Review Article - Interventional Cardiology (2012) Volume 4, Issue 1
Percutaneous coronary intervention in patients with prior coronary bypass surgery in 2012
- Corresponding Author:
- John S Douglas Jr
Green Lane Cardiovascular Service
Auckland City Hospital Park Road
Auckland, New Zealand
Tel: +1 404 727 7040
Fax: +1 404 712 1385
E-mail: jdoug01@emory.edu
Abstract
Keywords
coronary bypass surgery n drug-eluting stent n embolic protection n hybrid revascularization n percutaneous coronary intervention n saphenous vein graft
Coronary artery bypass graft surgery (CABG), one of the most commonly performed and expensive operations, provides excellent relief from anginal symptoms and prolongs life in patients with the most advanced disease. However, in spite of multiple improvements in coronary risk factor modification, antithrombotic drugs, use of arterial grafts and off-pump techniques, recurrent angina is a common problem following CABG. Poor performance of saphenous vein grafts (SVGs) subjected to arterial pressure and progressive atherosclerosis is the major limitation, but imprecise surgical technique, unbypassed or unbypassable diseased coronary arteries, graft thrombosis and progressive coronary artery atherosclerosis may also contribute. Due to the increased morbidity and mortality and reduced effectiveness of reoperative CABG, nonsurgical approaches are generally favored. The early experience with percutaneous coronary intervention (PCI) in 116 consecutive post-CABG patients at Emory University about 30 years ago was quite favorable with no in-hospital mortality, only three emergency operations, one Q-wave myocardial infarction (MI) and lasting symptom relief in over three-quarters of the patients [1]. This contrasted with the experience in the National Heart, Lung and Blood PTCA Registry, where 8.1% of the first 62 post-CABG patients died in-hospital. In the subsequent 20-year experience at Emory University (Atlanta, GA, USA) with over 34,000 PCIs, a history of prior CABG was not associated with an increased risk of death or Q-wave MI, but was associated with a reduced need for in-hospital CABG [2]. With the maturation of PCI technology and techniques, availability of stents and improved antithrombotic therapy, PCI has become a much more effective option and is clearly the dominant revascularization strategy in the post-CABG patient with recurrent anginal symptoms in 2012. However, selection of PCI or repeat CABG is influenced by a number of factors, with the status of the left anterior descending coronary artery (LAD) and its graft having major impact. Patients with a patent left internal mammary artery (LIMA) to LAD have been shown to have enhanced survival [3]. In a patient with a patent LIMA graft to the LAD, reoperation to bypass non-LAD coronary artery disease offers no additional survival benefit and may jeopardize the LIMA graft. For these reasons PCI is preferred. However, the presence of severe disease of a SVG to LAD, or a SVG supplying a large area of viable myocardium, multiple diseased SVGs, or multivessel coronary disease and moderately impaired left ventricular function, may warrant consideration for reoperation. The characteristics of coronary artery and bypass graft lesions obstructing coronary blood flow and producing myocardial ischemia, and the PCI treatment strategies utilized to relieve these obstructions, are significantly different depending on the length of time that has elapsed since CABG was performed. For this reason, selection for PCI and its performance will be discussed based on the time since surgery.
Early postoperative ischemia
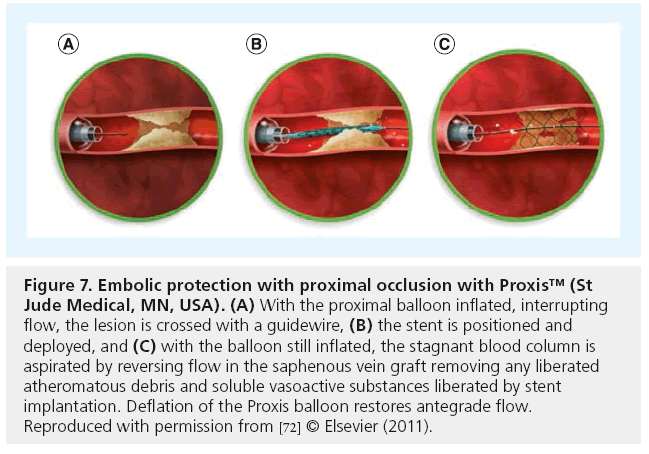
Three studies published in 1996 and 1997, reported the findings of coronary angiography performed in a total of 145 patients due to the development of myocardial ischemia within hours or days of CABG [4–6]. In approximately half of these patients, PCI or a return to the operating room was needed due to graft failure. In a more recent report, emergency coronary angiography was performed in 118 of 5427 consecutive isolated CABG patients, of whom 67 (57%) had graft failure and 40 (60%) underwent repeat revascularization [7]. Angiography and PCI in patients with recurrent ischemia soon after CABG is a Class I indication in the American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions PCI Guideline statement and the European Guidelines on Myocardial Revascularization and is highly recommended [8,9]. If a thrombosed graft is found, PCI of the native coronary artery is the preferred strategy. Use of thrombolytic agents or GPIIb/IIIa inhibitors risk major bleeding complications, but mechanical thrombectomy can be performed if the native vessel is not suitable for PCI, or a return to the operating room may be reasonable if the jeopardized vessel is important and cannot be effectively preserved with PCI. Significant distal anastomotic lesions of SVGs (Figure 1) or LIMA grafts, can be balloon dilated but balloon sizing should be conservative as suture line disruption has occurred. Stenting of distal anastomotic lesions is only required occasionally at this point. Mild to moderate anastomotic imperfections in the presence of excellent flow should not be treated, as they frequently disappear on subsequent angiography [10]. Whether this is due to resolution of edema, or some reparative quality of the LIMA, is unknown [11]. Some investigators have performed routine open-chest coronary angiography in a hybrid operating room, immediately after CABG, with interesting findings. Zhoa et al. studied 796 grafts in 366 consecutive patients, finding 12% of grafts had defects severe enough to warrant surgical revision (3.4%), intraoperative PCI (6%) or minor adjustment of the graft (2.8%) [12]. This relatively high frequency of technical problems is disturbing. Whether correction of these technical problems will lead to a better outcome, such as improved graft patency and heightened symptom relief, is unproven but is certainly a logical conclusion. Although routine completion angiography may not be possible, immediate coronary angiography is warranted in any early post-CABG patient suspected of having ischemia. Patients at increased risk of early graft failure include those with severe diffuse coronary atherosclerosis, patients undergoing minimally invasive CABG, those receiving offpump grafting and when arterial grafts other than the LIMA are constructed. In some cases, stenoses or total occlusions of LIMA or radial artery grafts have been successfully treated with balloon dilation plus stents, but graft rupture is a potential hazard (see Complications below). Hybrid revascularization (minimally invasive LIMA to LAD with stenting of non-LAD targets) is an attractive option for many patients with advanced disease and this represents a planned PCI following CABG (see ‘Hybrid coronary revascularization’ section below).
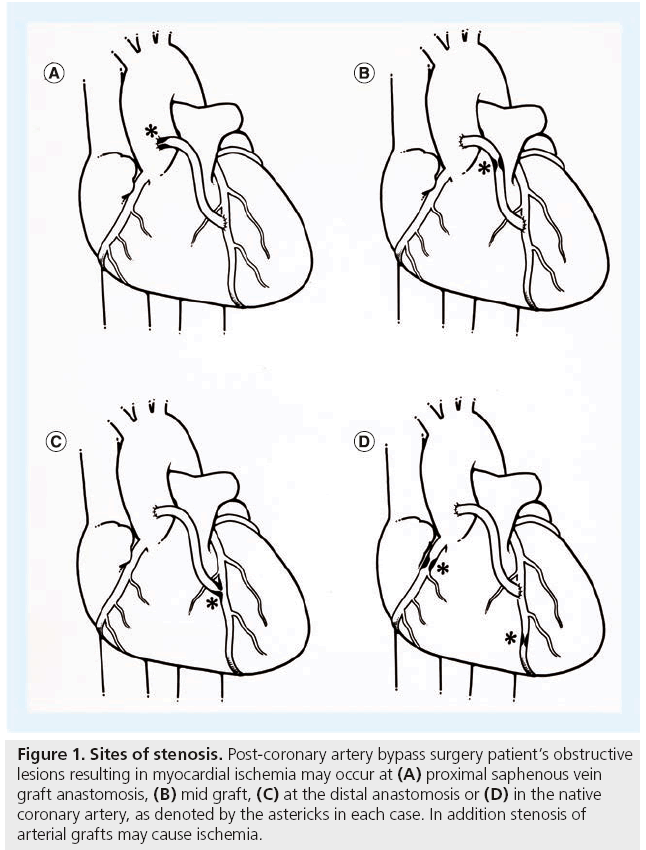
Figure 1: Sites of stenosis. Post-coronary artery bypass surgery patient’s obstructive lesions resulting in myocardial ischemia may occur at (A) proximal saphenous vein graft anastomosis, (B) mid graft, (C) at the distal anastomosis or (D) in the native coronary artery, as denoted by the astericks in each case. In addition stenosis of arterial grafts may cause ischemia.
