Review Article - Interventional Cardiology (2009) Volume 1, Issue 1
Percutaneous closure of the left atrial appendage in atrial fibrillation: an alternative if standard treatment fails?
- Corresponding Author:
- Werner Budts
Congenital & Structural Cardiology
University Hospitals Leuven
Herestraat 49, B-3000 Leuven, Belgium
Tel: +32 16 344 369
Fax: +32 16 344 240
E-mail: werner.budts@ uz.kuleuven.ac.be
Abstract
Keywords
atrial fibrillation, closure, left atrial appendage, stroke, thromboembolism
Atrial fibrillation (AF) is a common arrhythmia and its prevalence increases with age. Approximately 10% of all octogenarians suffer from AF [1]. Apart from the sense of palpitations and/or a lower exercise capacity, AF is responsible for 15% of all ischemic strokes and up to 25% of all ischemic strokes in octogenarians [2]. Chronic or paroxysmal AF might induce the formation of thrombi in the left atrium, of which, in nonrheumatic AF patients, more than 90% occur in the left atrial appendage (LAA) [3].
Oral anticoagulants (OACs) are currently the standard treatment in the prevention of thromboembolic events in AF patients and the CHADS2 score (C: congestive heart failure, H: hypertension, A: age greater than or equal to 75 years, D: diabetes, S: prior stroke or transient ischemic attack [TIA]) is a validated instrument to guide the usage of OACs in these patients [4]. Since OACs can cause numerous adverse events, as described below, the score provides a clinical guideline to determine in which patients the benefit outweighs the possible risk of complications. In general, OACs reduce the relative number of thromboembolic events by 68% [5]. The CHADS2 score is able to identify highrisk patients in whom this 68% risk reduction is important in absolute numbers as well.
However, OACs may fail to prevent thromboembolic events and may be contraindicated. The risk for hemorrhagic complications is the most important reason why physicians may be reluctant to start with OACs [6,7]. Antiplatelets are frequently used instead; but these appear to be less effective and studies with aspirin have demonstrated a risk reduction of only 22% [8].
Historically, some surgeons ligated or removed the LAA after mitral valve surgery, which was associated with a decreased risk for thromboembolic stroke [3]. All this led to the idea that exclusion of the LAA could be a therapeutic option to prevent thromboembolic events in patients with paroxysmal or chronic AF.
In the last decade, devices have been developed for percutaneous LAA closure. The feasibility, safety and efficacy of the closing procedure has been proven in several studies [9–11], and, recently, the results of a randomized trial have been reported showing an equivalent reduction of thromboembolic stroke for both OAC administration and percutaneous LAA closure.
Atrial fibrillation, outcome & thrombus formation
Compared with patients with no AF, mortality in AF patients is 1.5- to 1.9-times higher than in patients with no AF [12]. The latter is related to the AF itself or to the underlying disease that caused AF. In addition, the number of hospitalizations is higher in AF patients than in non-AF patients, indicating that AF cannot be considered to be benign, as formerly thought [12].
For patients with AF, the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial showed no difference in survival between patients treated based on rhythm versus rate control [13]. However, more recently, the placebo- controlled, double-blind, parallel-arm trial to Assess the Efficacy of Dronedarone 400 mg b.i.d. for the Prevention of Hospitalization or Death from any Cause in Patients with Atrial Fibrillation/Atrial Flutter (ATHENA) study indicated for the first time that maintenance of sinus rhythm with dronedarone reduced cardiovascular death and the number of hospitalizations [14]. An unpublished post hoc analysis even showed a reduction in the risk of stroke. Furthermore, AF patients have a lower exercise tolerance and many patients do have sporadic episodes of symptomatic AF with complaints such as palpitations or fatigue. Hence, from a viewpoint of quality of life, rhythm control remains the preferable option.
Apart from the symptoms related to the irregular rhythm and the decreased exercise capacity, paroxysmal or chronic AF is responsible for 15% of all strokes caused by thrombus formation in the left atrium and embolization into the cerebral circulation. This percentage rises up to 25% in octogenarians [1,2,15].
The reason why a thrombus is formed in patients with AF is multifactorial. Current knowledge of hemostasis and thrombogenesis has led to a better understanding of Virchow’s triad, already established in the 19th Century. Abnormal blood stasis, changes in hemostasis, changes in platelet function or inadequate fibrinolysis, and endothelial or endocardial damage are the current substitutes for the older features of the Virchow’s triad: abnormal changes in blood flow, vessel wall and blood constituents [16]. In AF patients, thrombus formation is enhanced by the structural and hemodynamic alterations in the left atrium, and more specifically, in the LAA. The anatomical features of the LAA, as described below, make it the preferred site of thrombogenesis. Both the left atrium and LAA enlarge in patients with AF and predispose for stasis. Furthermore, endothelial damage, such as small areas of endothelial denudation, occurs more often in patients with AF, and the LAA is mainly affected [17,18]. Failure of atrial systole, as a consequence of AF, also leads to stasis of blood in the left atrium and in the LAA [19]. In summary, stasis is more pronounced in the LAA so that most thrombi originate in the LAA. This was confirmed by an extensive review of 23 studies in which LAA was examined by autopsy, transesophageal echocardiography (TEE), or direct intraoperative inspection. Intracardiac thrombus was identified in 13% of both nonvalvular and valvular AF patients. Furthermore, 57% of atrial thrombi in valvular AF occurred in the appendage, whereas in nonvalvular AF, 90% of the left atrial thrombi were found in the LAA [3].
Transesophageal echocardiography remains the gold standard for visualizing a thrombus in the left atrium [20]. A few studies have reported on the detection of clot by MRI or spiral CT imaging, but these imaging techniques have not (yet) found their way into routine clinical practice for this indication [21,22]. In one study, TEE had a sensitivity of 93% and a specificity of 100% for thrombus detection [23]. However, trabeculations in the LAA remain difficult to distinguish from small thrombi. Tumoral masses are difficult to distinguish from larger thrombi. However, TEE might also detect abnormal stasis of blood or low blood flow in the left atrium and the LAA as a spontaneous echo contrast or smoke-like echos. Although this qualitative evaluation is strongly operator-dependent, it identifies patients at high risk for clot formation [24]. Pulsed Doppler studies of the LAA quantified more accurately the flow in the LAA. Flow velocities of less than 20–40 cm/s in the LAA are associated with a higher risk for thromboembolism [25,26]. In addition, a LAA area greater than 6 cm2 is also considered to be predictive for thromboembolic events [27,28]. Finally, increased prothrombotic indices, high fibrin D-dimers, congestive heart failure or a recent thromboemboligenic event were shown to independently predict LAA thrombi on TEE [29].
