Device Evaluations - Interventional Cardiology (2015) Volume 7, Issue 4
Percutaneous left atrial appendage closure: a review of the WATCHMAN clinical trial experience
- Corresponding Author:
- Brian
Whisenant
Intermountain Heart Institute, 5121 Cottonwood Street, Salt Lake City, UT 84107, USA
E-mail: brian.whisenant@imail.org
Abstract
Keywords
anticoagulation, atrial fibrillation, bleeding, left atrial appendage, stroke, WATCHMAN
The aging US population comes with an increasing burden of cardiovascular disease including atrial fibrillation (AF). Conservative estimates suggest that over 12 million Americans will be affected with AF over the next 15 years [1]. AF management includes the prevention of tachycardia [2] and stroke. Early Framingham data demonstrated that patients with AF have a fivefold increased incidence of stroke [3]. Oral anticoagulation is the standard of care for stroke prevention in AF, the mainstay being warfarin for patients at increased risk [4–7]. Among patients taking warfarin, even in sophisticated societies, anticoagulation is haphazard with a significant percentage of patients’ INR levels outside the indicated range. Furthermore, 45% of warfarin eligible patients with non-valvular AF do not receive oral anticoagulation for stroke prevention [8]. Although novel oral anticoagulants (NOACs) alleviate frequent monitoring and are associated with diminished intracerebral bleeding when compared with warfarin, major and minor bleeding including life-threatening bleeding remain a persistent threat [9–12]. Atrial fibrillation necessitating chronic anticoagulation is associated with persistent bleeding risk and is inherently undesirable for many patients, leaving a large unmet need for stroke prevention in AF. Left atrial appendage (LAA) closure is an attractive target for stroke prevention as over 90% of embolic strokes originate in that location in patients with a history of AF [13,14] and historical data suggest that surgical LAA exclusion may decrease stroke in nonvalvular AF [15].
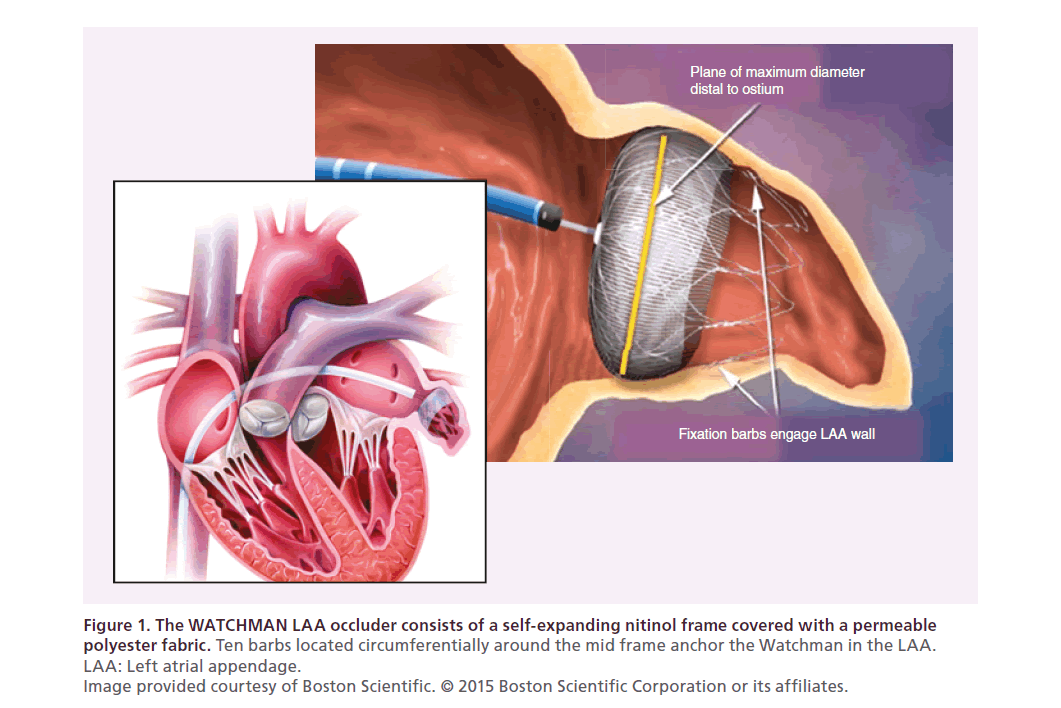
The WATCHMAN device was developed by Atritech Inc. (MN, USA) and acquired by Boston Scientific (MA, USA) in 2011. The WATCHMAN consists of a selfexpanding nitinol frame covered with a permeable polyester fabric. Ten barbs, located circumferentially around the mid frame, anchor the WATCHMAN in the LAA (Figure 1). It is available in five sizes with nominal diameters ranging from 21 to 33 mm. The WATCHMAN is delivered to the LAA via a transseptal approach using fluoroscopic and transesophageal echo guidance [16]. The WATCHMAN has been evaluated in a robust series of prospective randomized and nonrandomized tri-als including over 2400 patients with nearly 6000 years of patient follow-up (Table 1). This robust experience has been extensively published, dissected and debated. After three US FDA panel reviews, the WATCHMAN device was approved by the FDA in March 2015 with the following indication: the WATCHMAN device is indicated to reduce the risk of thromboembolism from the left atrial appendage in patients with nonvalvular atrial fibrillation who:
• Are at increased risk for stroke and systemic embolism based on CHADS2 or CHA2DS2-VASc scores and are recommended for anticoagulation therapy.