1–12 months post-CABG
Stenosis at the distal anastomosis of a SVG or LIMA graft is a common finding in patients with recurrent angina within the first year after CABG. Among 34 patients with distal anastomotic lesions reported from our experience, 22 presented within 6 months and 25 (74%) within 1 year of CABG [1]. Of the 32 patients successfully dilated, only four (12%) developed restenosis and three were redilated successfully. A total of 30 of the 32 patients (93%) had continued success at a mean follow-up of 10 months. It is important to note that this experience predated stents, which are now rarely used for treatment of early postoperative distal anastomosis lesions in our practice.
Vein graft thrombosis is also a common finding among patients with recurrent ischemia 1–12 months postoperatively. There is no prefered treatment in this situation, unless the native vessel is accessible. Attempts to administer low dose intragraft thrombolytic therapy for 4–12 h has been complicated by bleeding and little long term benefit. Several recently published studies analyzed angiographically assessed graft patency at 6–12 months following CABG and the findings were sobering. The most recent, the RIGOR study, reported that multidetector coronary computed tomography angiography (CTA) determined graft patency at 6 months in 229 patients and 497 SVGs, finding 21% of grafts to be occluded or containing >75% stenoses [13]. In 21 patients (9%), all implanted SVGs were occluded. In PRAGUE 4, angiographic follow-up at 1 year found over 40% of SVGs and 9% of LIMA grafts were occluded [14]. In PREVENT IV, performed in over 100 high volume centers, 29% of SVGs and 8% of LIMA grafts failed at 1 year, and graft failure was associated with death, new MI or repeat revascularization in 26% of patients [15]. In the ROOBY study, 23% of SVGs and 11% of LIMA grafts performed off-pump failed at 1 year [16]. These disappointing graft patency results, especially in SVGs, highlight the need for better antithrombotic measures, more frequent use of arterial grafts and support a practice of maintaining a low threshold for coronary angiography in postoperative patients with clues to possible ischemic symptoms. PCI of a graft stenosis, especially at a distal anastomosis, may confer long-term patency, whereas progression to thrombotic occlusion usually renders the graft unsalvageable.
Over 3 years following CABG
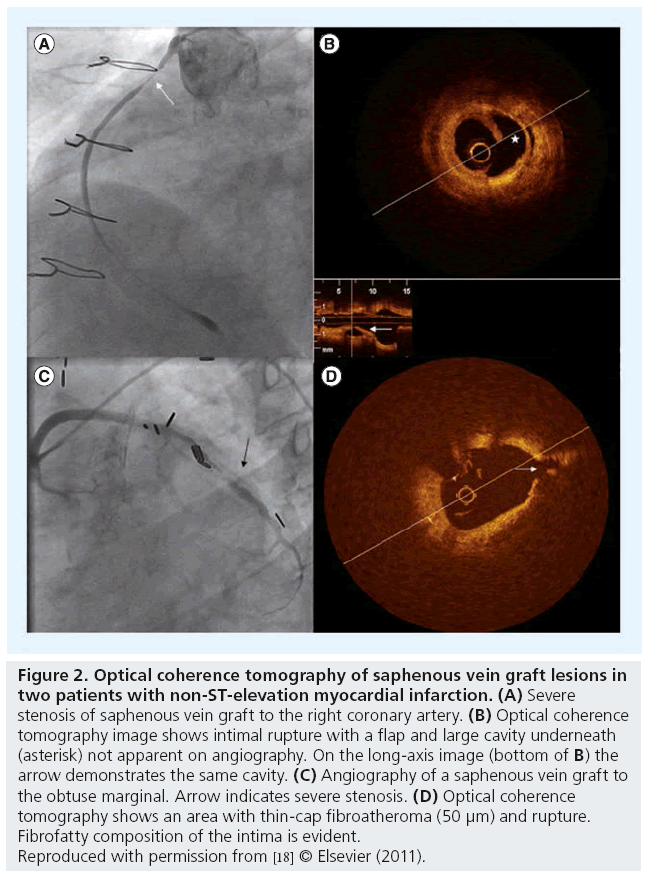
Autopsy studies have detected atheromatous plaques in SVGs within a year of surgery, but hemodynamically significant atheromatous lesions are uncommon before 3 years post- CABG. Neointimal growth, believed to be due to the high pressure environment in which the veins are placed, is the predominant early finding. After 3 years, the atherosclerotic process can be highly accelerated with development of a lipid core, a thin fibrous cap, plaque rupture, hemorrhage into the necrotic lipid core and graft thrombosis [17]. Recently reported optical coherence tomography (OCT) studies of SVGs in patients with acute coronary syndromes, demonstrated the enormous complexity of SVG lesions compared with the simple angiographic findings (Figure 2) and, along with pathology studies, explain the high frequency of atheroembolization and its potentially catastrophic results complicating SVG PCI [18]. Although SVG thrombosis or stenosis is the main cause for hospitalization, information from the National Cardiovascular Data Registry (NCDR) indicates that the PCI target in post-CABG patients was a native coronary artery in 62% of patients and a bypass graft in 37% of patients [19].
Figure 2: Optical coherence tomography of saphenous vein graft lesions in two patients with non-ST-elevation myocardial infarction. (A) Severe stenosis of saphenous vein graft to the right coronary artery. (B) Optical coherence tomography image shows intimal rupture with a flap and large cavity underneath (asterisk) not apparent on angiography. On the long-axis image (bottom of B) the arrow demonstrates the same cavity. (C) Angiography of a saphenous vein graft to the obtuse marginal. Arrow indicates severe stenosis. (D) Optical coherence tomography shows an area with thin-cap fibroatheroma (50 μm) and rupture. Fibrofatty composition of the intima is evident.
Reproduced with permission from [18] © Elsevier (2011).
ST-elevation MI presentation
Current guidelines do not provide specific recommendations regarding optimal reperfusion in patients with prior CABG and, in the past decade, few studies have addressed this issue. Early studies demonstrated a reduced efficacy of thrombolytic therapy in SVGs. Among 128 post- CABG patients in the recently reported APEXAMI trial, emergency angiography revealed that the infarct related vessel was a bypass graft in 63 individuals (41%). Patients with prior CABG were older, had more comorbidities, more coronary disease and increased 90-day death and major adverse cardiac events (MACE) compared with patients without prior CABG [20]. When post-CABG patient outcome was analyzed based on vessel type, 90-day death was significantly increased in patients receiving PCI of a bypass graft versus native coronary artery (19 vs 5.7%; p = 0.05). Among 128 prior CABG patients with STEMI who underwent PCI at the Mayo Clinic (MN, USA), treatment of a SVG was also independently associated with adverse cardiac events [21]. Confronted with a post-CABG patient with ST-elevation myocardial infarction (STEMI), most PCI operators pursue an invasive strategy with coronary angiography and PCI if feasible. Unfortunately, graft occlusion and a large thrombus burden are common findings and use of thrombectomy and embolic protection strategies may be needed (Figure 3). In a report of 192 post-CABG patients with acute MI who underwent SVG PCI at the Washington Hospital Center (Washington, DC, USA), 30-day mortality was also high compared with patients with native vessel PCI (14.3 vs 8.4%; p = 0.03) [22]. The American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) classified PCI of a SVG as Class IIa, ‘acceptable, of uncertain efficacy and may be controversial; weight of evidence in favor of usefulness/efficacy’ [23].
