Review Article - Interventional Cardiology (2011) Volume 3, Issue 1
Percutaneous approaches to closure of coronary artery fistulas
- Corresponding Author:
- Mehmet Ali Oto
Hacettepe University Faculty of Medicine
Department of Cardiology
Altındağ 06100, Ankara, Turkey
Tel: +90 312 305 1780
Fax: +90 312 305 4137
E-mail: alioto@superonline.com
Abstract
Keywords
coils, coronary artery fistula, detachable balloons, occluder devices, percutaneous closure, surgical repair
Coronary artery fistula (CAF) is an uncommon entity, which is associated with several potential complications, such as heart failure, myocardial ischemia, infective endocarditis and even rupture. Over the past few years a surgical approach has been the standard therapeutic option. However, recently, progress has been made in percutaneous interventional methods, with proven efficacy and safety.
In this article, we review the percutaneous methods used for the closure of a CAF. The comparison of several techniques is made to highlight the current treatment options in this rare abnormality.
Definition
Coronary artery fistula is defined as an abnormal communication – bypassing a capillary system – between a normal coronary artery and another cardiovascular structure, including cardiac chambers, veins or intrathoracic vessels. Previously, persistence of embryonal vascular sinusoids in the myocardium was the proposed mechanism for the formation of a CAF; however, the exact course of its embryogenesis is not yet clear [1]. According to the Hackensellner’s involution–persistence theory, persistence of pulmonary buds with accompanying involution of aortic buds results in the formation of coronary arteries from pulmonary buds [2]. CAFs may originate from any of the three major coronary arteries, including the left main trunk. The most frequent site of origin is the right coronary artery, followed by the left anterior descending artery. Over 90% of CAFs drain into the low-pressure venous system or right heart chambers [3].
Epidemiology, classification & pathophysiology
▪ Epidemiology
Coronary artery fistulas are rare abnormalities. The reported incidence of CAFs in the literature varies depending on the diagnostic method or ethnic group of the study population. As most CAFs are small or asymptomatic, true incidence is not known. Angiographic series reveal an incidence of 0.08–0.22% [4–7].
▪ Classification
Coronary artery fistulas are divided into two groups: solitary CAFs and coronary artery left ventricular multiple microfistulas. Most of the solitary CAFs are congenital and not gender-specific [8]. In addition to congenital CAFs, acquired forms can be developed due to chest trauma, Takayasu arteritis, endomyocardial biopsy, cardiac surgery, pacing-lead erosion and complications during percutaneous coronary angioplasty procedures [9–14]. Although the size of the CAF is an important determinant of intervention, there is no consensus regarding the absolute size of a CAF for categorization. Latson et al. deliberately classified the CAF as: small fistulas (not larger than twice the diameter of the coronary artery at any point and do not cause coronary artery dilatation); medium-sized fistulas (larger than twice the diameter of the coronary artery but less than three-times as big); and large fistulas (larger than three-times the expected proximal normal coronary artery diameter) [15]. In the Dutch Registry, Said et al. defined small fistulas as having a vessel diameter of less than 2 mm, medium-sized fistulas between 2 and 8 mm and large fistulas over 8 mm [16].
▪ Pathophysiology
‘The coronary steal phenomenon’ is the most widely accepted hypothesis for the ischemic symptoms of CAF. Myocardial segments distal to the fistula remain ischemic due to the diversion of coronary flow to the lower-pressure areas throughout the fistula and compensatory dilatation occurs in the proximal segment. High-flow fistula may also cause volume overload and increase pulmonary flow due to left-to-right shunt, resulting in heart failure or pulmonary hypertension. Usually, small CAFs have a benign natural history and even spontaneous regression has been reported [4,17]. However, large CAFs may progressively enlarge and the affected coronary artery and chamber may be dilated over time due to increased blood flow [18].
Clinical presentation
Although CAFs are uncommon and usually incidentally detected abnormalities, they may cause significant morbidity and mortality in any age group. The adult population is mostly asymptomatic unless the fistula causes significant hemodynamic shunt (pulmonary flow/systemic flow ≥2) or complications causing myocardial ischemia, heart failure, infective endocarditis or rupture of dilated fistulas. The symptoms and complications of medium- to large-sized CAFs tend to be seen in the adult age group [19]. They may present as angina pectoris, atypical chest pain, dyspnea, dizziness, syncope, fatigue, palpitations or even sudden cardiac death [8]. Patent ductus arteriosus, pulmonary arteriovenous fistula, ruptured sinus of Valsalva aneurysm, aortopulmonary window, internal mammary artery to pulmonary artery fistula, anomalous origin of left coronary artery or right coronary artery from the pulmonary artery and systemic arteriovenous fistula should be considered in differential diagnosis.
▪ Diagnostic evaluation
In children, the course and drainage sites of anomalous coronary arteries can be demonstrated by transthoracic echocardiography owing to an optimal acoustic window. In adults, transesophageal echocardiography is more useful, particularly in proximal lesions [20]. Although cardiac catheterization and coronary angiography is the gold-standard method to obtain the best hemodynamic data, shunt calculation and visualization of coronary anatomy, emerging imaging modalities such as multidetector computed tomography and MRI can accurately delineate the anatomy of coronary vessels and the origin, course and drainage site of the fistula [21,22].
Indications for intervention
Several factors including patient age, size, morphology and anatomy of a fistula and associated cardiac disorders should be considered for the best management of CAFs [23–27]. Traditionally, asymptomatic patients with small shunts are managed conservatively owing to the benign course and the possibility of spontaneous closure. In symptomatic patients presenting with myocardial ischemia, b-blockers or calcium channel blockers may relieve the symptoms by decreasing myocardial oxygen demand [28]. Symptomatic patients with left-to-right shunt and complications including myocardial ischemia, congestive heart failure, pulmonary hypertension, dysrhytmias and infective endocarditis should undergo interventional procedure. Percutaneous closure can be performed in the case of CAF associated with infective endocarditis. There is still controversy regarding intervention for large shunts in asymptomatic patients because of the lack of data about the natural history of unintervened CAF. Despite the case reports of spontaneous closure or nonprogression, several studies recommend closure of large CAFs owing to the risk of future complications [29–32]. According to the current American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for adults with congenital heart disease, a large CAF should be closed regardless of symptoms and small-to-moderate CAFs should be closed in the presence of documented myocardial ischemia, arrhythmia, otherwise unexplained ventricular systolic or diastolic dysfunction, enlargement or endarteritis [33]. Therefore, all of the major CAFs should be treated as soon as the diagnosis is made.