• Are deemed by their physicians to be suitable for warfarin.
• Have an appropriate rationale to seek a non-pharmacologic alternative to warfarin, taking into account the safety and effectiveness of the device compared with warfarin.
| PROTECT AF | CAP Registry | PREVAIL | CAP2 Registry | Totals | |
|---|---|---|---|---|---|
| Enrollment | 2005–2008 | 2008–2010 | 2010–2012 | 2012–2014 | – |
| Enrolled | 800 | 566 | 461 | 579 | 2406 |
| Mean Follow-up (years) | 4.0 | 3.7 | 2.2 | 0.58 | N/A |
| Patient-years | 2717 | 2022 | 860 | 332 | 5931 |
Table 1. Enrollment and follow-up in the WATCHMAN clinical trials.
Figure 1. The WATCHMAN LAA occluder consists of a self-expanding nitinol frame covered with a permeable
polyester fabric.
Ten barbs located circumferentially around the mid frame anchor the Watchman in the LAA.
LAA: Left atrial appendage.
Image provided courtesy of Boston Scientific. © 2015 Boston Scientific Corporation or its affiliates.
Emphasizing the subtle differences in interpretation of the data and its clinical application, The European Society of Cardiology guidelines state that ‘Interventional, percutaneous LAA closure may be considered in patients with a high stroke risk and contraindications for long-term oral anticoagulation’ [17].
The options for managing stroke risk in the setting of atrial fibrillation now include warfarin, novel anticoagulants and device therapy. Stroke and bleeding exist along a continuum of risk for each unique patient. These risks must be balanced along with lifestyle as physicians counsel their atrial fibrillation patients. The discussion below attempts to summarize key findings and conclusions from the WATCHMAN Trials which are essential to guide and discuss the option of LAA closure.
WATCHMAN feasibility experience
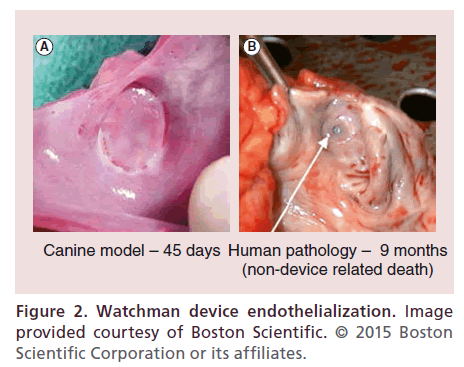
WATCHMAN feasibility was first reported in 2007 [18]. Sixty-six patients underwent device implantation and were followed for a mean of 740 +/- 341 days. At 45 days postimplant, 54 of 58 (93%) devices showed successful sealing of the LAA according to the protocol definition of no gap > 3 +/-2 mm. Two patients experienced device embolization, both successfully retrieved percutaneously. There were four major safety events (two tamponade, one air embolism, and one delivery wire fracture with surgical explantation). During follow up, two patients experienced transient ischemic attacks (TIA). There were two deaths, neither deemed device related. An autopsy performed on one patient who died with a ruptured abdominal aortic aneurysm nine months after device implantation revealed complete LAA closure and full device endothelialization (Figure 2). No strokes occurred during follow-up despite >90% of patients having discontinued oral anticoagulation. This feasibility trial led to the first large randomized trial of LAA closure, PROTECT AF.