Anatomy of the LAA
The LAA is sometimes considered as a minor extension of the left atrium. However, this blind-ended structure is embryologically distinct from the body of the left atrium. It is the remnant of the original embryonic left atrium that develops during the third week of gestation. The actual left atrium, which has a smooth wall, originates from the pulmonary veins later in development. The LAA lies in the left atrio–ventricular sulcus on top of the origin and proximal portion of the left circumflex artery. The LAA is characterized by lobes, and the number of lobes varies from one to four. It has been shown in a large autopsy study that 80% of all LAAs consist of two or more lobes and 54% are bilobar [30]. The LAA wall is made of muscular tissue and is trabeculated with parallel muscle bars, forming crypts. The size varies widely from 0.7 to 19.2 ml and the maximal diameter of the orifice ranges from 10 to 40 mm. The length of the LAA varies from 16 to 51 mm [31]. Given the narrow inlet for a relatively long structure, these features predispose for blood stasis, not only in AF, but also in patients in sinus rhythm [32].
Standard treatment of AF to prevent thromboembolism
Currently, the gold standard for the prevention of stroke are OACs or low-dose aspirin (75–150 mg) [4,5]. Whether OACs or aspirin should be administered is based on a validated risk score, the CHADS2 score [33]. Each risk factor accounts for one point except prior stroke or TIA, which account for two points. When the risk score equals zero, treatment with aspirin is sufficient to prevent thromboembolic events. When the risk score is two or more, the use of OACs is mandatory. With a risk score of one, both treatment options, aspirin or OACs, are valuable. In controlled settings, this treatment regimen gives a 60–70% risk reduction for thromboembolic stroke [5,8]. Therefore, this CHADS2 score is integrated into many guidelines.
Failure of standard treatment of AF
Nevertheless, numerous complications or difficulties can occur when OACs are administered. First, there is the risk of bleeding, especially of intracranial hemorrhage (ICH), which is the most serious complication with a mortality rate in excess of 50%. The risk of ICH when taking OACs is 0.3–0.6% per year while the estimated absolute rate of primary ICH is 0.5% per year in the general population (mean age: 70 years) [34]. Bleeding, however, can occur at virtually any site. The estimated annual incidence of bleeding varies, but is considered to be 0.6% for fatal bleeding, 3.0% for a major bleeding and 9.6% for major or minor bleeding. The risk of bleeding appears to be especially high during the first year of OAC utilization [35]. One group has attempted to identify clinical signs to predict bleeding, but none of them were sufficiently accurate predictors [36].
Recently, the implementation of warfarin pharmacogenetics has been thought to lower bleeding risks. Two genes are identified that influence the individual sensitivity for warfarin [37]. First, the VKORC1 gene, which codes for the vitamin K epoxide reductase, the enzyme where warfarin exerts its effect. Different genotypes appear to have variable sensitivity to the effect of OAC [38,39]. Patients with the AA genotype would require lower doses of warfarin compared with patients with the GG genotype. The second gene identified is the cytochrome P450 CYP2C9 gene, which codes for the liver enzyme that metabolizes warfarin. If a patient has a genetic variation in CYP2C9, he tends to metabolize warfarin more slowly, thus accumulating warfarin in the blood and hence leading to over-anticoagulation [40]. CYP2C9 genotype mutations can be identified so that, after initiation of OACs, a lower maintenance dose can be given to the patient, thus preventing over-anticoagulation [41]. However, numerous other factors play an important role in determining the necessary dose of OAC. It is thought that genetic variants only account for 39–56% of the variability in the warfarin dose [42–45]. Multiple other patient-specific factors such as smoking, weight, concomitant usage of other drugs, intake of vitamin K and ethnic origin also influence warfarin dosing [46]. Gage et al. has proposed an algorithm that explains 53–54% of the variability in the warfarin dose [47]. Although the implementation of pharmacogenetics does give more information to the clinician concerning adequate dosing, it has not yet found its way to routine clinical practice [48,49]. Apart from the risk of bleeding, there might be multiple contraindications for OAC therapy. AOCs are contraindicated in patients with hemorrhagic tendencies, therapeutic procedures with the potential for significant bleeding, recent or planned surgery of the eye or the CNS, fall risk, pregnancy and active pericarditis. Allergies for OAC have also been reported. Between 14 and 44% of patients with AF have one or more absolute or relative contraindications for OACs [50–53].
Other possible drawbacks for OAC therapy might be difficulties in monitoring of the therapy and the need for regular International Normalized Ratio (INR) control. Studies have demonstrated that up to 49% of patients with AF in whom OACs were indicated did not receive the requested treatment [54,55]. Furthermore, a recent meta-analysis showed that the INR of patients who receive OACs was only within the therapeutic range 63.6% of the time (range: 61.6–65.6%) [56].
Alternative treatment options: medication
▪ Aspirin monotherapy
In current practice, if OACs are contraindicated or considered to be too dangerous in a specific patient, aspirin monotherapy is often applied. Aspirin monotherapy has been proven to reduce the number of thromboembolic strokes by 22% compared with the 60–70% reduction with OACs [57].
▪ Clopidogrel + aspirin
Combination therapy of two antiplatelet agents has been attempted for the same reason. In one study, combination therapy with clopidogrel and aspirin was compared with the use of OACs. The study was terminated prematurely owing to the clear evidence of superiority of OACs (annual risk 3.93 vs 5.60%; p = 0.0003) [58]. The number of hemorrhagic complications was similar in the two groups. The addition of clopidogrel to aspirin gives a supplementary reduction in thromboembolic events in comparison to aspirin monotherapy, but at the cost of increased bleeding complications [59]. Hence, combination antiplatelet therapy is not considered to be an alternative to OAC.
▪ Direct thrombin inhibitors
To address the problem of monitoring, new anticoagulants have been developed that are easier to apply in clinical practice. With direct thrombin inhibitors (DTIs), coagulation monitoring is no longer necessary because of its independency of the CYP enzyme system. Clinical trials showed promising results with equal efficacy of DTIs in comparison to OACs. Major bleeding incidence was similar in the two groups. DTI therapy gave, however, a significantly lower number of minor bleedings. Unfortunately, with the first-generation DTIs, liver failure was a concern [60]. Given this adverse event, DTIs have not been approved by health authorities, but new generation DTIs are currently being tested in clinical practice.
▪ Idraparinux monotherapy
Monotherapy with specific factor Xa inhibitors has also been attempted to prevent thromboembolic events. In a large, randomized, noninferiority trial, the efficacy and safety of idraparinux administration was compared with the administration of vitamin K antagonists. The trial was stopped early because of the significant higher number of clinically significantly bleeding instances in comparison with vitamin K antagonists (19.7 vs 11.3 per 100 patient-years).
However, the noninferiority criterion was reached. Thromboembolism with idraparinux occurred in 0.9 versus 1.3% in the group treated with vitamin K antagonists. Although the number of bleedings was significantly higher in the idraparinux group, this was not associated with a higher mortality [61].
