Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. Engl. J. Med. 342(24), 1778–1785 (2000).
- Cecchi F, Olivotto I, Nistri S, Antoniucci D, Yacoub MH. Midventricular obstruction and clinical decision-making in obstructive hypertrophic cardiomyopathy. Herz. 31(9), 871–876 (2006).
- Kovacic JC, Khanna D, Kaplish D, Karajgikar R, Sharma SK, Kini A. Safety and efficacy of alcohol septal ablation in patients with symptomatic concentric left ventricular hypertrophy and outflow tract obstruction. Invasive Cardiol. 22(12), 586–591 (2010).
- Luria D, Klutstein MW, Rosenmann D, Shaheen J, Sergey S, Tzivoni D. Prevalence and significance of left ventricular outflow gradient during dobutamine echocardiography. Eur. Heart J. 20(5), 386–392 (1999).
- Fernandes VL, Nagueh SF, Wang W, Roberts R, Spencer WH 3rd. A prospective follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy – the Baylor experience (1996–2002). Clin. Cardiol. 28(3), 124–130 (2005).
- Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. One-year follow-up of percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy in 312 patients: predictors of hemodynamic and clinical response. Clin. Res. Cardiol. 96(12), 864–873(2007).
- Lakkis N, Plana JC, Nagueh S, Killip D, Roberts R, Spencer WH 3rd. Efficacy of nonsurgical septal reduction therapy in symptomatic patients with obstructive hypertrophic cardiomyopathy and provocable gradients. Am. J. Cardiol. 88(5), 583–586 (2001).
- Gietzen FH, Leuner CJ, Obergassel L, Strunk-Mueller C, Kuhn H. Role of transcoronary ablation of septal hypertrophy in patients with hypertrophic cardiomyopathy, New York Heart Association functional class III or IV, and outflow obstruction only under provocable conditions. Circulation 106(4), 454–459 (2002).
- Gietzen FH, Leuner CJ, Obergassel L, Strunk-Mueller C, Kuhn H. Transcoronary ablation of septal hypertrophy for hypertrophic obstructive cardiomyopathy: feasibility, clinical benefit, and short term results in elderly patients. Heart 90(6), 638–644 (2004).
- Veselka J, Duchonova R, Palenickova J et al. Age-related hemodynamic and morphologic differences in patients undergoing alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Circ. J. 70(7), 880–884 (2006).
- Faber L, Seggewiss H, Gietzen FH et al. Catheter-based septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: follow-up results of the TASH-registry of the German Cardiac Society. Z. Kardiol. 94(8), 516–523 (2005).
- Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J. Interv. Cardiol. 19(4), 319–327 (2006).
- Valeti US, Nishimura RA, Holmes DA et al. Comparison of surgical septalmyectomy and alcohol septal ablation with cardiac magnetic resonance imaging in patients with hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 49(3), 350–357 (2007).
- Faber L, Seggewiss H, Gleichmann U. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: results with respect to intraprocedural myocardial contrast echocardiography. Circulation 98(22), 2415–2421 (1998).
- Lakkis NM, Nagueh SF, Kleiman NS et al. Echocardiography-guided ethanol septal reduction for hypertrophic obstructive cardiomyopathy. Circulation 98(17), 1750–1755 (1998).
- Faber L, Seggewiss H, Ziemssen P, Gleichmann U. Intraprocedural myocardial contrast echocardiography as a routine procedure in percutaneous transluminal septal myocardial ablation: detection of threatening myocardial necrosis distant from the septal target area. Catheter Cardiovasc. Interv. 47(4), 462–466 (1999).
- Faber L, Seggewiss H, Welge D et al. Echo-guided percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: 7 years of experience. Eur. J. Echocardiogr. 5(5), 347–355 (2004).
- Monakier D, Woo A, Puri T et al. Usefulness of myocardial contrast echocardiographic quantification of risk area for predicting postprocedural complications in patients undergoing septal ethanol ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 94(12), 1515–1522 (2004).
- Baggish AL, Smith RN, Palacios I et al. Pathological effects of alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Heart 92(12), 1773–1778 (2006).
- Schulz-Menger J, Strohm O, Waigand J, Uhlich F, Dietz R, Friedrich MG. The value of magnetic resonance imaging of the left ventricular outflow tract in patients with hypertrophic obstructive cardiomyopathy after septal artery embolization. Circulation 101(15), 1764–1766 (2000).
- Veselka J, Duchonova R, Prochazkova S et al. The biphasic course of changes of left ventricular outflow gradient after alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Kardiol. Pol. 60(2), 133–136; discussion 137 (2004).
- Yoerger DM, Picard MH, Palacios IF, Vlahakes GJ, Lowry PA, Fifer MA. Time course of pressure gradient response after first alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 97(10), 1511–1514 (2006).
- Toledo E, Lang RM, Collins KA et al. Imaging and quantification of myocardial perfusion using real-time three-dimensional echocardiography. J. Am. Coll. Cardiol. 47(1), 146–154 (2006).
- Veronesi F, Caiani EG, Toledo E et al. Semi-automated analysis of dynamic changes in myocardial contrast from real-time three-dimensional echocardiographic images as a basis for volumetric quantification of myocardial perfusion. Eur. J. Echocardiogr. 10(4), 485–490 (2009).
- Ghersin E, Soto V, Heldman AW. Multidetector computerized tomography can guide and document alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Circulation 123(2), e5–e7 (2011).
- Sohns C, Sossalla S, Schmitto JD et al. Visualization of transcoronary ablation of septal hypertrophy in patients with hypertrophic obstructive cardiomyopathy: a comparison between cardiac MRI, invasive measurements and echocardiography. Clin. Res. Cardiol. 99(6), 359–368(2010).
- Lawrenz T, Borchert B, Leuner C et al. Endocardial radiofrequency ablation for hypertrophic obstructive cardiomyopathy: acute results and 6 months’ follow-up in 19 patients. J. Am. Coll. Cardiol. 57(5), 572–576 (2011).
- Sreeram N, Emmel M, De Giovanni JV. Percutaneous radiofrequency septal reduction for hypertrophic obstructive cardiomyopathy in children. J. Am. Coll. Cardiol. 58(24), 2501–2510 (2011).
▪ Description of alcohol septal ablation performed in the first three patients.
▪ The two preceding references frame the debate about choice of appropriate therapy for patients with hypertrophic obstructive cardiomyopathy and symptoms refractory to optimal medical therapy.
▪ The rationale for contrast echocardiography guidance during alcohol septal ablation.
▪ The rationale for contrast echocardiography guidance during alcohol septal ablation.