PROTECT AF
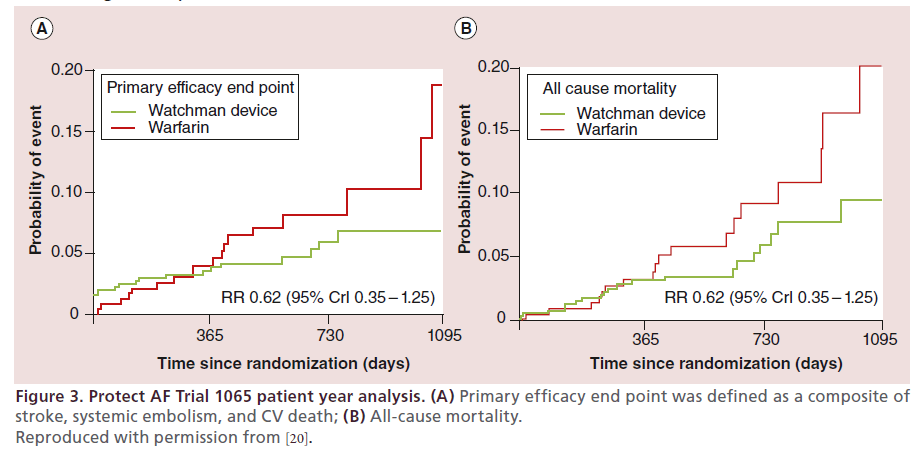
PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) remains the landmark trial demonstrating both efficacy of the WATCHMAN device and proof-of-principal for LAA closure [19,20]. PROTECT AF was a multicenter, nonblinded, randomized trial testing both the efficacy and safety of the WATCHMAN device. In addition to 93 nonrandomized roll-in patients, Protect AF randomized 463 patients to device and 244 patients to warfarin control in a 2:1 fashion with a noninferiority primary efficacy end point defined as all stroke (including ischemic and hemorrhagic), systemic embolism and cardiovascular death. The primary safety end point included procedure- related device events (pericardial effusion requiring drainage, stroke, or device embolization), and major bleeding (intracranial bleeding, or any bleeding requiring transfusion). Patients in the device group were prescribed daily low-dose aspirin and warfarin for the initial 45 days postimplant. Warfarin was stopped 45 days after device deployment at which time patients were transitioned to aspirin and clopidogrel. Clopidogrel was discontinued 6 months after device implantation and patients continued aspirin alone indefinitely. When first published with 1065 patient-years of followup the intention-to-treat analysis revealed the primary efficacy event rate was noninferior (3.0 per 100 patientyears in the device group and 4.9 per 100 patient-years in the control group (rate ratio [RR] 0.62, 95% Credible Interval [CrI] 0.35–1.25, Posterior probability [PP] >99.9%, noninferior). However, this came at a cost of significantly more safety end points in the intervention group compared with the control group (7.4 per 100 patient-years, 95% CrI 5.5–9.7, vs 4.4 per 100 patientyears, 95% CrI 2.5–6.7; RR 1.69, 1.01–3.19). All stroke and all-cause mortality also numerically favored the WATCHMAN device. (Figure 3)
Figure 3. Protect AF Trial 1065 patient year analysis. (A) Primary efficacy end point was defined as a composite of
stroke, systemic embolism, and CV death; (B) All-cause mortality.
Reproduced with permission from [20].
FDA nonapproval & updated analysis from Protect AF & CAP
Though PROTECT AF met its primary efficacy end point of non-inferiority, and received a favorable vote from a US FDA sponsored panel in 2009, the FDA ultimately issued a Not Approvable letter citing three major concerns: substantial enrollment of CHADS2 = 1 subjects (31%); concomitant use of chronic warfarin and clopidogrel therapy in both study groups (51% of follow-up time in device subjects and 16% of follow-up time in control subjects); and safety concerns regarding serious periprocedure adverse events including pericardial effusion and air embolism [21]. The result of this nonapproval eventually lead to the PREVAIL trial [22].