Principles of treatment
▪ Surgery versus interventional closure In a series examining surgical closure, good short- and long-term results were reported. In a surgical review of CAF in 17 patients consisting of infants and children, early surgical management of CAF was reported to be a safe and effective treatment resulting in a 100% survival and 100% closure rate [34]. In a study consisting of 41 patients with CAF, no operative mortality was reported after surgery and 96.9% of the patients were asymptomatic at a mean followup duration of 9.1 years [35]. In another surgical closure study by Wang et al. that included 52 patients, no residual shunt was reported before hospital discharge with good follow-up results for a mean period of 3.14 ± 1.84 years [36]. Although contemporary studies report no death after surgical closure, the surgical approach is not free of mortality and morbidity [19,23,36]. Accompanying congenital anomalies were more common in the surgical literature compared with percutaneous closure studies.
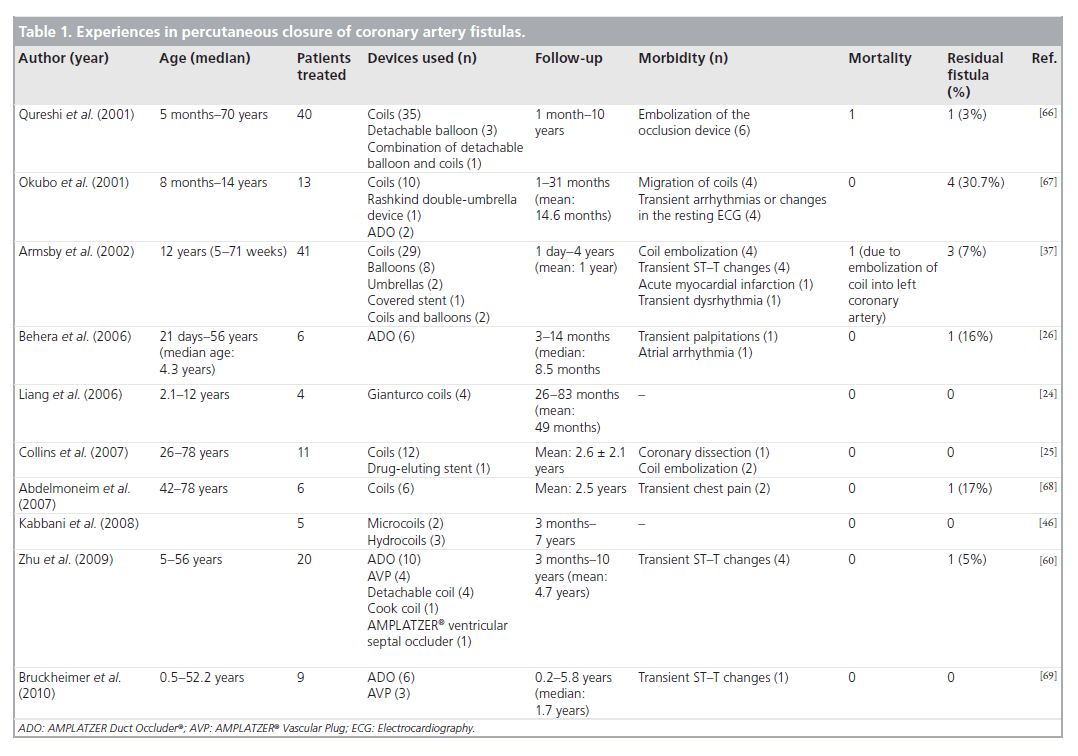
Despite the effectiveness of surgical closure, a similar success rate for transcatheter closure made the emerging percutaneous techniques an important alternative to surgery. In addition to the proven safety and efficacy, percutaneous techniques do not have many of the disadvantages of the surgical approach, which include sternotomy, cardiopulmonary bypass and longer hospital stay [37,38]. Although surgery seems to be associated with higher procedural success, it should be stressed that most of the available data were from single-center experiences in small patient groups. Furthermore, recurrence or residual shunts may also occur in approximately 10% of the patients who undergo surgery [35]. In a study including the report of short-term findings in 33 patients after the percutaneous closure of CAF by Armsby et al., complete occlusion was accomplished in 27 patients (82%) without procedural death or long-term morbidity in a median follow-up of 2.8 years [37]. These results showed similar early effectiveness, morbidity and mortality when compared with the surgical literature (Table 1).
Table 1. Experiences in percutaneous closure of coronary artery fistulas.