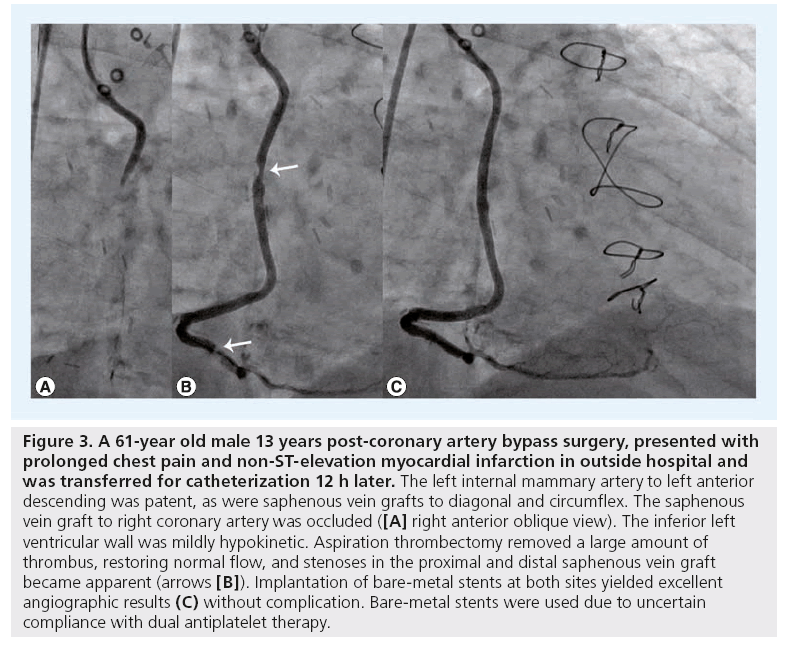
Figure 3: A 61-year old male 13 years post-coronary artery bypass surgery, presented with prolonged chest pain and non-ST-elevation myocardial infarction in outside hospital and was transferred for catheterization 12 h later. The left internal mammary artery to left anterior descending was patent, as were saphenous vein grafts to diagonal and circumflex. The saphenous vein graft to right coronary artery was occluded ([A] right anterior oblique view). The inferior left ventricular wall was mildly hypokinetic. Aspiration thrombectomy removed a large amount of thrombus, restoring normal flow, and stenoses in the proximal and distal saphenous vein graft became apparent (arrows [B]). Implantation of bare-metal stents at both sites yielded excellent angiographic results (C) without complication. Bare-metal stents were used due to uncertain compliance with dual antiplatelet therapy.
Revascularization results
▪ PCI compared with repeat CABG
The results of PCI and reoperative surgery were compared for over 4000 post-CABG patients treated at Emory University between 1980 and 1994. In-hospital results were better with PCI, with respect to mortality, Q-wave MI, stroke, length of stay and costs (US$8500 vs $24,200; p < 0.01). Survival at 10 years was better with PCI and superior with native coronary versus graft PCI [24]. This contrasted with the Mayo Clinic experience, where outcomes were similar with SVG and native vessel PCI [25]. Multivessel revascularization was performed in 2,191 post-CABG patients at the Cleveland Clinic (OH, USA) between 1995 and 2000. This study determined that 30-day outcomes were more favorable with PCI for mortality, Q-wave MI and stroke, and 5-year survival was better with surgery (79 vs 76%; p = 0.008) [26]. In the only randomized trial comparing PCI and CABG in post-CABG patients, Morrison et al. reported lower in-hospital mortality with PCI (0 vs 8%), but similar survival and symptomatic status at 3 years [27]. Among almost 2000 diabetic post-CABG patients requiring PCI (n = 1123) or reoperation (n = 589) at Emory University between 1985 and 1999, survival was similar at 5 years (62 and 61%) [28]. It is important to remember that all the published studies comparing PCI and repeat CABG were performed in the bare-metal stent (BMS) era. Use of drug-eluting stents (DES) and contemporary dual antiplatelet therapy may lead to better outcomes with PCI and, of course, surgical techniques have improved as well.
▪ Results of native coronary PCI
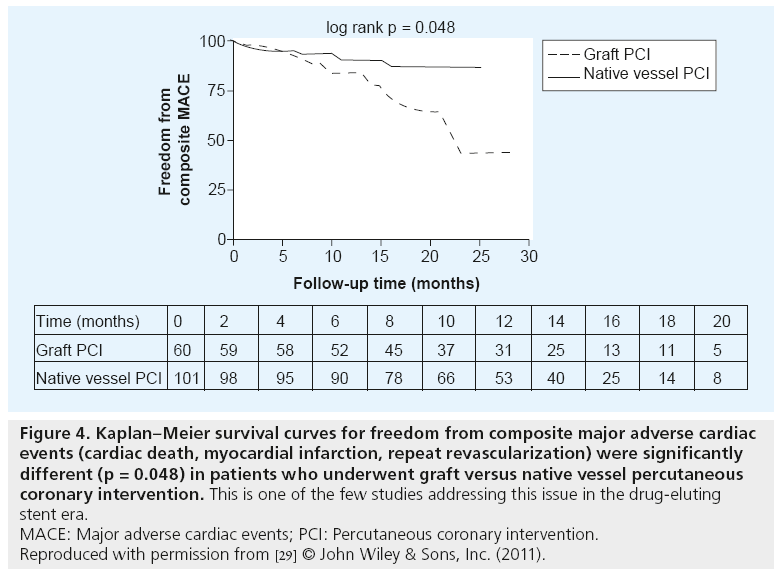
Recently published information from the National Coronary Data Registry, analyzing over 300,000 post-CABG patients who underwent PCI between 2004 and 2009, showed that post-CABG patients constitute over 17% of all PCIs and that native coronary artery PCIs were more frequent than graft PCI (62.5 vs 37.5%) [19]. There is very little information regarding outcomes of native vessel PCI in post-CABG patients in the DES era. Pre-DES era reports, however, indicate generally favorable outcomes [1,25–27]. In one of the few DES-era reports, Bundhoo et al. analyzed outcomes of 161 post- CABG patients [29]. Similar to the NCDR experience, 63% of patients underwent native vessel PCI and an additional 4% had both native vessel and graft PCI. DES were used in 85% of native vessel PCIs and a similar frequency in grafts. Results of native vessel PCI were favorable, with mean Canadian Cardiovascular Society angina classification reduced from 2.9 to 0.7 and composite MACE (cardiac death, MI, repeat revascularization) was significantly less than with graft PCI (8.9 vs 21.6%; p = 0.048) (Figure 4) at a mean follow-up of 13 months. There were no early or late cardiac deaths in the native vessel PCI group and only 4.9% required target vessel revascularization in a follow-up period of 13.5 months. Significantly lower in-hospital mortality and procedural complications were similarly reported for native vessel compared with PCI of grafts in the NCDR report (0.9 vs 1.5%; p < 0.001 and 4.7 vs 6.6%; p < 0.001, respectively) [19]. The available info rmation supports PCI of the native vessel rather than the graft when there is a choice.
Figure 4: Kaplan–Meier survival curves for freedom from composite major adverse cardiac events (cardiac death, myocardial infarction, repeat revascularization) were significantly different (p = 0.048) in patients who underwent graft versus native vessel percutaneous coronary intervention. This is one of the few studies addressing this issue in the drug-eluting stent era.
MACE: Major adverse cardiac events; PCI: Percutaneous coronary intervention.
Reproduced with permission from [29] © John Wiley & Sons, Inc. (2011).
▪ SVG PCI
US and EU guidelines [8,9] and appropriateness criteria for coronary revascularization [30] encourage the use of PCI in early graft failure, but are cautious regarding PCI in SVGs >3 years old due to the risk of periprocedural MI and poor long-term results of PCI in older grafts. SVG PCI is a Class IIa indication in severely symptomatic patients, those with preserved left venricular function, patent LIMA to LAD or in poor candidates for CABG. Criteria for determining significant stenosis in SVGs have generally mirrored those in native coronary artery stenosis. However, rapid progression of atherosclerosis in SVGs is well recognized, tempting some operators to intervene on less severe SVG lesions. Ellis et al. reported that patients with moderate, untreated stenoses in SVGs, had a 45% cardiac event rate compared with 2% in patients without moderate lesions, and suggested that a strategy of ‘sealing’ moderate lesions with stents be considered [31]. Rodes-Cabau et al. performed a pilot trial involving 57 patients, randomizing them to paclitaxel stent implantation in intermediate stenoses (30–60%) or medical therapy alone, and observed significantly lower MACE rates at 1 and 3 years in patients who underwent prophylactic plaque ‘sealing’ with paclitaxel stents [32,33]. These authors are currently conducting a larger trial to test this strategy (the VELETI II study).