The Bayesian statistical plan of the PROTECT AF Trial allowed for sequential analyses of the primary end points. Following FDA nonapproval, the PROTECT AF Trial was updated with 1588 patient years of follow-up (mean 2.3 years) which addressed many of the concerns noted by the FDA [23]. With continued follow up, the primary efficacy end point with device remained noninferior to warfarin (3.0% device vs 4.3% drug). When patients with a CHADS2 score = 1 were excluded from the analysis, primary efficacy event rates were 3.9% per year in the device group and 5.0% per year in the control group (probability of noninferiority = 0.999). The primary adverse event rate with device and aspirin was 2.3% per year compared with 4.1% per year with warfarin therapy (probability of superiority = 0.945) when the time when patients were on warfarin or clopidogrel was removed from the analysis. These results suggest that given a successful deployment, LAA closure with the WATCHMAN device was more effective than continued warfarin anticoagulation.
Evolving procedural safety was addressed in the Continued Access to Protect (CAP) registry of 566 patients. Procedural success, serious adverse events, periprocedural pericardial effusions and stroke were all much improved during the CAP experience when compared with PROTECT AF. While PROTECT AF represented the first WATCHMAN experience for the investigators, it also represented the first significant world-wide experience of endovascular LAA closure. Improved safety and procedural efficacy during PROTECT AF and in CAP represents growing understanding in the cardiology community of how to use the WATCHMAN device as well as an individual physician learning curve [24].
The most recent published update on PROTECT AF has extended follow up to 2621 patient years (mean 3.8 years) (Table 2) [25]. Keeping in mind that patients were randomized in a 2:1 device to medical therapy ratio, there were 39 events among 463 patients (8.4%) in the device group for a primary event rate of 2.3 events per 100 patient-years, compared with 34 events among 244 patients (13.9%) for a primary event rate of 3.8 events per 100 patient-years with warfarin (rate ratio, 0.60; 95% CrI, 0.41–1.05), PP 96%, superior). As with the original cohort report, patients in the device group demonstrated significantly lower rates of both cardiovascular mortality (3.7 vs 9%; HR, 0.40; 95% CI, 0.21–0.75; p = 0.005) and all-cause mortality (12.3 vs 18.0%; HR, 0.66; 95%CI, 0.45–0.98; p = 0.04). While all stroke was numerically lower with device than warfarin (1.5 vs 2.2%), ischemic stroke was numerically higher with device than warfarin (1.4 vs 1.1%). The warfarin arm of PROTECT AF suffered an unusually high rate of hemorrhagic stroke (1.1%) which contributed to both the primary safety and efficacy end points.
| Events | WATCHMAN Group (n = 463) | Warfarin Group (n = 244) | Posterior probabilities (%) | |
|---|---|---|---|---|
| Observed events per 100 | Observed events per 100 | Noninferior | Superiority | |
| patient years (95% CrI) | patient years (95% CrI) | |||
| Primary efficacy‡ | 2.3 (1.7–3.2) | 3.8 (2.5–4.9) | >99 | 96 |
| Stroke | 1.5 (1.0–2.2) | 2.2 (1.3–3.1) | >99 | 83 |
| Ischemic | 1.4 (0.9–2.1) | 1.1 (0.5–1.7) | 78 | 15 |
| Hemorrhagic | 0.2 (0.0–0.4) | 1.1 (0.5–1.8) | >99 | 99 |
| Disabling§ | 0.5 (0.2–0.8) | 1.2 (0.6–1.9) | >99 | 98 |
| CV or unexplained death | 1.0 (0.6–1.5) | 2.4 (1.4–3.4) | >99 | 99 |
| Primary Safety end point# | 3.6 (2.8–4.6) | 3.1 (2.0–4.3) | 98 | 20 |
§Modified Rankin Score of 3–6.
#Safety end point = procedural related (pericardial effusion requiring intervention, prolonged hospitalization, procedure-related stroke, or device embolization) and major bleeding (intracranial or transfusion). Adapted with permission from [25].
Table 2. Protect AF: 4-year (2621 Pt Yr) analysis.