▪ Technical details & devices
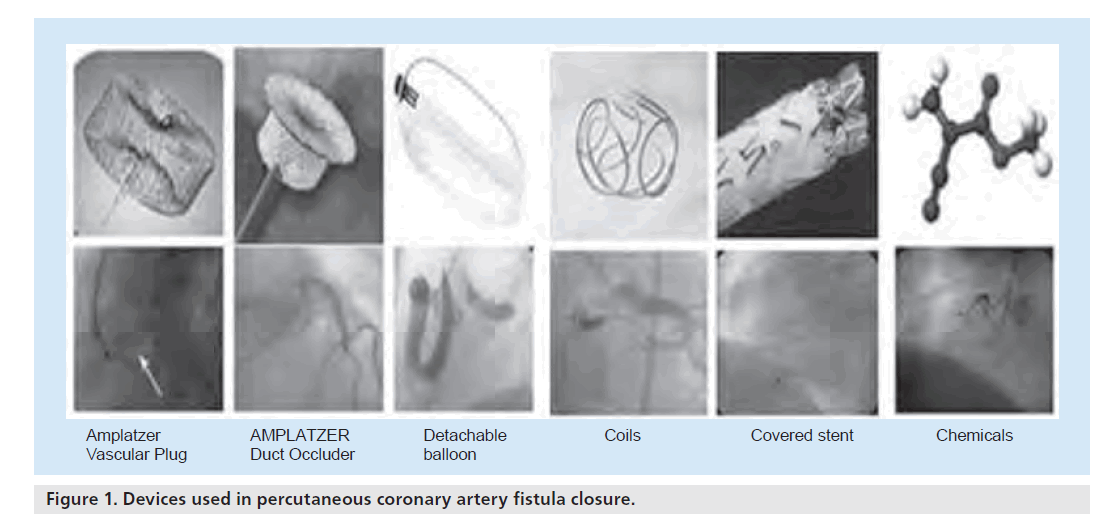
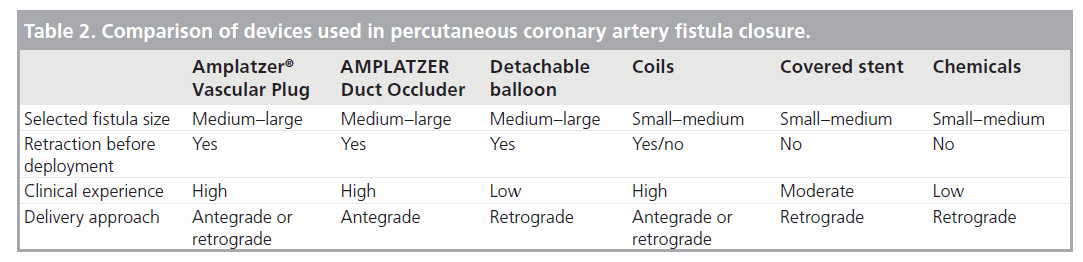
Since the first percutaneous intervention by Reidy et al. [39], various devices have been introduced for the transcatheter closure of CAFs, including Gianturco coils, stainless-steel coils, detachable balloons, platinum microcoils, double-umbrella devices, covered stents, AMPLATZER® Duct Occluder (ADO), AMPLATZER® Vascular Plug (AVP), polyvinyl alcohol foam and several chemicals including cyanoacrylate. The comparison of percutaneous methods used for the closure of CAF is illustrated in Figure 1 & Table 2. The most commonly used methods will be reviewed in the following sections.
Figure 1. Devices used in percutaneous coronary artery fistula closure.
Table 2. Comparison of devices used in percutaneous coronary artery fistula closure.
Coil embolization
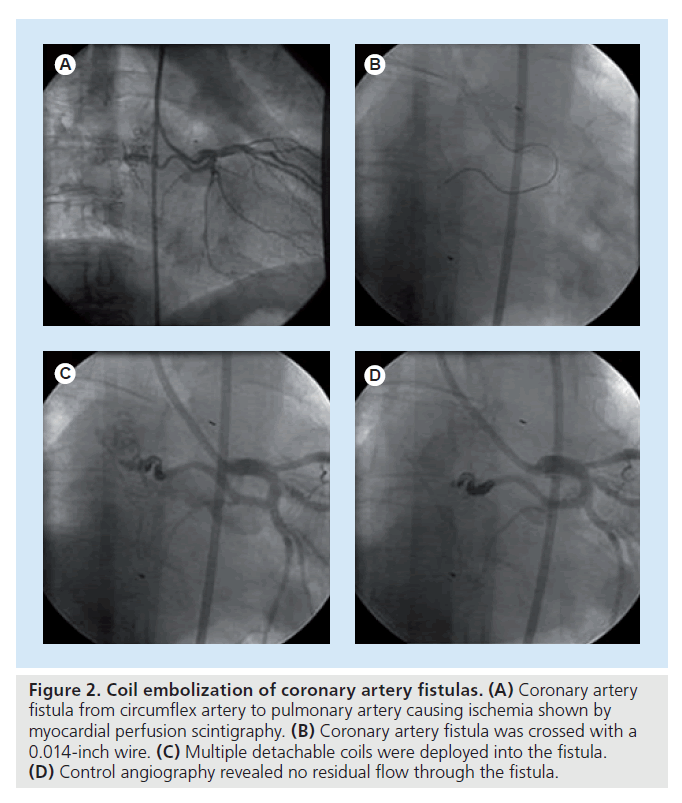
Currently, coil embolization is the most commonly preferred method for percutaneous closure of CAF either by retrograde or anterograde approaches (Figure 2). In case of multiple feeders, coil embolization should not be preferred because closure of CAF is difficult with a single intervention. Distally located fistulas, tortuous anatomy, an adjacent vessel at risk, the need for concomitant coronary bypass and larger fistulas do not favor coil embolization [34]. The potential complications are transient arrhythmias, coil embolization into the great vessels or recoil into the major coronary artery leading to acute myocardial infarction and, occasionally, sudden death. The coil needs to be up to 30% larger than the area of target vessel to avoid migration and embolization of the coil. After the implantation of the first coil in the correct position, different sizes of coils can be deployed until complete occlusion is achieved [40].
Figure 2. Coil embolization of coronary artery fistulas. (A) Coronary artery fistula from circumflex artery to pulmonary artery causing ischemia shown by myocardial perfusion scintigraphy. (B) Coronary artery fistula was crossed with a 0.014-inch wire. (C) Multiple detachable coils were deployed into the fistula. (D) Control angiography revealed no residual flow through the fistula.
Coils are made of metallic wires with variable size, shape and rigidity, which are either pushable or detachable. Coils are mainly divided into two groups: standard steel coils (Gianturco coils) and platinum microcoils [41,42]. Generally, coils are preferred in the occlusion of small fistulas; however, pushable coils such as Gianturco coils have been used in large and aneurysmal fistulas [43]. Platinum microcoils are available with or without fibers embedded into the device. Electrolytically detachable coils have also been used to occlude CAFs and can easily be retrieved from the delivery catheter until a precise location is found. These coils have the advantage of electrothrombosis to help the closure of the fistula [44]. Interlocking detachable coils have the advantage of retraction and repositioning before delivery, as do the electrolytically detachable coils. Until the optimal position is achieved, these coils can be easily manipulated, in contrast to the traditional platinium or steel coils, which cannot be retrieved or retracted before deployment. Furthermore, these coils contain Dacron® fibers to promote thrombogenicity. Flexibility and delivery in small catheters (<3 Fr) made those coils an attractive option, especially in tortuous fistula anatomy [45]. Hydrocoils are made of hybrid material and have the potential to expand up to nine-times their volume after contact with blood owing to an outer layer of hydrophilic, acrylic polymer gel, and are used successfully to occlude large CAFs [46].