▪ BMS versus balloon angioplasty in SVGs
Early observational studies of angioplasty in SVGs revealed restenosis in over 50% of patients with proximal and mid-lesions, but more favorable results with distal anastomotic lesions [1,34]. In a randomized comparison of Palmaz-Schatz™ stent implantation and standard balloon angioplasty (the SAVED trial) in 220 patients, outcomes were better with stenting with respect to angiographic success (97 vs 86%; p < 0.01), restenosis (37 vs 46%; p = 0.24) and MACE at 8 months (27 vs 42%; p = 0.03) [35]. Of note, the primary angiographic end point (binary restenosis) failed to be significantly better with stenting. Until the later availability of DES, BMS implantation became the default strategy in SVG PCI. However, it is important to note that even in the SAVED trial, where mostly simple lesions were treated, the mortality at 8 months was 7–9%. Among 2556 patients who underwent SVG PCI at Emory University, 5-year mortality was higher in diabetics compared with nondiabetics (37.1 vs 21.5%; p < 0.0001) again highlighting the relatively high mortality following SVG PCI [36].
▪ DES versus BMS in SVGs
Based on the favorable results with DES in native coronary arteries, many operators utilized DES in SVGs and a number of observational studies were reported with mixed results. In one of the largest reported, experiences of over 1000 patients, the Stent Group noted that DEStreated patients experienced fewer MACE (14 vs 21%; p = 0.001), lower frequency of death or MI, lower adjusted target vessel revascularization (TVR) and less stent thrombosis [37]. Almost a dozen meta-analyses comparing DES with BMS in SVG have been published, indicating that DES reduced MACE with preserved safety; an example is a meta-analysis of 20 studies including over 5000 patients with a 36% decrease in MACE associated with DES use [38].
Three randomized trials of DES versus BMS in SVGs have been published. One small trial randomized 75 patients to sirolimus-eluting stents or BMS [39]. Although 6-month angiographic follow-up showed reduced restenosis and TVR, at 3 years TVR rates were similar, suggesting a ‘late catch up’ and mortality was higher with sirolimus-eluting stents [40], raising a question as to the longer term value of DES in SVGs [41]. Brilakis et al. in a prospective, randomized trial of 80 patients with SVG lesions treated with paclitaxel-eluting stents or BMS, reported less TLR with DES at 1.5 years, with no difference in mortality [42] and significantly better clinical outcomes were documented to extend to 3 years [43].
The recently reported ISAR-CABG study is the first randomized comparison of DES and BMS in SVGs powered for clinical end points [44]. A total of 610 patients with de novo SVG lesions were randomly assigned to receive one of three DES or a BMS. The primary end point was a composite of death, MI or TLR at 1 year. Outcomes are reported in Table 1. DES reduced the incidence of the primary end point (15 vs 22%; p = 0.02) and the rate of TLR (7 vs 13%; p = 0.01). There were no differences in death, MI, or definite or probable stent thrombosis. A total of 438 patients (72%) underwent routine follow-up angiography (median 6.7 months), with less restenosis and graft occlusion in DEStreated patients (15 vs 29%; p < 0.0001 and 6 vs 12%; p = 0.008, respectively). The reduction in MACE of 36%, observed in the present study, is identical to that reported in a meta-analysis [38] and similar to that noted in the large STENT registry [37], and supports the use of DES in SVGs in patients who are candidates for prolonged dual antiplatelet therapy with no increased risk of death, MI or stent thrombosis. However, the follow-up period in ISAR-CABG was only 1 year and the favorable findings should be confirmed by a longer follow-up. In addition, the scheduled angiographic followup in ISAR-CABG may have magnified the apparent benefit of DES in SVGs by increasing TLR in the BMS group. A recent publication provided reassuring 4-year follow-up data in a large population-based cohort comprised of 709 propensity-score matched pairs of patients (n = 1418), who received DES or BMS in SVGs [45]. At 4-year follow-up, the rate of repeat TVR was 21% in DES-treated patients and 28% in the BMS group (24% reduction; p = 0.004). In patients with diabetes or receiving long stents (≥30 mm), the number of procedures needed to prevent a TVR at 4 years was 8 and 7, respectively. The risk of death or MI associated with the use of DES in this long follow-up study was less than with BMS, but it did not achieve statistical significance.
▪ Embolic protection
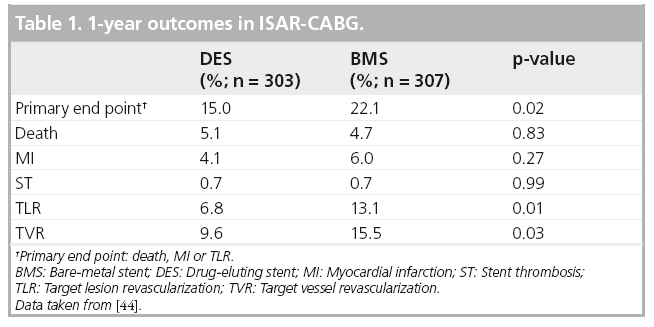
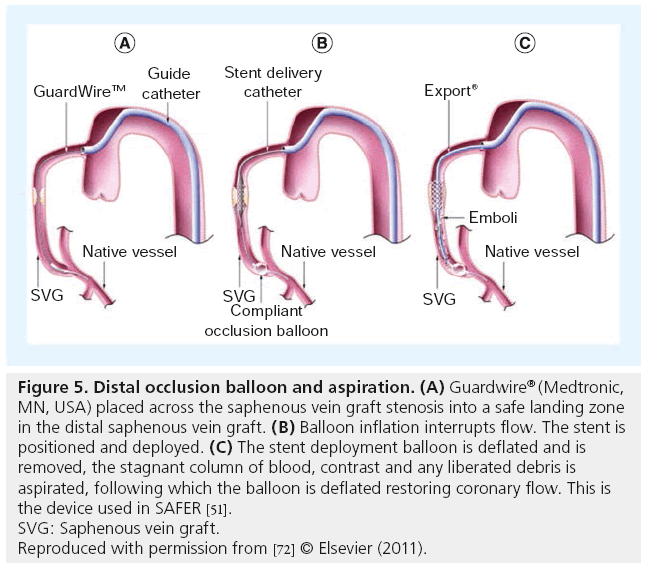
Saber et al. examined the hearts of five patients who died following SVG PCI, finding atheromatous plaque (foam cells, cholesterol clefts, blood elements and necrotic debris) obstructing intramural coronary arteries [46]. Subsequently, periprocedural MI and no-reflow were recognized as common complications of SVG PCI, predicted by angiographic estimates of plaque volume, SVG degeneration and presence of thrombus [47,48]. Efforts to mitigate the microvascular plugging and effects of soluble vasoconstrictors shown to be released during SVG PCI [49], include thrombus aspiration, microvascular dilators and embolic protection devices [50,51]. An analysis of five randomized trials showed no benefit with use of GPIIb/IIIa inhibitors administered during SVG PCI [52]; however, these agents are used in selected patients with large amounts of thrombus in our practice. There is only one randomized controlled trial of embolic protection devices in SVG PCI, the SAFER trial, which employed a distal occlusion balloon (Figure 5) which interrupted flow followed by stent implantation and aspiration of the suspended dye column and any debris generated [51]. Thirty-day MACE was reduced by 42% (16.5 to 9.6%; p = 0.004), primarily because of reduced non-Q-wave MI. Subsequently, several embolic protection devices employing filters (Figure 6) or proximal balloon occlusion (Figure 7) were tested against an active control arm demonstrating noninferiority. These comparisons were summarized by Coolong et al. who also expanded on the work of Liu et al. and developed a model to predict 30-day MACE following SVG PCI [47,48]. They noted that treatment benefit of embolic protection extended across all categories of risk, as occurred in SAFER [48,51]. This contrasts with the observation that embolic protection was used in only 22% of over 19,000 patients who underwent SVG PCI in the NCDR [53]. Clearly, there are limitations of embolic protection devices. Distal and proximal occlusion devices require interruption of flow and associated ischemia, cause reduced vessel visualization, involve crossing the lesion to place the distal balloon, but capture even very small particles and soluble elements. Filters are easier to use, permit flow during stenting with less ischemia and better visualization, but must cross the lesion, do not filter out small (<100 u) particles or soluble substances, may not protect both limbs of a Y graft and can be overwhelmed with large embolic loads. Although use of embolic protection has a Class I indication in US and European guideline statements [8,9], selective use of these devices is the norm, with about half of patients being protected in some recently published reports [54,55]. Interestingly, in the ISAR-CABG study, in which 610 patients with SVG lesions received stents (mean graft age 13.5 years and stent length ~27 mm), embolic protection was used in <5% and 30-day MACE was low (4%) [44]. This very selective use of embolic protection with apparent safety, suggests that patient selection factors or more effective modern pharmacotherapy, or both, may be in play.