PREVAIL
PREVAIL (Prospective Randomized Evaluation of the WATCHMAN Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long-Term Warfarin Therapy) was designed to confirm both the efficacy of the WATCHMAN device observed in PROTECT AF as well as the improved safety of the WATCHMAN device observed in CAP [22]. The Bayesian statistical model incorporated events from the PROTECT AF Trial in a discounted fashion as an informed prior event rate while randomizing additional patients. The first of three co-primary end points [a composite rate at 18-months of stroke, systemic embolism (SE), and cardiovascular/unexplained death] was 0.064 in the device group versus 0.063 in the control group (rate ratio 1.07 [95% CrI: 0.57–1.89]) and did not achieve the prespecified criteria for noninferiority (upper boundary of 95% CrI ≥1.75). The second primary end point (rate of stroke or SE >7 days postrandomization) was 0.0253 versus 0.0200 (risk difference 0.0053 [95% CrI: –0.0190 to 0.0273]), achieving noninferiority. The third co-primary end point was a safety end point consisting of a composite of all cause death, ischemic stroke, systemic embolism, or device/procedure-related events requiring open cardiovascular surgery or major endovascular intervention between randomization and within 7 days of the procedure or during the index hospitalization. There were 6 (2.2%) primary safety end points in the 269 patients with device implantation. As this was statistically less than the 2.67% performance goal, success for the safety end point was achieved. Even when using a broader definition of adverse effects, PREVAIL exhibited a marked reduction in safety end points when compared with PROTECT AF (4.2 vs 8.7%; p = 0.004). Pericardial effusions requiring surgical repair decreased from 1.6 to 0.4% (p = 0.027), and those requiring pericardiocentesis decreased from 2.9 to 1.5% (p = 0.36).
The authors pointed to the unusually low stroke rate among patients randomized to warfarin in the control arm of PREVAIL, explaining why PREVAIL failed to achieve its first primary end point. Among patients randomized to warfarin in PREVAIL, only 0.7 per 100 patient years experienced a stroke. This event rate was half or less than the event rate observed in the warfarin arms of contemporary NOAC trials [9–12]. With only 138 patients in the warfarin arm of PREVAIL, this low event rate was surrounded by a large confidence interval. Furthermore, the PREVAIL statistical model was not designed to compare newly randomized patients independent of the PROTECT AF Trial, but rather was a composite of newly randomized PREVAIL patients with discounted PROTECT AF patients.
An FDA panel voted in December 2013 in favor of WATCHMAN safety and efficacy [26]. However, with new events during ongoing follow up (mean 25.9 months), the FDA convened yet a third panel to review the latest data. At the time of the third FDA panel in October 2014, there were 24 primary end point events among the 269 patients randomized to WATCHMAN and 9 primary end point events among the 138 patients randomized to warfarin in PREVAIL. The 269 device patients experienced 13 ischemic strokes, 2 hemorrhagic strokes, 1 systemic embolism and 8 cardiovascular or unexplained deaths. Among the 138 patients randomized to warfarin, there was one ischemic stroke, with two hemorrhagic strokes and six cardiovascular or unexplained deaths. With a mean CHADS2 score of 2.6, the rate of ischemic stroke in the WATCHMAN patients was consistent with the rate of ischemic stroke observed in a similar population of patients treated in PROTECT AF or with oral anticoagulation in the NOAC Trials. Once again, the single ischemic stroke observed in the warfarin group (0.3 events per 100 patient years) is dramatically lower than observed in any contemporary warfarin trials (Table 3). However, as PREVAIL was not powered for analysis independent of PROTECT AF and was certainly not powered to evaluate ischemic stroke as a stand-alone end point, such an anomaly should not be surprising. After considering the totality of data including the updated analysis from PREVAIL, the complete 5-year dataset from PROTECT AF as well as the two continued access registries, the FDA Panel once again concluded that the benefits of the WATCHMAN Device outweigh the potential risks [27].
| Study | WATCHMAN strokes (per 100 pt-yrs) | Control strokes (per 100 pt-yrs) |
|---|---|---|
| Prevail (new pts only) | 13 (2.3) | 1 (0.34) |
| Protect AF | 24 (1.3) | 10 (1.1) |
| CAP | 24 (1.2) | – |
| CAP2 | 9 (2.7) | – |
| Total | 70 (1.5) | 11 (0.9) |
Table 3. Ischemic stroke across WATCHMAN studies.
Boston Scientific and the FDA compared the stroke rates in PROTECT AF, PREVAIL and CAP to an imputed placebo group (Table 4). A placebo control event rate was calculated based on validated CHADS2 risk classification scheme and compared with the observed event rate of patients in the WATCHMAN trials. With device group ischemic stroke rates of 1.3, 1.2 and 2.3% in PROTECT AF, CAP and PREVAIL, respectively; CHADS2 predicted stroke rates between 5.6 and 6.7% with placebo. Therefore, LAA closure conveyed a 65–81% relative risk reduction compared with the predicted rate of stroke in the absence of anticoagulation.
| Study (dataset lock | Average CHADS2 Score | Imputed untreated | Observed WATCHMAN | Relative risk |
|---|---|---|---|---|
| date) | WATCHMAN patients | ischemic stroke rate | ischemic stroke rate | reduction (%) |
| Protect AF (3/3/2014) | 2.2 | 5.65 | 1.3 | 77 |
| CAP (3/7/2014) | 2.5 | 6.4 | 1.1 | 83 |
| PREVAIL (4/18/2014) | 2.6 | 6.65 | 2.5 | 62 |
Table 4. Ischemic stroke in the WATCHMAN trials compared with an imputed rate of ischemic stroke in the absence of anticoagulation imputed from the validated CHADS Score literature.