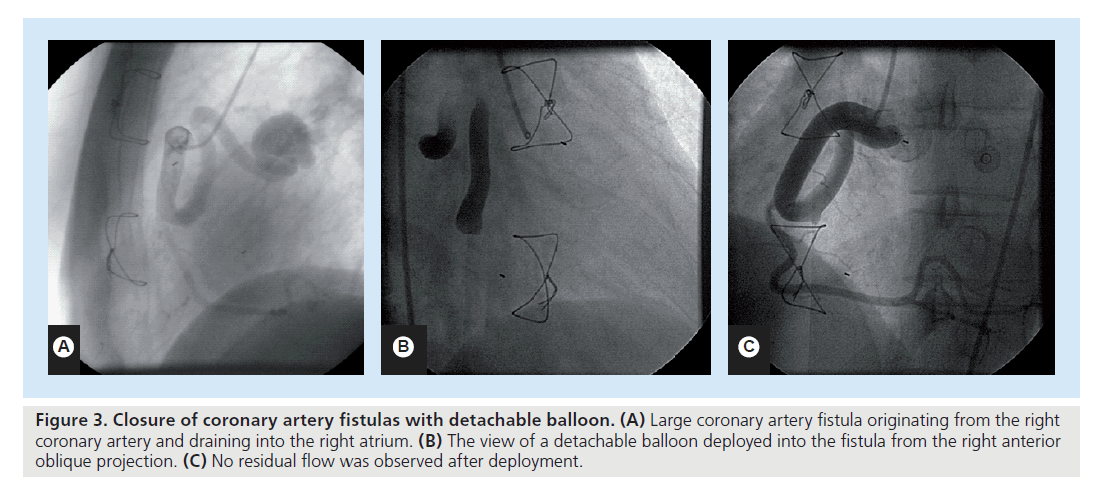
Detachable balloons
Detachable balloons are no longer preferred in the closure of CAFs since the advent of newer methods. They require large introducer catheters and are inflated with contrast medium or silicone material (Figure 3). They are floated within the arterial vessel with the direction of blood flow and immediate occlusion is achieved when the balloon is inflated and detached. Detachable balloons are a reliable and safe technique for occluding large CAF, with several advantages over other methods, which are:
Figure 3. Closure of coronary artery fistulas with detachable balloon. (A) Large coronary artery fistula originating from the right coronary artery and draining into the right atrium. (B) The view of a detachable balloon deployed into the fistula from the right anterior oblique projection. (C) No residual flow was observed after deployment.
▪The effects of occlusion on myocardium can be assessed before the final release of the balloon;
▪Detachable balloons can be easily inflated and constructed to fit the size of the fistula;
▪Balloons can be easily controlled;
▪They can be directed to the lesion site easily from the coronary artery because of the same direction of blood flow.
Immediate and reversible occlusion can be achieved with these balloons during detachment. After the withdrawal of the microcatheter, the balloons are closed by a valve. Detachable balloon is a reliable and safe way to occlude medium- to large-sized CAFs. However, owing to infrequent use, available data are mainly limited to occasional case reports [47,48].
AMPLATZER® Vascular Plug
The AMPLATZER® Vascular Plug is a selfexpandable, cylindrical device made of nitinol mesh wires and is used in a variety of lesions including CAFs. As with other devices, the diameter of the device should be sized at 30–50% larger than the diameter of the reference vessel at the intended point of occlusion [27]. Moreover, unlike other AMPLATZER occlusion devices, the the AVP has no occlusive material. The main advantage of AVP is the high rate of occlusion without the need for delivery of multiple occlusion devices, as complete occlusion can be achieved with a single device.
AMPLATZER® Duct Occluder
The ADO is a self-expandable device used in the closure of patent ductus arteriosus, made of nitinol wire mesh. Polyester material within the device causes thrombosis and closure of the fistula. The device size should be 30% greater than the vessel diameter. The ADO should be placed at a location in the proximal portion of the fistula at a narrow site, so the device can be anchored to the area of interest. The reported advantages of ADOs in CAFs include single device usage, a high ratio of complete occlusion, antegrade placement, ease of placement and improved control during deployment of the device.
Covered stents
Covered stents are made of a membrane integrated into a coronary stent. These stents are mostly used to seal degenerated vein grafts and cover coronary artery perforations, aneurysms and fistulas [49,50]. Mostly, stents are covered with polytetrafluoroethylene material. Recently, covered stents have been introduced as an important alternative technique for closure of CAFs. Covered stents preserve the main vessel patency while occluding flow through the fistula. Covered stents are mainly preferred in cases of an accompanying coronary atherosclerotic lesion near to the origin of the CAF and may also be useful in plexus-like CAFs, in which the implantation of a single device is insufficient for the closure of the CAF [51,52]. The main limitations of covered stents are side branch occlusion, mismatch between proximal and distal vessel diameter (as seen in many patients with large CAFs) and a high risk of in-stent restenosis and stent thrombosis [53,54].
Chemicals
Embolization of fistulas with chemical materials is an alternative method for the percutaneous closure of CAFs. Mainly particulate materials such as gel foam and polyvinyl alcohol foam or liquid materials such as N-butyl cyanoacrylate have been used. Gel foam is composed of gelatin sponge, and polyvinyl alcohol foam is composed of synthetic plastic foam. Thrombus formation around those particles is the mainstay of the embolization procedures. N-butyl cyanoacrylate is a type of glue and this agent polymerizes immediately after contact with blood, which causes vessel occlusion. Successful results of percutaneous embolization of CAF with cyanoacrylate [55], polyvinyl alcohol foam [56] and gel foam [43] have been reported. In addition to isolated use in occluded CAFs, cyanoacrylate may be combined with coils in the case of residual flow after coil embolization.