Figure 5: Distal occlusion balloon and aspiration. (A) Guardwire® (Medtronic,
MN, USA) placed across the saphenous vein graft stenosis into a safe landing zone
in the distal saphenous vein graft. (B) Balloon inflation interrupts flow. The stent is positioned and deployed. (C) The stent deployment balloon is deflated and is removed, the stagnant column of blood, contrast and any liberated debris is aspirated, following which the balloon is deflated restoring coronary flow. This is the device used in SAFER [51].
SVG: Saphenous vein graft.
Reproduced with permission from [72] © Elsevier (2011).
Figure 6: Embolic protection with a filter. Following placement of the constrained filter (A) across the stenosis, the sheath is withdrawn deploying the filter (B). The device shown is FilterWire EZ™ (Boston Scientific, MA, USA), which is one of several filters available for saphenous vein graft percutaneous coronary intervention. Filters have the major advantage of preserving coronary flow during percutaneous coronary intervention.
Reproduced with permission from [72]
© Elsevier (2011).
Figure 7: Embolic protection with proximal occlusion with Proxis™ (St Jude Medical, MN, USA). (A) With the proximal balloon inflated, interrupting flow, the lesion is crossed with a guidewire, (B) the stent is positioned and deployed, and (C) with the balloon still inflated, the stagnant blood column is aspirated by reversing flow in the saphenous vein graft removing any liberated atheromatous debris and soluble vasoactive substances liberated by stent implantation. Deflation of the Proxis balloon restores antegrade flow.
Reproduced with permission from [72] © Elsevier (2011).
▪ Procedural issues in SVG PCI
Clot avoidance strategies incorporating thrombin and platelet inhibitors have not been well studied in SVG PCI. Dual antiplatelet therapy is routine, but patients undergoing SVG PCI were excluded from most trials, so optimal dosing and duration of therapy are unknown. Unfractionated heparin was used in most reports of SVG PCI. Outcomes following use of unfractionated heparin or bivalirudin, were compared in a randomized trial in 403 patients undergoing SVG PCI [56]. There was no difference in ischemic end points or major bleeding, but less minor bleeding with bilvalirudin (14.8 vs 22.7%; p = 0.037). Among 329 patients with acute coronary syndromes randomized to heparin plus GPIIb/IIIa inhibitors, bivalirudin plus GPIIb/IIIa inhibitors, or bivalirudin alone, ischemic end points and major bleeding were similar but minor bleeding was less with bivalirudin [57]. Given the increased risk of SVG perforation, many operators prefer unfractionated heparin, which is reversible, but bivalirudin may be preferred in some patients with high risk of bleeding.
The use of abciximab in a Mayo Clinic analysis, increased major bleeding without improvement in acute or long-term outcomes of SVG PCI [58]. In EPIC and EPILOG, degenerated SVG was the only lesion type that failed to benefit from abciximab therapy [59] and when the analysis was extended to five large studies of GPIIb/IIIa inhibitors, including 600 SVG PCIs, there was no demonstrable benefit of GPIIb/ IIIa inhibitor use [52]. In a meta-analysis of over 3000 patients, GPIIb/IIIa inhibitor use was a univariate, but not multivariate, predictor of periprocedural infarction [60]. In a trial of filterbased embolic protection, GPIIb/IIIa antagonist use was associated with significantly lower MACE, but increased bleeding [61]. Whether reduced no reflow and better filter patency favoring debris capture accounted for these observations is uncertain but plausible. Questionable benefit and increased bleeding has led most operators to reserve GPIIb/IIIa inhibitor use to highly selected cases with large thrombus burden. The choice of arterial access in SVG PCI is open to femoral or radial artery routes. However, when there is a need to visualize a LIMA graft, the left radial artery is preferred. In SVGs with superiorly oriented takeoff, Amplatz guide catheters are preferred.
Use of intravascular ultrasound (IVUS) in SVGs has proved useful to accurately determine SVG size, especially when the diameter is ≥4 mm. Hong et al. used IVUS in DES implantation in over 200 SVGs, reporting less plaque extrusion through the stent struts and less MI when stents were sized to the reference vessel or slightly smaller, with no loss of clinical benefit [62]. As a result of these observations, and to minimize the risk of perforation, most experienced operators avoid oversizing stents in SVGs. A very limited experience with the use of OCT in SVGs has been reported. Among 26 patients with acute coronary syndromes undergoing OCT of SVGs at the time of PCI, a thin fibrous cap was more frequent in STEMI (100%) compared with non-STEMI (53%) and unstable angina (20%; p = 0.03) [18]. Plaque rupture and thrombus were common and lesions were more complex than they appeared on angiography (Figure 2). Plaque debulking strategies, such as atherectomy and laser ablation, have not improved outcomes in SVG PCI. Many operators prefer direct stenting without predilation, hoping to minimize atheroembolization, but this has not been adequately studied. In an observational study, Leborgne et al. reported that direct stenting resulted in less non-Q wave MI (11 vs 18%; p < 0.02) and lower CK-MB levels [63].
▪ Arterial graft PCI
Stenoses encountered in LIMA grafts are frequently at the anastomosis with the coronary artery and these respond similarly to SVG distal anastomosis lesions with low restenosis. Lesions in mid-graft are rare but may occur as the result of surgical trauma, with early postoperative presentation and a risk of perforation with PCI (Figure 8). Gruberg et al. reported outcomes in 174 patients, 63% with anastomotic lesions treated mostly with balloon angioplasty, whereas proximal lesions were often treated with stents (11 of 16 [92%]) [64]. Procedural success was very high (97%) and TLR at 1 year was 7.4%. There is very little pubished data regarding PCI of other arterial grafts. The author has observed good long-term patency in a small number of radial artery grafts treated with DES.
Figure 8: A 74-year-old female with recurrent angina was found to have severe left anterior descending and circumflex coronary artery stenosis. Hybrid revascularization with minimally invasive left internal mammary artery (LIMA) to left anterior descending was planned followed by percutaneous coronary intervention to the circumflex the next day. (A) Angiographic visualization of the LIMA graft 1 day post-coronary artery bypass surgery revealed occlusion in its mid portion. (B) Intravascular ultrasound was interpreted as showing dissection and two 3.0mm stents were deployed. (C) Following deployment of the second stent, the presence of a large perforation of the LIMA graft was recognized. The stent deployment balloon was reinflated at the site of perforation, controlling it, but it was not effective in sealing it. A second larger guiding catheter (7 Fr) was introduced from the contralateral femoral artery loaded with a 3.0 x 26 Jo Med PTFE covered stent. (D) Brief deflation of the occluding balloon permitted a second wire to cross the site of perforation and following withdrawal of the 6 Fr guide catheter, the covered stent was advanced quickly to the site of perforation and deployed. The perforation was immediately and permanently sealed. (E) Surveillance angiography at 6 weeks confirmed a widely patent graft. A stress myocardial perfusion scan was normal at 3 years and at last followup 6 years post-percutaneous coronary intervention, the patient was asymptomatic. A dual guiding catheter approach is a superb strategy when the initial catheter ≤6 Fr is too small for delivery of a covered stent. Image courtesy of Anna Kalynych (Piedmont Heart Institute, Atlanta, GA, USA).
▪ Complications
With the wide spread use of coronary stents, need for emergency reoperation for failed PCI in a post-CABG patient has dramatically decreased from approximately 3.5% observed in the 1980s to <1% currently [65]. The most frequent complication is atheroembolic MI discussed above and no reflow, while the most feared cardiac complication is vessel perforation. When no reflow occurs, or is anticipated, intracoronary administration of adenosine, nitroprusside or a calcium channel blocking drug is frequently effective [49,50,66]. Intracoronary administration of nicardipine for example in 100 μg boluses every 2–5 min through the guide catheter or a distal infusion catheter, is usually well tolerated with no hypotension or heart block. Japanese investigators reported a decade ago that aspiration of the stagnant dye column was more effective than calcium-channel blocking agents; aspirates often contained plaque gruel and probably contained soluble vasoconstrictors shown to be liberated during SVG PCI [49]. The possible role of GPIIb/IIIa inhibitors in no reflow encountered in SVG PCI has not been adequately tested.