During the October, 2014 FDA panel meeting, Boston Scientific proposed the following Indications for Use (IFU): ‘The WATCHMAN LAAC Device is indicated to prevent thromboembolism from the left atrial appendage. The device may be considered for patients with nonv-alvular atrial fibrillation who, based on CHADS2 or CHA2DS2-VASc scores, would be recommended for warfarin therapy to reduce the risk of stroke and systemic embolism.’ The Panel was uncomfortable with the proposed IFU statement and how patients would be educated to make an informed decision when considering the WATCHMAN device as an option versus oral anticoagulation. Several panelists who voted in favor of device noted that they anticipated a more limited, revised indication. While several of the panel members who did not vote in favor of the device noted that they would have voted positively had the indication been limited to a more specific patient population.
LAA closure for patients with reasons to avoid chronic oral anticoagulation
Anticoagulation associated bleeding is a major risk and concern for many patients and their physicians. Three sequential FDA panels have concluded that LAA closure is an important option to address the major unmet need of stroke prevention in AF patients at increased risk for stroke who have reasons not to take oral anticoagulants but should not be used as a broad first line therapy [26,27]. As anticoagulation associated bleeding risk exists along a difficult to delineate continuum, defining a population of patients who would be best served with LAA closure is challenging. Furthermore, the US WATCHMAN protocols have required 45 days of aspirin and warfarin following device implantation. The large PROTECT AF, PREVAIL and CAP registries anecdotally enrolled patients who were warfarin eligible but who had reasons to avoid chronic oral anticoagulants which may explain the higher incidence of hemorrhagic stroke observed among patients randomized to warfarin in PROTECT AF [20]. This experience demonstrates both the clinical need for an alternative to chronic oral anticoagulation and the natural instinct of physicians to use LAA closure as second line therapy.
The European ASAP registry was designed as a feasibility study of WATCHMAN LAA closure in warfarin contraindicated patients. Unlike the aforementioned WATCHMAN trials which required 45 days of warfarin following device deployment, ASAP enrolled 150 patients who had objective contraindications to oral anticoagulants but who could tolerate 6 months of thienopyridine therapy (clopidogrel or ticlopidine) [28]. The mean CHADS2 score and CHA2DS2-VASc scores were 2.8 ±1.2 and 4.4 ± 1.7, respectively. Serious procedure or device-related safety events occurred in 8.7% of patients (n = 13). All-cause stroke or systemic embolism occurred in 4 patients (2.3% per year): ischemic stroke in 3 patients (1.7% per year) and hemorrhagic stroke in 1 patient (0.6% per year). While not powered for a stroke end point, the ischemic stroke rate of 1.7% suggests that LAA occlusion with the WATCHMAN device may be safely implanted and provide some degree of stroke prevention in high risk patients who are ineligible for oral anticoagulants.
Further trials with sufficient power are needed to confidently understand the utility of LAA closure in this patient population without post procedure warfarin.
Future perspective
The WATCHMAN clinical trial experience is nuanced and critically important to understanding the role of LAA closure as an option for stroke reduction in AF. LAA closure with the WATCHMAN device appears to be associated with a higher incidence of ischemic stroke, but lower mortality and hemorrhagic stroke than warfarin. The low cardiovascular mortality observed in all of the WATCHMAN Trials has not only been less than the mortality observed among patients randomized to warfarin but is less than the cardiovascular mortality observed among patients randomized to either warfarin or a NOAC [9–12] (Table 5). Bleeding is clearly associated with excess mortality in numerous clinical trials providing a solid hypothesis for why WATCHMAN may be associated with diminished mortality when compared with warfarin. However, caution must be exercised before making firm conclusions regarding these secondary end point observations. For now it is safe to conclude that LAA closure is an appropriate option for patients with reasons to avoid chronic oral anticoagulation.