Alternative treatment options: interventions
▪ Radiofrequency catheter ablation for AF
In recent years, radiofrequency catheter ablation (RFA) has been introduced in the treatment of AF and reduces the risk of AF recurrence by 65% at 1 year compared with antiarrhythmic drugs [62,63]. RFA could obviate the need for OACs, but ceasing therapy with OACs is not recommended for numerous reasons. First, the risk of stroke rises peri-procedurally because of the endocardial lesions provoked by the ablation technique [64,65]. Second, it is difficult to identify those patients in whom AF has truly been ‘cured’. Several patients still experience paroxysms of AF [66,67]. Furthermore, RFA is often reserved for patients who are younger and have less risk factors for thromboembolic events [68]. Continuation of OACs after RFA is recommended if the patient meets the CHADS2 criteria. However, if there is no evidence of recurrent AF after 3–6 months, discontinuation of OACs can be attempted, but the safety of this approach requires further study [68].
▪ Surgical exclusion
Exclusion of the LAA from the systemic circulation was suggested when it was observed that in rheumatic heart disease, the major site of thrombi is the left atrium and its appendage [69–72]. After the feasibility of canine LAA resection was demonstrated by Hellerstein and his associates, [70], Madden performed an LAA excision in two patients with AF during mitral valve surgery in 1949 [71]. In 1950, Beal et al. described two cases of left and one case of right atrial appendectomy for prophylaxis of thromboembolism [72]. Johnson et al. reported prophylactic LAA excision in 437 patients undergoing open heart surgery, including 17 patients with preoperative AF [73]. No later strokes were attributed to AF. In subsequent years, no patients were found to have atrial clots on TEE; however, routine TEE examination at prespecified times postintervention was not defined in the study design. The authors concluded that routine LAA excision is safe and should be considered whenever the chest is opened. Consistent with this, the absence of LAA ligation and the presence of left atrial thrombus were the only independent predictors of an embolic event during a mean follow-up of 69.4 months in patients with previous mitral valve replacement [69,74]. However, there has never been a randomized study regarding safety and effectiveness of the surgical LAA.
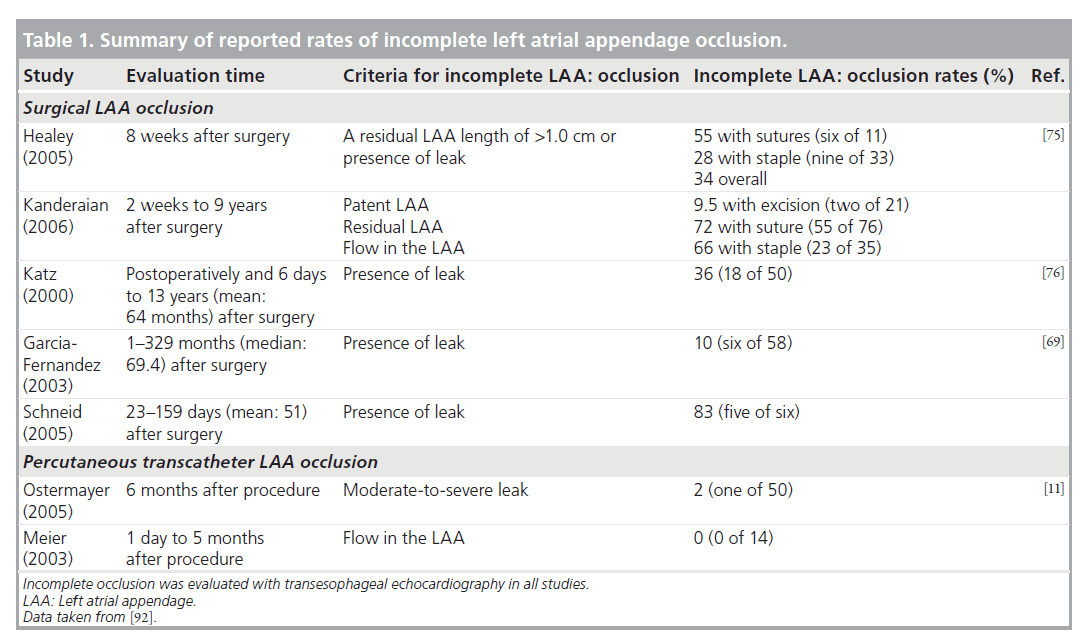
More recently, surgical ligation of the LAA was attempted in patients undergoing coronary artery bypass graft operation (LAA Occlusion Study [LAAOS]), who were at risk for developing AF. Safety and efficacy of the procedure were assessed [75]. The study showed that complete occlusion of the LAA is quite challenging and the result is highly dependent on the experience of the surgeon and the techniques used. Among patients having a postoperative TEE, complete occlusion of the LAA was achieved in 45% of cases using sutures and in 72% (24 out of 33) using a stapler.
Indeed, the success rate of closure might be severely overestimated, and in studies where mitral valve surgery was accompanied by LAA closure, a success rate of only 64% was reported [76]. Nevertheless, successful closure can be achieved in up to 90% of the procedures if the surgeon is more experienced (more than four cases) and with the use of stapling devices. Hence, the reason why surgical exclusion is so often unsuccessful is related to experience of the surgeon as well as to technical issues [69,75]. In many centers, the LAA is removed during mitral valve surgery (Table 1).
▪ Thoracoscopic techniques
Odell et al. performed the first thoracoscopic obliteration of the LAA in dogs and human cadavers and demonstrated the feasibility of this method [77]. Johnson et al. performed a thoracoscopic LAA excision in patients with chronic AF as an isolated surgical procedure [73]. Blackshear et al. used a loop snare in eight patients and a stapler in seven patients for thoracoscopic obliteration of the LAA [78]. The procedure was completed in 14 out of 15 patients and they confirmed that the procedure was technically feasible, but its effect on stroke prevention was limited. Newly developed minimally invasive/ thoracoscopic tools for LAA occlusion have emerged recently [79,80]. The tools are either a stapler [80,81] or clipping device [79]. Accordingly, numerous reports suggest a recent expansion of minimal invasive/thoracoscopic LAA occlusion, especially when performed in conjunction with the minimally invasive MAZE procedure for ablation of AF [74,81]. Most studies, however, were performed with small numbers of patients and could not prove a significant reduction of thromboembolic events after the procedure.
▪ Percutaneous LAA closure
Since at present no alternative is available when OACs are contraindicated, new methods for prevention of thromboembolic stroke were looked for. Percutaneous closure of the LAA has been attempted and for this purpose different LAA closure devices have been developed. In European countries, the PLAATO device (ev3 Endovascular, Inc., North Plymouth, MN, USA) was particularly popular. It was implanted from 2001 until 2007, but then removed from the market. This was not due to safety or efficacy issues. Since the PLAATO device did not receive US FDA approval, based on the study results discussed below, the device was only available on the European market and ev3 decided to stop production for economic reasons. Another, similar device, WATCHMAN® (Atritech Inc., Plymouth, MN, USA), has identical therapeutic objectives, but has some structural differences when compared with the PLAATO device. It has been implanted since 2002 in Europe and since 2003 in the USA. WATCHMAN is most often used in the USA. The WATCHMAN device is still available in studies.