Perforations continue to be a problem in all vessels (Figure 8), but especially in old SVGs where slight balloon or stent oversizing may produce a tear in the SVG that is difficult or impossible to seal, even with a covered stent. Due to the poor performance of covered stents in three prospective randomized trials of elective use in SVGs, covered stents are generally withheld and used only when a perforation does not seal with balloon occlusion. Features unique to the most commonly used polytetrafluroethylene-covered JOSTENT® Graftmaster (Abbott Vascular, Redwood City, CA, USA) include the need for a 7Fr guiding catheter in most cases, good guide catheter and guidewire support to deliver the bulky, inflexible device and high pressure deployment using IVUS guidance to adequately expand the device [67].
▪ Closure of SVG aneurysms
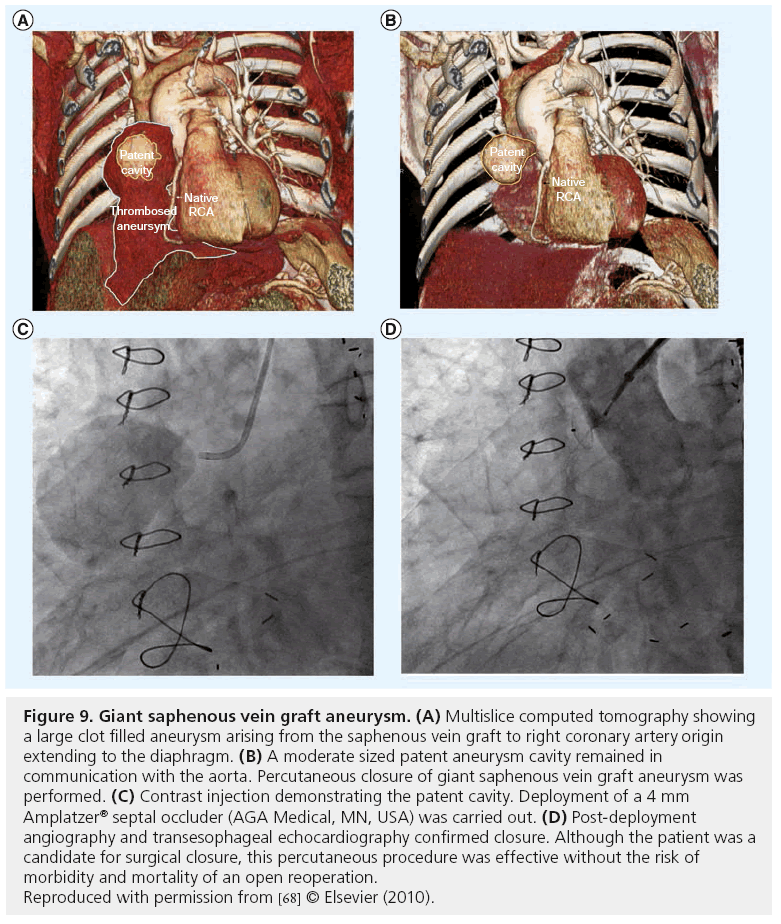
Aortocoronary SVG aneurysms and pseudoaneurysms, are a late complication of SVG degeneration that commonly presents as a mediastinal mass and may rupture. These aneurysms may require surgery, but some can be closed with coils, covered stents, or septal occluder devices (Figure 9) [68].
Figure 9: Giant saphenous vein graft aneurysm. (A) Multislice computed tomography showing a large clot filled aneurysm arising from the saphenous vein graft to right coronary artery origin extending to the diaphragm. (B) A moderate sized patent aneurysm cavity remained in communication with the aorta. Percutaneous closure of giant saphenous vein graft aneurysm was performed. (C) Contrast injection demonstrating the patent cavity. Deployment of a 4 mm Amplatzer® septal occluder (AGA Medical, MN, USA) was carried out. (D) Post-deployment angiography and transesophageal echocardiography confirmed closure. Although the patient was a candidate for surgical closure, this percutaneous procedure was effective without the risk of morbidity and mortality of an open reoperation. Reproduced with permission from [68] © Elsevier (2010).
▪ Hybrid coronary revascularization
A relatively new and attractive alternative for patients with advanced coronary disease including the LAD, is a minimally invasive LIMA graft to the LAD followed by DES treatment of non-LAD lesions; a so-called hybrid revascularization. In our institution, we prefer to perform the surgery first, followed by PCI within 24–48 h in the catheterization laboratory, effectively separating the two procedures whose antithrombotic requirements are quite different [69]. This represents a planned PCI in a post-CABG patient. This hybrid approach was first described in 1996 by Angelini, who also reported that in one of two patients who underwent a ‘one-stop’ procedure experienced acute stent thrombosis, leading him to question the safety of performing both revascularizations in a single procedure [70]. Subsequently, newer endoscopic and robotic techniques, coupled with the improved efficacy of DES, have popularized hybrid revascularization, which is performed in some institutions as a ‘one-stop’ procedure in a hybrid operating room [12]. In this ‘one-stop’ procedure, angiographic assessment of the LIMA graft to LAD can be made, surgical revisions performed if needed and non-LAD targets stented. However, optimal strategies to avoid bleeding on one hand and stent thrombosis on the other have yet to be established. Hybrid revascularization may permit safe, less invasive therapy of patients with left main or complex multivessel disease with high SYNTAX scores, where results of PCI alone may be inferior to open CABG [71].
Conclusion
PCI is a useful technique to relieve symptoms and improve the quality of life of post-CABG patients whose operation has failed due to early technical issues or late disease progression. The most severely diseased patients may require repeat CABG, but the majority can be successfully palliated with PCI. DES and improved PCI technology have made the procedure safer and more durable.
Future perspective
As the huge population of post-CABG patients ages, there will be an increasing need to treat the symptoms and signs of graft failure and progressive native coronary disease. Apart from the need for improved preventive methods, the greatest challenge is a more effective method of dealing with degenerating SVGs, which remain the dominant conduit used in CABG. New techniques similar to the relining of old sewer pipes by plumbers, are needed to treat old SVGs. Further enhancements in the ability to perform PCI of chronic total occlusions of native coronary arteries are expected and will provide new opportunities for revascularizing post-CABG patients. Improved pharmacotherapy to safely prevent thrombosis and restenosis will hopefully be commonplace in the future. Whether the trend towards the use of more arterial grafts will improve outcomes of post-CABG patients remains to be determined.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Executive summary
Recurrent ischemia after coronary artery bypass surgery is common
▪ Approximately 20–40% of saphenous vein grafts (SVGs) fail within a year.
▪ Approximately Half of SVGs are occluded by 5–10 years.
▪ Approximately 20% of percutaneous coronary interventions are in post-coronary artery bypass graft (CABG) surgery patients.
▪ The more widespread use of arterial grafts is recommended.
PCI is favored over repeat CABG
▪ Reoperative CABG has more risk and less benefit.
▪ In-hospital risk of percutaneous coronary intervention is less than CABG.
▪ Death or myocardial infarction on follow-up is similar.
Results of post-CABG PCI have improved
▪ Embolic protection reduces periprocedural myocardial infarction.
▪ Drug-eluting stents have improved outcomes compared with bare-metal stents.
▪ Complications can be minimized by attention to details such as stent sizing, direct stenting, and use of intravascular ultrasound and embolic protection devices.
Novel approaches provide less invasive options
▪ SVG aneurysms and pseudoaneurysms may be closed percutaneously.
▪ Hybrid revascularization offers the best of the two revascularization worlds (a left internal mammary artery to left anterior descending and drug-eluting stents to non-left anterior descending targets).
References
Papers of special note have been highlighted as:
▪ of interest
- Douglas JS Jr, Gruentzig AR, King SB III et al. Percutaneous transluminal coronary angioplasty in patients with prior coronary bypass surgery. J. Am. Coll. Cardiol. 2, 745–754 (1983).
- Douglas JS, Ghazzal ZMB, Morris DC, King SB III. Twenty years of angioplasty at Emory University. Circulation 102(Suppl. II), II753 (2000).
- Loop FD, Lytle BW, Cosgrove DM et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 314, 1–6 (1986).
- Reifart N, Haase J, Storger H et al. Interventional standby for cardiac surgery. Circulation 94(Suppl. I), I186 (1996).
- Rasmussen C, Thiis JJ, Clemmensen P et al. Significance and management of early graft failure after coronary artery bypass grafting. Feasibility and results of acute angiography and re-revascularization. Eur. J. Cardiothorac. Surg. 12, 847–852 (1997).