▪ Tips & tricks
Percutaneous closure of CAFs is mainly preferred in cases of proximal origin of fistula, the absence of multiple feeders, with a lack of adjacent coronary artery branches and for drainage to lowpressure areas [37,42]. Multiple drainage sites, extreme vessel tortuosity, aneurysm formation, acute angulation, close location of a side branch to a drainage site and distal location of the lesion not allowing delivery of a catheter are the main limitations for the percutaneous approach [34,37]. The selection of occlusion devices mostly depends on the age of the patient, associated cardiac diseases and the anatomic characteristics of the fistula. The therapeutic approach does not differ according to the type of CAF (either congenital or acquired).
Selective catheterization of the fistula and imaging the entire coronary anatomy is the most important step in percutaneous intervention. In cases of high-flow fistulas, balloon occlusion angiography can be used to identify all coronary and fistula anatomy and drainage sites. Selective cannulation of fistulas with small diameter-guiding catheters (e.g., 4 or 5 Fr) is a widely used method for proper positioning [25]. In cases of tortuous anatomy, cannulation of the coronary ostium with a large diameter-guiding catheter (e.g., 8 Fr) and afterwards, either hightorque floppy wires or a delivery catheter passing through this large guiding catheter can be used for positioning at the closure site of the fistula. In these cases, the 3-Fr Tracker (Target Therapeutics, CA, USA) or Micro Ferret catheter (Cook Medical, IN, USA) are commonly used to reach the target area and, afterwards, detachable microcoils can be deployed with the guidance of a 0.014-inch coronary guidewire. To prevent device migration, positioning the catheter proximal to the narrowest area of the fistula is essential for procedural success. Temporary balloon occlusion may be needed for the appropriate positioning of coils in high-flow CAF. Another strategy is to create an arteriovenous loop with a wire by crossing through the entire fistula from arterial site to venous site with a snare, so as to carry the devices in a large CAF. Coils are mostly preferred in cases of small-to-moderate CAF.
Deployment of a coil that is too large for the vessel results in insufficient wrapping and the device remains elongated, therefore causing insufficient closure. Conversely, coils that are too small carry the risk of embolization. The size of the AVPs and ADOs should be 30% greater than the target vessel area, as with other devices, for appropriate deployment. These devices have the advantage of closure with a single device and can be used in large fistulas compared with coils. AMPLATZER devices are MRI compatible and may be selected in patients in whom medical status may require additional imaging. Currently, AVPs and ADOs are preferred in cases of large CAFs instead of detachable balloons. AVPs and ADOs also have the advantage of fistula occlusion with a single device. The AVP can be used in cases of multiple CAFs draining into a single area. The main advantage of covered stents is the exclusion of fistulous anatomy in device selection. Furthermore, covered stents can be used in patients with coexistent coronary atherosclerosis at the target occlusion area.
The main aim of catheter intervention in CAF is to occlude the fistula artery at a precise point, away from the native coronary artery. However, sometimes, if the embolization of CAF is too distal, it may cause migration of an occluding device or material into the relevant cardiac structures and pulmonary circulation. Closure device delivery should be distal enough within the fistula that the native coronary circulation is protected from device migration, clot propagation and side branch occlusion. Distal location of the fistula, presence of multiple feeders, tortuosity, side branch lesion, acute angulation, aneurysm formation and accompanying cardiac anomalies favor surgical intervention. However, percutaneous intervention can be performed in most of these cases with appropriate equipment. For example, in cases of high flow across the fistula, flow can be stopped with a balloon prior to intervention or superfloppy guidewires can be used in tortuous vessels. Despite early reports stating that a CAF with multiple drainage sites precluded percutaneous intervention, progress in experience and improvements in equipment and occlusion devices have enabled the use of transcatheter closure in CAFs with multiple drainage sites [37]. In cases where the fistula is located near to the origin of an adjacent vessel, percutaneous closure can be safely performed when the fistula is selectively and distally cannulated. The anatomical structure of fistulous vessel aneurysm, atherosclerosis and histologic abnormalities in the vessel wall may lead to complications [57]. Therefore, particularly in percutaneous interventions in large fistulas, gentle manipulation of catheters and devices is essential to prevent dissection or vessel rupture [37].
Multiple CAFs constitute 10% of all CAFs [58]. There are controversies about the optimal management of multiple CAFs, either percutaneous or surgical interventions. Mostly, multiple CAFs are referred for surgical closure; however, in some cases, percutaneous closure can be performed [27].
Mostly, retrograde approaches are used in percutaneous intervention to occlude CAFs. The main advantage of a retrograde approach is familiarity with this approach but, in some cases, an antegrade approach can be performed to occlude the fistula (e.g., short feeding vessel). The use of larger catheters, a straighter catheter course and avoiding femoral artery access are the potential advantages of the antegrade approach, despite the risk of device embolization due to lack of flow control.
A thorough analysis of past reports indicates that all symptomatic patients and asymptomatic patients with large CAFs should undergo closure of CAFs. Owing to the unusual nature of this abnormality, firm recommendations relevant to the optimal management are difficult to make. However, progress in technical and interventional tools extended the current concept of percutaneous intervention to CAFs. Furthermore, percutaneous interventions can be performed in cases with challenging coronary anatomy.
Complications, outcomes & follow-up
Early complications related to occlusion procedures are transient myocardial ischemia or dysrhythmias, myocardial infarction, distal coronary spasm, fistula dissection, device embolization and, very rarely, procedural death [37]. Residual flow is seen in approximately 10% of patients [59]. Zhu et al. reported that the trivial–mild residual incidence was 25% and recurrence was 5% among 24 patients after percutaneous closure in a mean follow-up of 4.7 ± 3.2 years [60]. Examining the use of a surgical approach, Rittenhouse et al. reported a recurrence incidence of 4% [61]. In fact, most of the recurrences were small and did not require additional intervention [62].
Coronary artery dilatation may persist after intervention, either with percutaneous closure or surgical ligation, in intermediate to long-term follow-up [63]. Rupture of CAFs may occur in patients, independent of preceding dilation. Therefore, close follow-up is warranted, especially for thin-walled ectatic arteries. Although there is not a consensus regarding the antithrombotic or anticoagulant therapy for dilated coronary arteries after closure, some authors advocate anti-platelet therapy for dilated coronary arteries and anticoagulation with warfarin in severe coronary artery dilatation (>10 mm), especially in patients with sluggish coronary flow [63].