Nevertheless, interest in the technique of percutaneous closure has never completely disappeared. Percutaneous closure of the LAA with devices that were not even developed for this purpose has occasionally been carried out. Two case reports of closure of the LAA with the AMPLATZER® septal occluder (AGA Medical, Plymouth, MN, USA) have been published [82,83]. Recently, the new AMPLATZER cardiac plug (AGA Medical) has been developed for the percutaneous closure of cardiac structures, including the LAA. Given its very recent introduction, no studies have been published to evaluate this new device. This device is currently commercially available in Europe.
▪ Composition & implantation technique
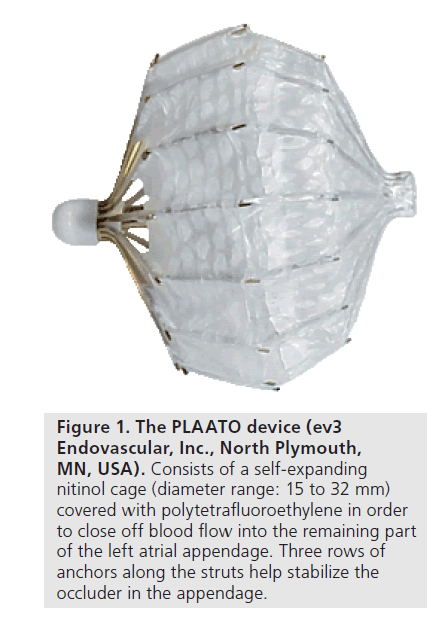
The PLAATO implant was composed of a selfexpanding nitinol cage that was covered with a polytetrafluoroethylene (ePTFE) membrane. This membrane was shaped and constructed in such a way that it could have close contact with the inner surface of the LAA. The device was anchored into the LAA with small metal anchors attached to the struts of the device and piercing the membrane (Figure 1). After deployment, the purpose of the device’s membrane was double. It had to both occlude the LAA as well as allow neo-endothelization and tissue incorporation in the device. The device was delivered with a specific trans-septal sheath (14 Fr) that was designed to point at the LAA after introduction and passing the interatrial septum. Under TEE guidance and by means of trans-septal access, the size and shape of the orifice of the LAA was evaluated by angiography, after which the appropriate size of the implant was chosen. After withdrawal of the delivery system and the trans-septal sheath, but with the device still attached to the catheter piercing the interatrial wall, radiocontrast was injected proximal of, thus in the left atrium, and distal of the implant to evaluate the position of the device and the adequate sealing of the LAA. If an insufficiently stable position of the implant was found, the device could either be collapsed and repositioned, or could be safely withdrawn from the LAA.
Figure 1. The PLAATO device (ev3 Endovascular, Inc., North Plymouth, MN, USA). Consists of a self-expanding nitinol cage (diameter range: 15 to 32 mm) covered with polytetrafluoroethylene in order to close off blood flow into the remaining part of the left atrial appendage. Three rows of anchors along the struts help stabilize the occluder in the appendage.
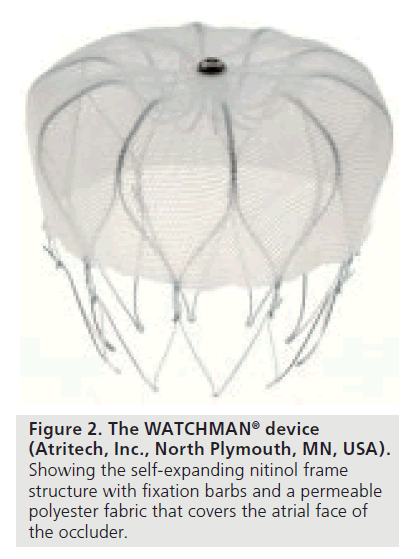
The WATCHMAN implant also consisted of a nitinol cage that was self-expanding and had a permeable polyester membrane. The polyester membrane covered the distal, thus the atrial facing side of the LAA. Anchors on the side of the device ensured a sufficiently stable anchoring into the LAA (Figure 2). The delivery technique of the WATCHMAN system was similar to the technique of the PLAATO device. If an insufficiently stable position was observed after implantation the device could be collapsed and redeployed, as well as completely retrieved and replaced by another device with a different diameter. TEE guidance was also needed and angiography was performed systematically to evaluate the stability and localization of the implant.
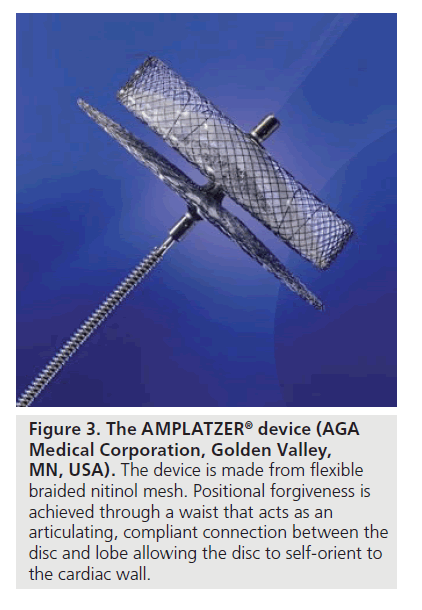
The recently developed AMPLATZER cardiac plug consists of a nitinol mesh (Figure 3). It is designed to conform to the inner wall of the LAA with a minimal depth of 10 mm. In between the lobe and the disc of the implant, there is a waist that acts as an articulating connection and automatically adapts to the geometry of the LAA. The disc itself is designed to completely cover the orifice of the cardiac structure and provide apposition against the chamber wall.
Figure 3. The AMPLATZER® device (AGA Medical Corporation, Golden Valley, MN, USA). The device is made from flexible braided nitinol mesh. Positional forgiveness is achieved through a waist that acts as an articulating, compliant connection between the disc and lobe allowing the disc to self-orient to the cardiac wall.
Given the anatomic variation in the human LAA, devices need a wide range of sizes to be available. The PLAATO implant size ranged from 15 to 32 mm, the WATCHMAN device from 21 to 33 mm and the new AMPLATZER device from 16 to 30 mm. It is preferable that patients take aspirin lifelong and clopidogrel for 3 months after the procedure. The combined use of antiplatelets is important to avoid thrombus formation on the device during the endothelization process.