- Cutlip DE, Dauerman HL, Carrozza JP. Recurrent ischemia within thirty days of coronary artery bypass surgery: angiographic findings and outcomes of percutaneous revascularization. Circulation 94(Supp. I), I249 (1996).
- Thielmann M, Massoudy P, Jaeger BR et al. Emergency re-revascularization with percutaneous coronary intervention, reoperation or conservative treatment in patients with acute perioperative graft failure following coronary artery bypass surgery. Eur. J. Cardiothorac. Surg. 30, 117–125 (2006).
- Smith SC Jr, Feldman TF, Hirschfeld JW et al. ACC/AHA/SCAI 2005 guideline update for percutaneous intervention: a report of the American College of Cardiology/American Heart Association Task Force on the Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention). J. Am. Coll. Cardiol. 47, 121 (2006).
- Wijns W, Kolh P, Dandin N et al. Guidelines in myocardial revascularization. Eur. Heart J. 31, 2501–2555 (2010).
- Izumi C, Hayashi H, Ueda Y et al. Late regression of left internal thoracic artery graft stenosis at the anastomotic site without intervention therapy. J. Thorac. Cardiovasc. Surg. 130, 1661–1667 (2005).
- Kitamura S. Does the internal thoracic artery graft have self-reparative ability? J. Thorac. Cardiovasc. Surg. 130, 1494–1495 (2005).
- Zhoa DX, Leacche M, Balaguer JM et al. Routine intraoperative completion angiography after coronary artery bypass grafting and 1-stop hybrid revascularization: results from a fully integrated hybrid catheterization laboratory/operating room. J. Am. Coll. Cardiol. 53, 232–241 (2009).
- Gluckman TJ, McLean RC, Schulman SP et al. Effects of aspirin responsiveness and platelet reactivity on early vein graft thrombosis after coronary artery bypass graft surgery. J. Am. Coll. Cardiol. 57, 1069–1077 (2011).
- Widimsky P, Straka Z, Stros P et al. One-year coronary bypass graft patency: a randomized comparison between off-pump and on-pump surgery angiographic results of the PRAGUE-4 trial. Circulation 110, 3418–3423 (2004).
- Alexander JH, Hafley G, Harrington RA et al. Efficacy and safety of edifoligide, an F2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 294, 2446–2454 (2005).
- Shroyer AL, Grover FL, Hattler B et al. On-pump versus off-pump coronary artery bypass surgery. N. Engl. J. Med. 361, 1827–1837 (2009).
- Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition and prevention. Circulation 97, 916–931 (1998).
- Davlouros P, Damelou A, Karantalis V et al. Evaluation of culprit saphenous vein graft lesions with optical coherence tomography in patients with acute coronary syndromes. J. Am. Coll. Cardiol. Interv. 4, 683–693 (2011).
- Brilakis ES, Rao SV, Banerjee S et al. Percutaneous coronary intervention in native coronary arteries versus bypass grafts in prior coronary artery bypass grafting patients: a report from the National Cardiovascular Data Registry. J. Am. Col. Cardiol. Interv. 4, 844–850 (2011).
- Welsh RC, Granger CB, Westerhout CM et al. Prior coronary artery bypass graft patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J. Am. Coll. Cardiol. Interv. 3, 343–351 (2010).
- Suwaidi JA, Velianou JI, Berger PB et al. Primary percutaneous coronary intervention in patients with acute myocardial infarction and prior coronary artery bypass grafting. Am. Heart J. 142, 452–459 (2001).
- Gaglia MA, Torguson R, Xue Z et al. Outcomes of patients with acute myocardial infarction from a saphenous vein graft culprit undergoing percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 78, 23–29 (2011).
- Ryan TJ, Anderson JL, Antman EM et al. ACC/AHA guidelines for the management of patients with acute myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction). J. Am. Coll. Cardiol. 28, 1328–1428 (1996).
- Weintraub WS, Jones EL, Morris DC et al. Outcome of reoperative coronary bypass surgery versus coronary angioplasty after bypass surgery. Circulation 95, 868–877 (1997).
- Mathew V, Clavell AL, Lennon RJ et al. Percutaneous coronary interventions in patients with prior coronary bypass surgery: changes in patient characteristics and outcome during two decades. Am. J. Med. 108, 127–135 (2000).
- Brener SJ, Lytle BW, Casserly IP et al. Predictors of revascularization method and long-term outcome of percutaneous coronary intervention or repeat coronary bypass surgery in patients with multivessel coronary disease and previous coronary bypass surgery. Eur. Heart J. 27, 413–418 (2006).
- Morrison DA, Sethi G, Sacks J et al. Percutaneous coronary intervention versus repeat bypass surgery for patients with medically refractory myocardial ischemia: AWESOME randomized trial and registry experience with post-CABG patients. J. Am. Coll. Cardiol. 40, 1951–1954 (2002).
- Cole JH, Jones EL, Craver JM et al. Outcomes of repeat revascularization in diabetic patients with prior coronary surgery. J. Am. Coll. Cardiol. 40, 1968–1975 (2002).
- Bundhoo SS, Kalla M, Anantharaman R et al. Outcomes following PCI in patients with previous CABG: a multicenter experience. Catheter. Cardiovasc. Interv. 78, 169–176 (2011).
- Patel MR, Dehmer GJ, Hirshfeld JW et al. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization. J. Am. Coll. Cardiol. 53, 530–553 (2009).
- Ellis SG, Brener SJ, DeLuca S et al. Late myocardial ischemic events after saphenous vein graft intervention: importance of initially ‘non-significant’ vein graft lesions. Am. J. Cardiol. 79, 1460–1464, (1997).
- Rodes-Cabau J, Bertrand OF, Larose E et al. Comparison of plaque sealing with paclitaxel-eluting stents versus medical therapy for the treatment of moderate non-significant saphenous vein graft lesions: the Moderate Vein Graft Lesion Stenting with the Taxus Stent and Intravascular Ultrasound (VELETI) pilot trial. Circulation 120, 978–986 (2009).
- Rodes-Cabau J. Plaque sealing with paclitaxel-eluting stents for the treatment of moderate non-significant saphenous vein graft lesions. Three-year follow-up of the VELETI (moderate vein graft lesion stenting with the Taxus stent and intravascular ultrasound) trial. Presented at: i2 Summit 2010. Atlanta, GA, USA, 14 March 2010.
- Douglas JS Jr, Weintraub WS, King SB III. Changing prospective in vein graft angioplasty. J. Am. Coll. Cardiol. 25 (Suppl. A), 78A (1995).
- Savage MP, Douglas JS Jr, Fischman DL et al. Stent placement compared with balloon angioplasty for obstructed bypass grafts. Saphenous vein de novo trial investigators. N. Engl. J. Med. 33, 740–747 (1997).
- Ashfaq S, Ghazzal Z, Douglas JS et al. Impact of diabetes on five-year outcomes after vein graft intervention performed prior to the drug-eluting stent era. J. Invas. Cardiol. 18, 100–105 (2006).
- Brodie BR, Wilson H, Stuckey T et al. for the STENT Group. Outcomes with drug-eluting versus bare-metal stents in saphenous vein graft intervention: results from the STENT (strategic transcatheter evaluation of new therapies) Group. J. Am. Coll. Cardiol. Interv. 2, 1105–1113 (2009).
- Mamas MA, Foley J, Nair S et al. Comparison of drug-eluting stents versus bare-metal stents in saphenous vein graft PCI outcomes: a meta-analysis. J. Interv. Cardiol. 24, 172–180 (2011).
- Vermeersch P, Agostoni P, Verheye S et al. Randomized double-blind comparison of sirolimus-eluting stent versus bare-metal stent implantation in diseased saphenous vein grafts. Six-month angiographic, intravascular ultrasound, and clinical follow-up of the RRISC trial. J. Am. Coll. Cardiol. 48, 2423–2431 (2006).
- Vermeersch P, Agostoni P, Verheye S et al. Increased late mortality after sirolimuseluting stents versus bare-metal stents in diseased saphenous vein grafts: results from the randomized DELAYED RRISC Trial. J. Am. Coll. Cardiol. 50, 261–267 (2007).
- Douglas JS Jr. Are our patients better off with drug-eluting stents in saphenous vein grafts? J. Am. Coll. Cardiol. Interv. 2, 1113–1115 (2009).