A small CAF has the most favorable course. Sherwood et al. reported that a small asymptomatic CAF had a benign natural history and patients were free of complications at a mean follow-up of 9.3 years [64]. Cheung and coauthors concluded that the benefits of surgery are uncertain for asymptomatic patients with mild shunting and regular follow-up has been recommended; surgical correction should be planned when symptoms develop or shunt increases [35]. In cases of asymptomatic small fistulas, routine clinical evaluation combined with imaging including both ventricular dimensions and fistula morphology is reasonable because of the risk of increase in size with time.
The follow-up algorithm is limited for CAF after intervention. Long-term follow-up is essential owing to the possibility of postprocedural recanalization or residual flow, persistent dilation of the coronary artery, late thrombosis and myocardial ischemia. Therefore, even though most patients become asymptomatic after intervention, they should not be dismissed from follow-up. Clinical evaluation with chest x-ray, electrocardiogram and echocardiography should be used in the follow-up. When myocardial ischemia is suspected, myocardial perfusion scintigraphy should be performed. In our institution, followup visits were performed at 1, 3, 6 and 12 months during the first year and annually thereafter. In the majority of studies, color-Doppler study was integrated into echocardiographic analysis of both intervened and unintervened CAFs. The changes in affected chamber dimensions should be checked at each visit. Coronary angiography should be performed in symptomatic patients if the clinical condition is attributed to fistula recanalization or other complications. However, the necessity for coronary angiography in long-term follow-up in asymptomatic patients is not clear. Asymptomatic patients should be followed- up with noninvasive tests. Newer imaging modalities such as multidetector computed tomography and cardiac MRI may produce valuable data regarding the patency or residual flow of the fistula in those cases [65]. Although reported long-term outcomes after percutaneous closure are promising, routine follow-up with either invasive or noninvasive tests should be the standard of care in both intervened and unintervened cases.
Future perspective
Currently, percutaneous management of CAFs provides a high degree of procedural success with a very low risk of serious complications. Device selection and delivery technique should be based on the anatomic and morphologic characteristics of the fistula. Although surgical ligation has previously been the standard treatment for CAF, specialized techniques, equipment and newer devices have made the percutaneous approach a safe and effective first-line treatment modality in most patients with suitable anatomy, with good follow-up results. In our opinion, with the advent of new devices and equipment, outcomes of the percutaneous approach will be improved, and most of the CAFs are amenable to percutaneous closure. More studies evaluating the efficacy and safety of percutaneous approaches with long-term follow-up results will provide valuable data in the near future.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Indications for intervention
▪ Symptomatic patients with left-to-right shunt and complications including myocardial ischemia, congestive heart failure, pulmonary hypertension, dysrhythmia and infective endocarditis should undergo percutaneous intervention.
Principles of treatment
▪ Percutaneous closure of a coronary artery fistula is recommended at the proximal origin of the fistula, in the absence of multiple feeders and if there is a lack of adjacent coronary arterial branches. The selection of occlusion device mostly depends on the cost of the device, the operator’s experience, the age of the patient, associated cardiac diseases and the anatomic characteristics of the fistula.
Tips & tricks
▪ Positioning the catheters as proximal as possible to the narrowest area of the fistula is integral to procedural success to prevent device migration. The selected device should be slightly larger than the target vessel diameter to allow proper positioning of the device.
Complications, outcomes & follow-up
▪ Long-term follow-up is essential owing to the possibility of postprocedural recanalization or residual flow, persistent dilation of the coronary artery, late thrombosis and myocardial ischemia after percutaneous intervention.
References
Papers of special note have been highlighted as:
▪ of interest
- Grant RT: An unusual anomaly of the coronary vessels in the malformed heart of a child. Heart 13, 273–283 (1926).
- Heifetz SA, Robinowitz M, Mueller KH, Virmani R: Total anomalous origin of the coronary arteries from the pulmonary artery. Pediatr. Cardiol. 7(1), 11–18 (1986).
- Levin DC, Fellows KE, Abrams HL: Hemodynamically significant primary anomalies of the coronary arteries. Angiographic aspects. Circulation 58(1), 25–34 (1978).
- Yamanaka O, Hobbs RE: Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet. Cardiovasc. Diagn. 21(1), 28–40 (1990).
- Gillebert C, Van Hoof R, Van De Werf F, Piessens J, De Geest H: Coronary artery fistulas in an adult population. Eur. Heart J. 7(5), 437–443 (1986).
- Sercelik A, Mavi A, Ayalp R, Pestamalci T, Gumusburun E, Batiraliev T: Congenital coronary artery fistulas in Turkish patients undergoing diagnostic cardiac angiography. Int. J. Clin. Pract. 57(4), 280–283 (2003).
- Baltaxe HA, Wixson D: The incidence of congenital anomalies of the coronary arteries in the adult population. Radiology 122(1), 47–52 (1977).
- Said SA, Lam J, Van Der Werf T: Solitary coronary artery fistulas: a congenital anomaly in children and adults. A contemporary review. Congenit. Heart Dis. 1(3), 63–76 (2006).
- Pattee PL, Chambers RJ: Acquired postoperative coronary arteriovenous fistula. Cathet. Cardiovasc. Diagn. 26(2), 140–142 (1992).
- Rangel A, Badui E, Verduzco C, Valdespino A, Enciso R: Traumatic coronary arteriovenous fistula communicating the left main coronary artery to pulmonary artery, associated with pulmonary valvular insufficiency and endocarditis: case report. Angiology 41(2), 156–160 (1990).
- Endo M, Tomizawa Y, Nishida H et al.: Angiographic findings and surgical treatments of coronary artery involvement in takayasu arteritis. J. Thorac. Cardiovasc. Surg. 125(3), 570–577 (2003).
- Noble S, Frangos C, Romeo P: Coronary artery-middle cardiac vein fistula after endomyocardial biopsy in a heart transplant patient. Can. J. Cardiol. 25(9), E334 (2009).
- Saeian K, Vellinga T, Troup P, Wetherbee J: Coronary artery fistula formation secondary to permanent pacemaker placement. Chest 99(3), 780–781 (1991).