▪ Feasibility, safety & efficacy
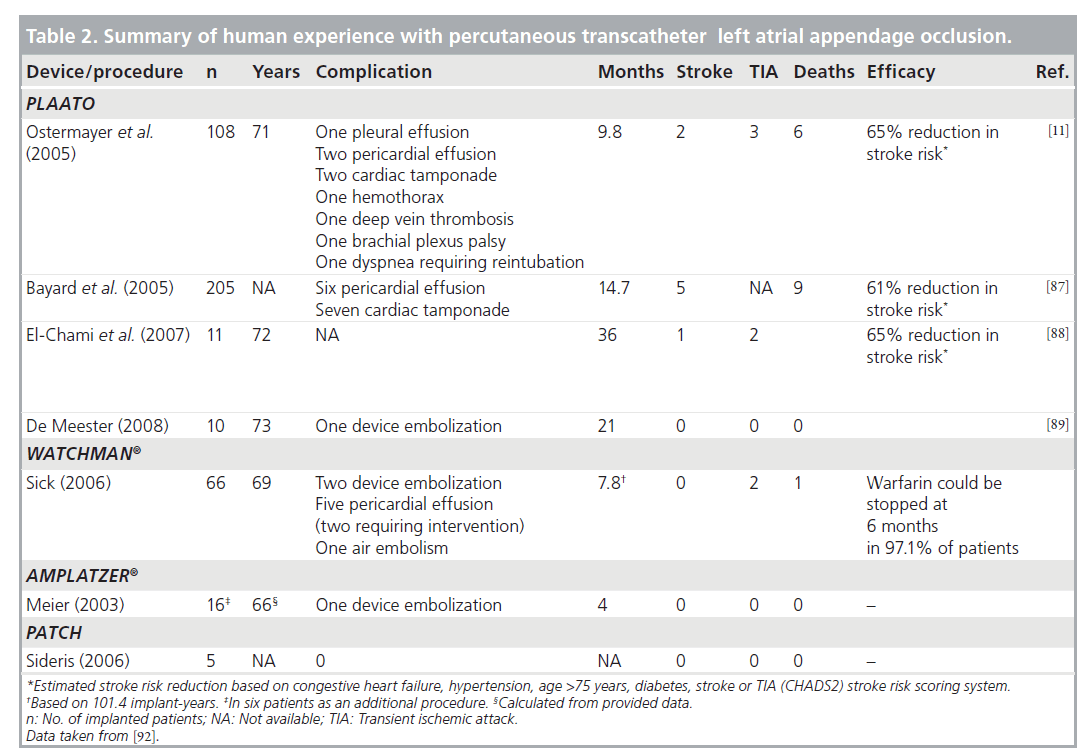
Feasibility has been well established in different studies. First, the technique was attempted in canine models. Percutaneous closure with the PLAATO implant was performed in 25 dogs. On gross anatomy examination after the dogs were euthanized, there was evidence of tissue attachment at 1 month and complete healing over the membrane surface at 3 months when the device was completely incorporated into the LAA wall. These macroscopic findings were confirmed on histological examination on which an organized neointima on the implant surface was visualized [84]. In addition, early clinical experience has shown the feasibility of the PLAATO procedure [10]. Two larger feasibility studies were conducted, one in Europe and one in North America [11]. The LAA occlusion with the PLAATO device was successful in 108 out of 111 (97.3%) patients with nonrheumatic AF. A total of nine procedure- related serious adverse events occurred in seven patients. None of the serious adverse events were considered to be device-related. The major observed complications were pericardial effusion and groin hematoma. Successful LAA occlusion was achieved in 97.7, 100 and 98% of patients immediately after the procedure, at 1 and 6 months, respectively. There was no mobile thrombus, mitral valve damage or pulmonary vein obstruction seen on TEE performed up to 6 months after the procedure. No device migration or dislodgement was noted. There were seven major adverse events in five patients, including two strokes and four cardiac or neurologic deaths, during the average followup of 9.8 months (90.7 documented implant years). A total of six patients (5.4%) died during the study period and none were adjudicated as device- or procedure-related.
Efficacy of percutaneous LAA closure with the PLAATO device has also been studied by means of echocardiography. This showed complete closure of the LAA in 87–97% of patients [11,85]. One post-mortem study illustrated that neo-endothelization of the device also occurred in humans. This study concerned an 84‑year-old woman who died at 1 year after implantation of a PLAATO device. A neointima was formed on the surface of the device and the device occluded the complete orifice of the LAA [86].
The WATCHMAN device has been implanted since 2002 [9]. Patients were assessed at 45 days and 6 months post-procedure with TEE and chest x-ray and had annual clinical assessments thereafter. In 66 patients worldwide, the device has been implanted successfully with 101.4 cumulative implant years. Two patients experienced device embolization. Five pericardial effusions and one major air embolism occurred without long-term sequelae. At follow-up, if the device was found to be stable and no thrombus was detected on TEE, then warfarin was stopped. In 97.1% of patients warfarin could be discontinued at 6 months. Four patients, however, had a flat thrombus on the device at 6 months and one of these patients suffered from a TIA. Another patient with a history of TIA experienced the event without visible LAA thrombus. No ischemic stroke or systemic embolism has occurred. One patient died after 9 months as a result of an ascending aortic dissection. Autopsy documented a stable, well-endothelialized device. These data suggested that the WATCHMAN LAA system was feasible and safe.
▪ Reduction of thromboembolic events
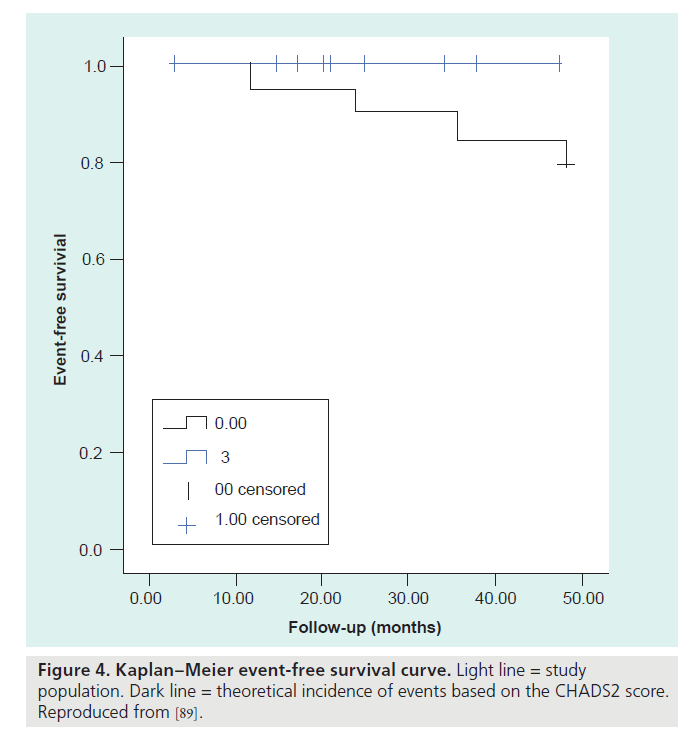
Of the 111 enrolled patients in the PLAATO study [11], two experienced a stroke 173 and 215 days after the implant procedure, respectively. Three TIAs occurred in two patients. The observed and estimated annual stroke rate was 2.2 and 6.3%, respectively, representing a 65% relative stroke risk reduction with the PLAATO procedure. Bayard et al. reported that device implantation was successful in 205 out of 210 (97.6%) patients [87]. In a follow-up of 258 patient-years (mean follow-up: 14.7 months), five patients had a stroke with an estimated 61% reduction from the expected annual risk of stroke. Nine patients died for various reasons not related to the procedure or the device. Whether the percutaneous closure of the LAA gives a long-term reduction of the number of thromboembolic events in patients with AF has not been established yet. However, two small studies suggested promising results. El-Chami et al. followed 11 patients (age 72 ± 9 years) for 36 ± 1.4 months [88]. The predicted stroke risk for their cohort was 8.6% per year as calculated using the CHADS2 score. There was only one stroke during follow-up; the stroke risk in the treated population was 3% per year. No systemic embolic events were noted and no long-term complications related to PLAATO were observed. De Meester et al. followed their patients for a median follow-up term of 21 months, and no thromboembolic events occurred during this follow-up period (Figure 4) [89]. This study was conceived as a proof of principle to reinvigorate interest in the LAA closure technique (summary in Table 2).