- Brilakis ES, Lechtenwalter C, de Lemos JA et al. A randomized controlled trial of a paclitaxel-eluting stent versus a similar bare-metal stent in saphenous vein graft lesions the SOS (stenting of saphenous vein grafts) trial. J. Am. Coll. Cardiol. 53, 919–928 (2009).
- Brilakis ES, Lichtenwalter C, Abdel-karim AR et al. Continued benefit from paclitaxeleluting compared with bare-metal stent implantation in saphenous vein graft lesions during long-term follow-up of the SOS (stenting of saphenous vein grafts) trial. J. Am. Coll. Cardiol. Interv. 4, 176–182 (2011).
- Mehilli J, Pache J, Abdel-Wahab M et al. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomized controlled superiority trial. Lancet 378, 1071–1078 (2011).
- Ko DT, Guo H, Wijeysundera HC et al. Long-term safety and effectiveness of drug-eluting stents for the treatment of saphenous vein grafts disease: a populationbased study. J. Am. Coll. Cardiol. Interv. 4, 965–973 (2011).
- Saber RS, Edwards WD, Holmes DR Jr et al. Balloon angioplasty of aortocoronary saphenous vein bypass grafts: a histopathologic study of six grafts from five patients, with emphasis on restenosis and embolic complications. J. Am. Coll. Cardiol. 12, 1501–1509 (1988).
- Liu MW, Douglas JS Jr, Lembo NJ, King SB. Angiographic predictors of a rise in serum creatine kinase (distal embolization) after balloon angioplasty of saphenous vein coronary artery bypass grafts. Am. J. Cardiol. 72, 514–517 (1993).
- Coolong A, Baim DS, Kuntz RE et al. Saphenous vein graft stenting and major adverse cardiac events. A predictive model derived from a pooled analysis of 3958 patients. Circulation 117, 790–797 (2008).
- Salloum J, Tharpe C, Vaughan D et al. Release and elimination of soluble vasoactive factors during percutaneous intervention of saphenous vein grafts: analysis using the PercuSurge GuardWire distal protection device. J. Invas. Cardiol. 17, 575–579 (2005).
- Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstructionrelated myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. JACC Cardiovasc. Interv. 3, 695–704 (2010).
- Baim DS, Wahr D, George B et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation 105, 512–590 (2002).
- Roffi M, Mukherjee D, Chew DP et al. Lack of benefit from intravenous platelet glycoprotein GPIIb/IIIa receptor inhibition as adjunctive treatment for percutaneous interventions of aortocoronary bypass grafts: a pooled analysis of five randomized clinical trials. Circulation 106, 3063–3067 (2002).
- Mehta SK, Frutkin AD, Milford-Beland S et al. Utilization of distal embolic protection in saphenous vein graft interventions (an analysis of 19,546 patients in the American College of Cardiology-National Cardiovascular Data Registry). Am. J. Cardiol. 100, 1114–1118 (2007).
- Lavi S, Ivanov J, Appleby CE et al. Selective use of embolic protection devices during saphenous vein grafts interventions: a single-center experience. Catheter. Cardivasc. Interv. 75, 1037–1044 (2010).
- Badhey N, Lichtenwalter C, de Lemos JA et al. Contemporary use of embolic protection devices in saphenous vein graft interventions: insights from the stenting of saphenous vein grafts trial. Catheter. Cardiovasc. Interv. 76, 263–269 (2010).
- Kao J, Lincoff AM, Topol EJ et al. Direct thrombin inhibition appears to be a safe and effective anticoagulant for percutaneous bypass graft interventions. Catheter. Cardiovasc. Interv. 68, 352–356 (2006)
- Kumar D, Dangas G, Mehran R et al. Comparison of bivalirudin versus bivalirudin plus glycoprotein GPIIb/IIIa inhibitor versus heparin plus glycoprotein GPIIb/IIIa inhibitor in patients with acute coronary syndromes having percutaneous intervention for narrowed saphenous vein aorto-coronary grafts (the ACUITY Trial Investigators). Am. J. Cardiol. Cardiol 106, 941–945 (2010).
- Mathew V, Grill DE, Scott CG et al. The influence of abciximab use on clinical outcomes after aortocoronary vein graft interventions. J. Am. Coll. Cardiol. 34, 1163–1169 (1999).
- Ellis SG, Lincoff AM, Miller D et al. Reduction in complications of angioplasty with abciximab occurs largely independently of baseline lesion morphology. EPIC and EPILOG Investigators. Evaluation of 7E3 for the prevention of ischemic complications. Evaluation of PTCA to improve long-term outcome with abciximab GPIIb/IIIa receptor blockade. J. Am. Coll. Cardiol. 32, 1619–1623 (1998).
- Hillegass WB, Lyerly MJ, Patel NJ et al. Frequency and predictors of post-procedural myonecrosis in saphenous vein graft intervention in the stent era: a metaregression. Circulation 114(Suppl. 2), 733 (2006).
- Jonas M, Stone GW, Mehran R et al. Platelet glycoprotein GPIIb/IIIa receptor inhibition as adjunctive treatment during saphenous vein graft stenting: differential effects after randomization to occlusion or filter-based embolic protection. Eur. Heart J. 27, 920–928 (2006).
- Hong YJ, Pichard AD, Mintz GS et al. Outcome of undersized drug-eluting stents for percutaneous coronary intervention of saphenous vein graft lesions. Am. J. Cardiol. 105, 179–185 (2010).
- Leborgne L, Cheneau E, Pichard A et al. Effect of direct stenting on clinical outcome in patients treated with percutaneous coronary intervention on saphenous vein graft. Am. Heart J. 146, 501–506 (2003).
- Gruberg L, Dangas G, Mehran R et al. Percutaneous revascularization of the internal mammary artery graft: short- and long-term outcomes. J. Am. Coll. Cardiol. 35, 944–948 (2000).
- Weintraub WS, Cohen CL, Curling PE et al. Results of coronary surgery after failed elective coronary angioplasty in patients with prior coronary surgery. J. Am. Coll. Cardiol. 16, 1341–1347 (1990).
- Fischell TA, Subraya RG, Ashraf K et al. ‘Pharmacologic’ distal protection using prophylactic, intragraft nicardipine to prevent no-reflow and non-Q-wave myocardial infarction during elective saphenous vein graft intervention. J. Invas. Cardiol. 19, 58–62 (2007).
- Romaguera R, Waksman R. Covered stents for coronary perforations: is there enough evidence? Catheter. Cardiovasc. Interv. 78, 246–253 (2011).
- Sura AC, Douglas JS Jr. Percutaneous closure of giant saphenous vein graft aneurysm. J. Am. Coll. Cardiol. Interv. 3, 784–785 (2010).
- Vassiliades TA Jr, Douglas JS, Morris DC et al. Integrated coronary revascularization with drug-eluting stents: immediate and seven-month outcome. J. Thorac. Cardiovasc. Surg. 131, 956–962 (2006).
- Angelini GD, Wild P, Salerno TA et al. Integrated left small thoracotomy and angioplasty for multivessel coronary artery revascularization. Lancet 347, 757–758 (1996).
- Rab ST, Douglas JS Jr, Lyons E et al. Hybrid coronary revascularization for the treatment of left main coronary stenosis: a feasibility study. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.23312 (2011) (Epub ahead of print).
- Lee MS, Park SJ, Kandzari D et al. Saphenous vein graft intervention. J. Am. Coll. Cardiol. Interv. 4(8), 831–843 (2011).
▪ Study providing early reassurance that percutaneous coronary intervention (PCI) post-coronary artery bypass graft (CABG) surgery was safe and effective. It documents good results with balloon dilation of distal anastomotic saphenous vein graft (SVG) lesions, native vessels and higher recurrence in mid-SVG lesions.
▪ Report of optical coherence tomography (OCT) images in SVGs indicating that the complexity of SVG lesions is greatly underestimated by angiography.
▪ Provocative pilot trial suggesting that 30–60% SVG stenoses are stabilized by paclitaxel-eluting stent implantation. A large trial of this strategy is ongoing (VELETI II).
▪ Large observational study indicating that drug-eluting stent (DES) use in SVGs is safe but that the benefit may only be short term.
▪ Largest and most definitive study comparing DES and bare-metal stent (BMS) use in SVGs, showing significantly better outcomes at 1 year related primarily to reduced need for repeat revascularization.
▪ 4-year outcomes in propensity score matched patients were better in DES compared to BMS. This is the longest follow-up available and counters, to a degree, the concerns over ‘late catch up’ of target lesion revascularization in DES compared to BMS.