- Bata IR, Macdonald RG, O’Neill BJ: Coronary artery fistula as a complication of percutaneous transluminal coronary angioplasty. Can. J. Cardiol. 9(4), 331–335 (1993).
- Latson LA: Coronary artery fistulas: how to manage them. Cathet. Cardiovasc. Interv. 70(1), 110–116 (2007).
- Said SA, Van Der Werf T: Dutch survey of coronary artery fistulas in adults: congenital solitary fistulas. Int. J. Cardiol. 106(3), 323–332 (2006).
- Hackett D, Hallidie-Smith KA: Spontaneous closure of coronary artery fistula. Br. Heart J. 52(4), 477–479 (1984).
- Edis AJ, Schattenberg TT, Feldt RH, Danielson GK: Congenital coronary artery fistula. Surgical considerations and results of operation. Mayo Clin. Proc. 47(8), 567–571 (1972).
- Liberthson RR, Sagar K, Berkoben JP, Weintraub RM, Levine FH: Congenital coronary arteriovenous fistula. Report of 13 patients, review of the literature and delineation of management. Circulation 59(5), 849–854 (1979).
- Vitarelli A, De Curtis G, Conde Y et al.: Assessment of congenital coronary artery fistulas by transesophageal color doppler echocardiography. Am. J. Med. 113(2), 127–133 (2002).
- Dodd JD, Ferencik M, Liberthson RR et al.: Evaluation of efficacy of 64-slice multidetector computed tomography in patients with congenital coronary fistulas. J. Comput. Assist. Tomogr. 32(2), 265–270 (2008).
- Weymann A, Lembcke A, Konertz WF: Right coronary artery to superior vena cava fistula: imaging with cardiac catheterization, 320-detector row computed tomography, magnetic resonance imaging, and transoesophageal echocardiography. Eur. Heart J. 30(17), 2146 (2009).
- Kamiya H, Yasuda T, Nagamine H et al.: Surgical treatment of congenital coronary artery fistulas: 27 years’ experience and a review of the literature. J. Card. Surg. 17(2), 173–177 (2002).
- Liang CD, Ko SF: Midterm outcome of percutaneous transcatheter coil occlusion of coronary artery fistula. Pediatr. Cardiol. 27(5), 557–563 (2006).
- Collins N, Mehta R, Benson L, Horlick E: Percutaneous coronary artery fistula closure in adults: technical and procedural aspects. Catheter Cardiovasc. Interv. 69(6), 872–880 (2007).
- Behera SK, Danon S, Levi DS, Moore JW: Transcatheter closure of coronary artery fistulae using the amplatzer duct occluder. Catheter Cardiovasc. Interv. 68(2), 242–248 (2006).
- Brown MA, Balzer D, Lasala J: Multiple coronary artery fistulae treated with a single amplatzer vascular plug: check the back door when the front is locked. Catheter Cardiovasc. Interv. 73(3), 390–394 (2009).
- Duckworth F, Mukharji J, Vetrovec GW: Diffuse coronary artery to left ventricular communications: an unusual cause of demonstrable ischemia. Cathet. Cardiovasc. Diagn. 13(2), 133–137 (1987).
- Macri R, Capulzini A, Fazzini L, Cornali M, Verunelli F, Reginato E: Congenital coronary artery fistula: report of five patients, diagnostic problems and principles of management. Thorac. Cardiovasc. Surg. 30(3), 167–171 (1982).
- Lowe JE, Oldham HN Jr, Sabiston DC Jr: Surgical management of congenital coronary artery fistulas. Ann. Surg. 194(4), 373–380 (1981).
- Shubrooks SJ Jr, Naggar CZ: Spontaneous near closure of coronary artery fistula. Circulation 57(1), 197–199 (1978).
- Francis CK, Sacheti CK, Cohen RB: Fistulous communication between the left coronary artery and main pulmonary artery: a thirteen-year follow-up. Cathet. Cardiovasc. Diagn. 5(4), 357–366 (1979).
- Warnes CA, Williams RG, Bashore TM et al.: ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults with Congenital Heart Disease). Developed in Collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 52(23), E1–E121 (2008).
- Mavroudis C, Backer CL, Rocchini AP, Muster AJ, Gevitz M: Coronary artery fistulas in infants and children: a surgical review and discussion of coil embolization. Ann. Thorac. Surg. 63(5), 1235–1242 (1997).
- Cheung DL, Au WK, Cheung HH, Chiu CS, Lee WT: Coronary artery fistulas: long-term results of surgical correction. Ann. Thorac. Surg. 71(1), 190–195 (2001).
- Wang S, Wu Q, Hu S et al.: Surgical treatment of 52 patients with congenital coronary artery fistulas. Chin. Med. J. (Engl.) 114(7), 752–755 (2001).
- Armsby LR, Keane JF, Sherwood MC, Forbess JM, Perry SB, Lock JE: Management of coronary artery fistulae. Patient selection and results of transcatheter closure. J. Am. Coll. Cardiol. 39(6), 1026–1032 (2002).
- Oto A, Aytemir K, Cil B et al.: Percutaneous closure of coronary artery fistulae in adults with intermediate term follow-up results. J. Interv. Cardiol. DOI: 10.1111/j.1540-8183.2010.00623.x. (2011) (Epub ahead of print).
- Reidy JF, Sowton E, Ross DN: Transcatheter occlusion of coronary to bronchial anastomosis by detachable balloon combined with coronary angioplasty at same procedure. Br. Heart J. 49(3), 284–287 (1983).
- Qureshi SA: Coronary arterial fistulas. Orphanet. J. Rare Dis. 1, 51 (2006).
- Perry SB, Rome J, Keane JF, Baim DS, Lock JE: Transcatheter closure of coronary artery fistulas. J. Am. Coll. Cardiol. 20(1), 205–209 (1992).
- Reidy JF, Anjos RT, Qureshi SA, Baker EJ, Tynan MJ: Transcatheter embolization in the treatment of coronary artery fistulas. J. Am. Coll. Cardiol. 18(1), 187–192 (1991).
- Reidy JF, Jones OD, Tynan MJ, Baker EJ, Joseph MC: Embolisation procedures in congenital heart disease. Br. Heart J. 54(2), 184–192 (1985).