In the Embolic Protection in Patients with Atrial Fibrillation (PROTECT AF) trial, researchers compared the current standard of therapy, anticoagulation with warfarin, to the WATCHMAN device [90,91]. The results were recently communicated during the American College of Cardiology meeting in 2009. For this study, 707 patients with nonvalvular AF were randomly assigned to closure of the LAA with the WATCHMAN device (463 patients), followed by discontinuation of warfarin, or to long-term treatment with warfarin (244 patients). The study found that the combined rate of stroke (ischemic and hemorrhagic) and cardiovascular death – the primary measures of effectiveness – was 3.4 per 100 patient-years in the device group versus 5.0 per 100 patient-years in the warfarin group, a relative risk reduction of 32%. As for the safety of the device, the researchers observed more procedure-related complications in patients treated with the device (8.7 vs 4.2 per 100 patientyears). However, after successful implantation of the WATCHMAN and discontinuation of warfarin therapy, complication rates were significantly lower with device therapy (1.7 vs 4.2 per 100 patient-years) (Table 2) [91].
Conclusion
To date, OACs have been considered the standard treatment for AF. However, numerous complications can occur if OACs are administered. If standard treatment fails, the clinician has no good alternative treatment. Different treatment regimens have been used but none gives a solution for the problems addressed above. However, several new therapies are being studied. New anticoagulants have been developed, surgical techniques have been refined and percutaneous LAA closure with specially designed devices has been attempted. Results vary widely between the different techniques. Preliminary studies show especially promising results for LAA closure. It obviates the need for OAC in patients with AF and, thus, lowers the risk of bleeding. Further monitoring and frequent INR control will no longer be necessary. The technique can be applied in patients in whom OACs are contraindicated. In comparison with other LAA exclusion techniques (e.g., surgical techniques, either open or thoracoscopic), percutaneous LAA closure is less invasive. On the other hand, percutaneous LAA closure requires catheterization of the heart, which is an invasive procedure and has inherent risks. In addition, the implantation of the device does not protect against other sources of thromboembolic events (e.g., the left atrium). Most importantly, however, more randomized controlled trials to examine the clinical efficacy of the percutaneous closure of the LAA are needed to at least prove the equal potency of the technique compared with the established treatment regimens.
Future perspective
Given their numerous contraindications and adverse events, OACs are bound to disappear. Several new techniques are being studied. It is still unclear which new therapy will become the gold standard for stroke prevention in AF. Given the promising results, albeit preliminary, percutaneous LAA closure could be a good alternative for OAC administration in the near future. It addresses the problem of avoiding thromboembolic events, the difficulties and adverse events encountered with OACs, while being a safe and minimally invasive procedure. Further studies and clinical trials should be performed to establish the true effectiveness of LAA closure. Currently, the procedure is only indicated in high-risk patients with contra-indications for, or failure of, OACs.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Oral anticoagulants/aspirin is the gold standard for treatment of atrial fibrillation in patients with CHADS2 greater than or equal to 1
▪ Proven in experimental studies as well as in clinical practice.
▪ Wide experience with the technique.
▪ Tools for determining when to administrate oral anticoagulants (OACs) have been developed.
OAC/aspirin treatment has numerous complications and problems in clinical medicine
▪ Difficult international normalized ratio control.
▪ Bleeding complications.
▪ Numerous contraindications.
▪ Not all patients who should receive OACs are treated with OACs.
▪ What if the patient with atrial fibrillation has contraindications for OACs?
Alternative medication treatment
▪ No equivalent results compared with OACs.
▪ Addresses problem of monitoring of international normalized ratio.
▪ Does not address the problem of bleeding complications.
Surgical ligation of the left atrial appendage
▪ This technique has proven to be favorable.
▪ It is an invasive procedure.
▪ Thoracoscopic techniques have been developed.
Percutaneous closure of the left atrial appendage
▪ New devices have been developed.
▪ Feasibility, safety and efficacy has been established.
▪ Reduction in the number of thromboembolic events; results of the first randomized controlled trial are now available.
▪ Promising results in longer-term, small, single-center studies.
▪ The technique warrants further investigation.
Conclusion
▪ Percutaneous closure has captured the attention of interventional cardiologists.
▪ Preliminary studies show promising results.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Wolf PA, Abbott RD, Kannel WB: Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22, 983–988 (1991).
- Sandercock P, Bamford J, Dennis M et al.: Atrial fibrillation and stroke: prevalence in different types of stroke and influence on early and long-term prognosis (Oxfordshire community stroke project). BMJ 305, 1460–1465 (1992).
- Blackshear JL, Odell JA: Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 61, 755–759 (1996).
- Fuster V, Ryden LE, Cannom DS et al.: ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J. Am. Coll. Cardiol. 48, 854–906 (2006).
- Rockson SG, Albers GW: Comparing the guidelines: anticoagulation therapy to optimize stroke prevention in patients with atrial fibrillation. J. Am. Coll. Cardiol. 43, 929–935 (2004).
- Levine MN, Raskob G, Landefeld S, Kearon C: Hemorrhagic complications of anticoagulant treatment. Chest 119, 108S–121S (2001).
- Lechat P, Lardoux H, Mallet A et al.: Anticoagulant (fluindione)–aspirin combination in patients with high-risk atrial fibrillation. A randomized trial (Fluindione, Fibrillation Auriculaire, Aspirin et Contraste Spontane; FFAACS). Cerebrovasc. Dis. 12, 245–252 (2001).
- Hart RG, Benavente O, McBride R, Pearce LA: Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann. Intern. Med. 131, 492–501 (1999).
- Sick PB, Schuler G, Hauptmann KE et al.: Initial worldwide experience with the WATCHMAN® left atrial appendage system for stroke prevention in atrial fibrillation. J. Am. Coll. Cardiol. 49, 1490–1495 (2007).
- First experience with the WATCHMAN® device for left atrial appendage closure. 10 Sievert H, Lesh MD, Trepels T et al.: Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation 105, 1887–1889 (2002).
- Ostermayer SH, Reisman M, Kramer PH et al.: Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J. Am. Coll. Cardiol. 46, 9–14 (2005).
- Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D: Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98, 946–952 (1998).
- Wyse DG, Waldo AL, DiMarco JP et al.: A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 347, 1825–1833 (2002).
- Hohnloser SH, Crijns HJ, van Eickels M et al.: Effect of dronedarone on cardiovascular events in atrial fibrillation. N. Engl. J. Med. 360, 668–678 (2009).
- Kannel WB, Wolf PA, Benjamin EJ, Levy D: Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am. J. Cardiol. 82, 2N–9N (1998).
- Brotman DJ, Deitcher SR, Lip GY, Matzdorff AC: Virchow’s triad revisited. South Med. J. 97, 213–214 (2004).
- Goldsmith IR, Blann AD, Patel RL, Lip GY: von Willebrand factor, fibrinogen, and soluble P-selectin levels after mitral valve replacement versus mitral valve repair. Am. J. Cardiol. 85, 1218–1222 (2000).
- Masawa N, Yoshida Y, Yamada T, Joshita T, Ooneda G: Diagnosis of cardiac thrombosis in patients with atrial fibrillation in the absence of macroscopically visible thrombi. Virchows Arch. A. Pathol. Anat. Histopathol. 422, 67–71 (1993).
- Sanfilippo AJ, Abascal VM, Sheehan M et al.: Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 82, 792–797 (1990).
- Klein AL, Grimm RA, Murray RD et al.: Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N. Engl. J. Med. 344, 1411–1420 (2001).
- Ohyama H, Hosomi N, Takahashi T et al.: Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke 34, 2436–2439 (2003).
- Jaber WA, White RD, Kuzmiak SA et al.: Comparison of ability to identify left atrial thrombus by three-dimensional tomography versus transesophageal echocardiography in patients with atrial fibrillation. Am. J. Cardiol. 93, 486–489 (2004).
- Hwang JJ, Chen JJ, Lin SC et al.: Diagnostic accuracy of transesophageal echocardiography for detecting left atrial thrombi in patients with rheumatic heart disease having undergone mitral valve operations. Am. J. Cardiol. 72, 677–681 (1993).
- Daniel WG, Nellessen U, Schroder E et al.: Left atrial spontaneous echo contrast in mitral valve disease: an indicator for an increased thromboembolic risk. J. Am. Coll. Cardiol. 11, 1204–1211 (1988).
- Bartel T, Muller S, Nesser HJ, Mohlenkamp S, Bruch C, Erbel R: Usefulness of motion patterns indentified by tissue Doppler echocardiography for diagnosing various cardiac masses, particularly valvular vegetations. Am. J. Cardiol. 84, 1428–1433 (1999).
- Agmon Y, Khandheria BK, Gentile F, Seward JB: Echocardiographic assessment of the left atrial appendage. J. Am. Coll. Cardiol. 34, 1867–1877 (1999).
- Valocik G, Kamp O, Mihciokur M et al.: Assessment of the left atrial appendage mechanical function by three-dimensional echocardiography. Eur. J. Echocardiogr. 3, 207–213 (2002).
- Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann. Intern. Med. 116, 6–12 (1992).
- Habara S, Dote K, Kato M et al.: Prediction of left atrial appendage thrombi in nonvalvular atrial fibrillation. Eur. Heart J. 28, 2217–2222 (2007).
- Veinot JP, Harrity PJ, Gentile F et al.: Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation 96, 3112–3115 (1997).
- Al-Saady NM, Obel OA, Camm AJ: Left atrial appendage: structure, function, and role in thromboembolism. Heart 82, 547–554 (1999).
- Pollick C, Taylor D: Assessment of left atrial appendage function by transesophageal echocardiography. Implications for the development of thrombus. Circulation 84, 223–231 (1991).
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ: Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285, 2864–2870 (2001).
- Hart RG, Tonarelli SB, Pearce LA: Avoiding central nervous system bleeding during antithrombotic therapy: recent data and ideas. Stroke 36, 1588–1593 (2005).
- Landefeld CS, Beyth RJ: Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am. J. Med. 95, 315–328 (1993).
- Dahri K, Loewen P: The risk of bleeding with warfarin: a systematic review and performance analysis of clinical prediction rules. Thromb. Haemost. 98, 980–987 (2007).
- Carlquist JF, Horne BD, Muhlestein JB et al.: Genotypes of the cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. J. Thromb. Thrombolysis 22, 191–197 (2006).
- Wadelius M, Chen LY, Downes K et al.: Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 5, 262–270 (2005).
- Rost S, Fregin A, Ivaskevicius V et al.: Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427, 537–541 (2004).
- Furuya H, Fernandez-Salguero P, Gregory W et al.: Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics 5, 389–392 (1995).
- Takahashi H, Wilkinson GR, Nutescu EA et al.: Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African–Americans. Pharmacogenet. Genomics 16, 101–110 (2006).
- Gage BF, Eby C, Milligan PE, Banet GA, Duncan JR, McLeod HL: Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb. Haemost. 91, 87–94 (2004).
- Hillman MA, Wilke RA, Yale SH et al.: A prospective, randomized pilot trial of model-based warfarin dose initiation using CYP2C9 genotype and clinical data. Clin. Med. Res. 3, 137–145 (2005).
- Wadelius M, Chen LY, Eriksson N et al.: Association of warfarin dose with genes involved in its action and metabolism. Hum. Genet. 121, 23–34 (2007).
- Aquilante CL, Langaee TY, Lopez LM et al.: Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin. Pharmacol. Ther. 79, 291–302 (2006).
- Miao L, Yang J, Huang C, Shen Z: Contribution of age, body weight, and CYP2C9 and VKORC1 genotype to the anticoagulant response to warfarin: proposal for a new dosing regimen in Chinese patients. Eur. J. Clin. Pharmacol. 63, 1135–1141 (2007).
- Gage BF, Eby C, Johnson JA et al.: Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin. Pharmacol. Ther. 84, 326–331 (2008).
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G: Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133, 160S–198S (2008).
- Bussey HI, Wittkowsky AK, Hylek EM, Walker MB: Genetic testing for warfarin dosing? Not yet ready for prime time. Pharmacotherapy 28, 141–143 (2008).
- Brass LM, Krumholz HM, Scinto JM, Radford M: Warfarin use among patients with atrial fibrillation. Stroke 28, 2382–2389 (1997).
- Carroll K, Majeed A: Comorbidity associated with atrial fibrillation: a general practicebased study. Br. J. Gen. Pract. 51, 884–886, 889–891 (2001).
- Albers GW, Yim JM, Belew KM et al.: Status of antithrombotic therapy for patients with atrial fibrillation in university hospitals. Arch. Intern. Med. 156, 2311–2316 (1996).
- Bradley BC, Perdue KS, Tisdel KA, Gilligan DM: Frequency of anticoagulation for atrial fibrillation and reasons for its non-use at a Veterans Affairs medical center. Am. J. Cardiol. 85, 568–572 (2000).
- Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S: Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke 37, 1075–1080 (2006).
- Somerfield J, Barber PA, Anderson NE et al.: Not all patients with atrial fibrillationassociated ischemic stroke can be started on anticoagulant therapy. Stroke 37, 1217–1220 (2006).
- van Walraven C, Jennings A, Oake N, Fergusson D, Forster AJ: Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest 129, 1155–1166 (2006).
- Benavente O, Hart R, Koudstaal P, Laupacis A, McBride R: Antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database Syst. Rev. 2, CD001925 (2000).