- Goldberg SL, Makkar R, Duckwiler G: New strategies in the percutaneous management of coronary artery fistulae: a case report. Catheter Cardiovasc. Interv. 61(2), 227–232 (2004).
- Qureshi SA, Reidy JF, Alwi MB et al.: Use of interlocking detachable coils in embolization of coronary arteriovenous fistulas. Am. J. Cardiol. 78(1), 110–113 (1996).
- Kabbani Z, Garcia-Nielsen L, Lozano ML, Febles T, Febles-Bethencourt L, Castro A: Coil embolization of coronary artery fistulas. A single-centre experience. Cardiovasc. Revasc. Med. 9(1), 14–17 (2008).
- Godart F, Rey C, Cajot MA et al.: [Value of detachable silicone balloon in the closure of coronary artery fistula. Apropos of 3 cases]. Arch. Mal. Coeur Vaiss. 90(5), 611–616 (1997).
- Skimming JW, Gessner IH, Victorica BE, Mickle JP: Percutaneous transcatheter occlusion of coronary artery fistulas using detachable balloons. Pediatr. Cardiol. 16(1), 38–41 (1995).
- Jamshidi P, Mahmoody K, Erne P: Covered stents: a review. Int. J. Cardiol. 130(3), 310–318 (2008).
- Mullasari AS, Umesan CV, Kumar KJ: Transcatheter closure of coronary artery to pulmonary artery fistula using covered stents. Heart 87(1), 60 (2002).
- Bonello L, Com O, Gaubert JY, Sbraggia P, Paganelli F: Covered stent for closure of symptomatic plexus-like coronary fistula. Int. J. Cardiol. 109(3), 408–410 (2006).
- Ahn Y, Park OY, Jeong MH: Combined membrane covered and uncovered stents for coronary arteriovenous fistula associated with atherosclerotic plaque in a patient with acute myocardial infarction. Heart 90(2), 150 (2004).
- Gercken U, Lansky AJ, Buellesfeld L et al.: Results of the jostent coronary stent graft implantation in various clinical settings: procedural and follow-up results. Catheter Cardiovasc. Interv. 56(3), 353–360 (2002).
- Stankovic G, Colombo A, Presbitero P et al.: Randomized evaluation of polytetrafluoroethylene-covered stent in saphenous vein grafts: the Randomized Evaluation of polytetrafluoroethylene COVERed stent in Saphenous vein grafts (RECOVERS) trial. Circulation 108(1), 37–42 (2003).
- Karagoz T, Celiker A, Cil B, Cekirge S: Transcatheter embolization of a coronary fistula originating from the left anterior descending artery by using n-butyl 2-cyanoacrylate. Cardiovasc. Intervent. Radiol. 27(6), 663–665 (2004).
- Justo RN, Slaughter RE, Whight CM, Radford DJ: Transcatheter embolisation of coronary artery fistulae. Heart Lung Circ. 10(2), 53–57 (2001).
- Maleszka A, Kleikamp G, Minami K, Peterschroder A, Korfer R: Giant coronary arteriovenous fistula. A case report and review of the literature. Z Kardiol. 94(1), 38–43 (2005).
- Luo L, Kebede S, Wu S, Stouffer GA: Coronary artery fistulae. Am. J. Med. Sci. 332(2), 79–84 (2006).
- Kung GC, Moore P, McElhinney DB, Teitel DF: Retrograde transcatheter coil embolization of congenital coronary artery fistulas in infants and young children. Pediatr. Cardiol. 24(5), 448–453 (2003).
- Zhu XY, Zhang DZ, Han XM et al.: Transcatheter closure of congenital coronary artery fistulae: immediate and long-term follow-up results. Clin. Cardiol. 32(9), 506–512 (2009).
- Rittenhouse EA, Doty DB, Ehrenhaft JL: Congenital coronary artery–cardiac chamber fistula. Review of operative management. Ann. Thorac. Surg. 20(4), 468–485 (1975).
- Aggoun Y, Bonhoeffer P, Sidi D, Bonnet D, Acar P, Kachaner J: [Congenital coronarycardiac fistula in children. Effects of surgical occlusion and percutaneous embolization]. Arch. Mal. Coeur Vaiss. 90(5), 605–609 (1997).
- Mcmahon CJ, Nihill MR, Kovalchin JP, Mullins CE, Grifka RG: Coronary artery fistula. Management and intermediate-term outcome after transcatheter coil occlusion. Tex. Heart Inst. J. 28(1), 21–25 (2001).
- Sherwood MC, Rockenmacher S, Colan SD, Geva T: Prognostic significance of clinically silent coronary artery fistulas. Am. J. Cardiol. 83(3), 407–411 (1999).
- Yorgun H, Hazirolan T, Karcaaltincaba M et al.: The role of multidetector computed tomography coronary angiography in the follow up of coronary artery fistula after closure by percutaneous intervention. Presented at: 6th Congress of Update in Cardiology and Cardiovascular Surgery in conjunction with 59th International Congress of the European Society for Cardiovascular Surgery. İzmir, Turkey, 15–18 April 2010. 66 Qureshi SA, Tynan M: Catheter closure of coronary artery fistulas. J. Interv. Cardiol. 14(3), 299–307 (2001).
- Okubo M, Nykanen D, Benson LN: Outcomes of transcatheter embolization in the treatment of coronary artery fistulas. Catheter Cardiovasc. Interv. 52(4), 510–517 (2001).
- Abdelmoneim SS, Mookadam F, Moustafa S et al.: Coronary artery fistula: single-center experience spanning 17 years. J. Interv. Cardiol. 20(4), 265–274 (2007).
- Bruckheimer E, Harris M, Kornowski R, Dagan T, Birk E: Transcatheter closure of large congenital coronary–cameral fistulae with amplatzer devices. Catheter Cardiovasc. Interv. 75(6), 850–854 (2010).
▪ Largest registry investigating the incidence of coronary artery anomalies.
▪ Provides important data regarding long-term follow-up results after surgical correction of coronary artery fistulas.
▪ Important study revealing results of a percutaneous approach.
▪ First report of percutaneous closure of coronary artery fistula.
▪ Suggests an excellent outcome of a percutaneous approach to coronary artery fistulas with favorable long-term follow-up results.