- Connolly S, Pogue J, Hart R et al.: Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 367, 1903–1912 (2006).
- Connolly SJ, Pogue J, Hart RG et al.: Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N. Engl. J. Med. 360, 2066–2078 (2009).
- Ford GA, Choy AM, Deedwania P et al.: Direct thrombin inhibition and stroke prevention in elderly patients with atrial fibrillation: experience from the SPORTIF III and V Trials. Stroke 38, 2965–2971 (2007).
- Bousser MG, Bouthier J, Buller HR et al.: Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open-label, non-inferiority trial. Lancet 371, 315–321 (2008).
- Lubitz SA, Fischer A, Fuster V: Catheter ablation for atrial fibrillation. BMJ 336, 819–826 (2008).
- Nair GM, Nery PB, Diwakaramenon S, Healey JS, Connolly SJ, Morillo CA: A systematic review of randomized trials comparing radiofrequency ablation with antiarrhythmic medications in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 20, 138–144 (2009).
- Bertaglia E, Zoppo F, Tondo C et al.: Early complications of pulmonary vein catheter ablation for atrial fibrillation: a multicenter prospective registry on procedural safety. Heart Rhythm 4, 1265–1271 (2007).
- Oral H, Chugh A, Ozaydin M et al.: Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 114, 759–765 (2006).
- Hindricks G, Piorkowski C, Tanner H et al.: Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation 112, 307–313 (2005).
- Seow SC, Lim TW, Koay CH, Ross DL, Thomas SP: Efficacy and late recurrences with wide electrical pulmonary vein isolation for persistent and permanent atrial fibrillation. Europace 9, 1129–1133 (2007).
- Natale A, Raviele A, Arentz T et al.: Venice Chart international consensus document on atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 18, 560–580 (2007).
- Garcia-Fernandez MA, Perez-David E, Quiles J et al.: Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J. Am. Coll. Cardiol. 42, 1253–1258 (2003).
- Hellerstein HK, Sinaiko E, Dolgin M: Amputation of the canine atrial appendages. Proc. Soc. Exp. Biol. Med. 66, 337 (1947).
- Madden JL: Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J. Am. Med. Assoc. 140, 769–772 (1949).
- Beal JM, Longmire WP Jr, Leake WH: Resection of the auricular appendages. Ann. Surg. 132, 517–530 (1950).
- Johnson WD, Ganjoo AK, Stone CD, Srivyas RC, Howard M: The left atrial appendage: our most lethal human attachment! Surgical implications. Eur. J. Cardiothorac. Surg. 17, 718–722 (2000).
- Yilmaz A, Van Putte BP, Van Boven WJ: Completely thoracoscopic bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J. Thorac. Cardiovasc. Surg. 136, 521–522 (2008).
- Healey JS, Crystal E, Lamy A et al.: Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am. Heart J. 150, 288–293 (2005).
- Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I: Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J. Am. Coll. Cardiol. 36, 468–471 (2000).
- Odell JA, Blackshear JL, Davies E et al.: Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann. Thorac. Surg. 61, 565–569 (1996).
- Blackshear JL, Johnson WD, Odell JA et al.: Thoracoscopic extracardiac obliteration of the left atrial appendage for stroke risk reduction in atrial fibrillation. J. Am. Coll. Cardiol. 42, 1249–1252 (2003).
- Kamohara K, Fukamachi K, Ootaki Y et al.: A novel device for left atrial appendage exclusion. J. Thorac. Cardiovasc. Surg. 130, 1639–1644 (2005).
- Wolf RK, Schneeberger EW, Osterday R et al.: Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J. Thorac. Cardiovasc. Surg. 130, 797–802 (2005).
- Balkhy HH, Chapman PD, Arnsdorf SE: Minimally invasive atrial fibrillation ablation combined with a new technique for thoracoscopic stapling of the left atrial appendage: case report. Heart Surg. Forum 7, 353–355 (2004).
- Chiam PT, Ruiz CE: Percutaneous transcatheter left atrial appendage exclusion in atrial fibrillation. J. Invasive Cardiol. 20, E109–113 (2008).
- Cruz-Gonzalez I, Cubeddu RJ, Sanchez- Ledesma M et al.: Left atrial appendage exclusion using an Amplatzer device. Int. J. Cardiol. 134, E1–E3 (2009).
- Nakai T, Lesh MD, Gerstenfeld EP, Virmani R, Jones R, Lee RJ: Percutaneous Left Atrial Appendage Occlusion (PLAATO) for preventing cardioembolism: first experience in canine model. Circulation 105, 2217–2222 (2002).
- Hanna IR, Kolm P, Martin R, Reisman M, Gray W, Block PC: Left atrial structure and function after percutaneous left atrial appendage transcatheter occlusion (PLAATO): six-month echocardiographic follow-up. J. Am. Coll. Cardiol. 43, 1868–1872 (2004).
- Omran H, Schmidt H, Hardung D et al.: Post-mortem analysis of a left atrial appendage occlusion device (PLAATO) in a patient with permanent atrial fibrillation. J. Interv. Card. Electrophysiol. 14, 17–20 (2005).
- Bayard Y, Omran H, Kramer P et al.: Worldwide experience of percutaneous left atrial appendage transcatheter occlusion (PLAATO). J. Neurol. Sci. 238, S70 (2005).
- El-Chami MF, Grow P, Eilen D, Lerakis S, Block PC: Clinical outcomes three years after PLAATO implantation. Catheter Cardiovasc. Interv. 69, 704–707 (2007).
- De Meester P, Thijs V, Van Deyk K, Budts W: Prevention of stroke by percutaneous left atrial appendage closure: short term follow-up. Int. J. Cardiol. DOI: 10.1016/j.ijcard2008.11.112 (2009) (Epub ahead of print).
- Fountain R, Holmes DR Jr, Hodgson PK, Chandrasekaran K, Van Tassel R, Sick P: Potential applicability and utilization of left atrial appendage occlusion devices in patients with atrial fibrillation. Am. Heart J. 152, 720–723 (2006).
- Fountain RB, Holmes DR, Chandrasekaran K et al.: The PROTECT AF (WATCHMAN® Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) trial. Am. Heart J. 151, 956–961 (2006).
- Onalan O, Crystal E: Left atrial appendage exclusion for stroke prevention in patients with nonrheumatic atrial fibrillation. Stroke 38, 624–630 (2007).
▪ Describes epidemiological data about atrial fibrillation and stroke.
▪▪ Overview of the guidelines for the management of patients with atrial fibrillation.
▪ Describes use of oral anticoagulants in patients with atrial fibrillation.
▪ First experience with the WATCHMAN® device for left atrial appendage closure.
▪ First experience with the PLAATO device for left atrial appendage closure.
▪▪ Detailed feasibility trial of the PLAATO device.
▪▪ Compares rate versus rhythm control in patients with atrial fibrillation.
▪ Surgical left atrial appendage obliteration in patients with mitral valve surgery.
▪ Surgical left atrial appendage occlusion in patients with coronary bypass surgery.