| Protect AF | Prevail (new patients) | CAP 1 | CAP 2 | RE-LY | Engage AF | Averroes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WATCHMAN | warfarin | WATCHMAN | warfarin | WATCHMAN | WATCHMAN | Dabigitran | Warfarin | Edoxaban | Warfarin | Apixiban | |
| 150 mg | 60 mg | ||||||||||
| CV death | 1.0 | 2.33 | 1.38 | 2.30 | 1.3 | 0.0 | 2.28 | 2.69 | 2.74 | 3.17 | 2.7 |
Table 5. Cardiovascular mortality across WATCHMAN and NOAC studies.
While the WATCHMAN clinical trial experience is robust, the research performed to date and FDA approval represent merely a beginning for the field of LAA closure. The ongoing LAAOS 3 Trial is randomizing AF patients undergoing cardiac surgery to either LAA excision or usual therapy but calls for usual post procedure anticoagulation in both groups [29]. The statistical model assumes that a portion of patients will discontinue oral anticoagulants. This trial emphasizes that local therapy directed at the left atrial appendage does not preclude pharmacologic antithrombotics. The AF population is highly heterogeneous. There is little available evidence regarding the comparative efficacy of LAA closure and oral anticoagulants, new or old, stratified according to clinical factors which may influence the efficacy of each therapy such as heart failure and atherosclerosis. The combination of pulmonary vein isolation and left atrial appendage closure may have synergistic benefits and warrants further study. Finally, additional LAA occlusion technologies may each convey unique advantages, risks and benefits.
Conclusion
The WATCHMAN device, through multiple robust trials, has been shown to be non-inferior to warfarin for the combined primary end point of stroke, systemic embolism and cardiovascular death with consistent statistically superior all-cause and cardiovascular mortality observed with long-term follow-up. While WATCHMAN largely eliminates the risk of anticoagulation associated hemorrhagic stroke and is also associated with diminished cardiovascular mortality, the device is associated with numerically higher risk of ischemic stroke compared with warfarin. The available data strongly support LAA closure with the WATCHMAN device as an alternative to oral anticoagulants in patients with a reason to avoid chronic oral anticoagulants.
Executive summary
• The WATCHMAN device is a percutaneous left-atrial appendage closure device recently approved for use by the US FDA.
• The WATCHMAN device can be deployed with excellent rates of left atrial appendage closure and low periprocedural adverse events.
• The WATCHMAN device has been shown to be noninferior to warfarin when evaluated with a primary composite end point of death, systemic embolism and all stroke.
• Secondary end point observations include a significant mortality benefit of the WATCHMAN device when compared with warfarin and a lower risk of hemorrhagic stroke, but a numerically increased risk of ischemic stroke with WATCHMAN when compared with warfarin.
Financial & competing interests disclosure
Brian Whisenant: Director and stockholder of Coherex Medical, Consultant for Boston Scientific. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
In addition to the peer-review process, with the authors’ consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made at the discretion of the authors and based on scientific or editorial merit only.
References
Papers of special note have been highlighted as: • of interest.
- Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 112(8), 1142–1147 (2013).
- Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J. Am. Coll. Cardiol. 29(4), 709–715 (1997).
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22(8), 983–988 (1991).
- Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. J. Am. Coll. Cardiol. 18(2), 349–355 (1991).
- Randomised double-blind trial of fixed low-dose warfarin with aspirin after myocardial infarction. Coumadin Aspirin Reinfarction Study (CARS) Investigators. Lancet 350(9075), 389–396 (1997).
- Ezekowitz MD, Bridgers SL, James KE et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N. Engl. J. Med. 327(20), 1406–1412 (1992).
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 146(12), 857–867 (2007).
- Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann. Intern. Med. 131(12), 927–934 (1999).
- Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361(12), 1139–1151 (2009).
- Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 365(10), 883–891 (2011).
- Granger CB, Alexander JH, Mcmurray JJ et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 365(11), 981–992 (2011).
- Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 369(22), 2093–2104 (2013).
- Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event:
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 61(2), 755–759 (1996).
- Cairns JA. Stroke prevention in atrial fibrillation trial. Circulation 84(2), 933–935 (1991).
- Saw J, Lempereur M. Percutaneous left atrial appendage closure: procedural techniques and outcomes. JACC. Cardiovasc. Interv. 7(11), 1205–1220 (2014).
- Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 33(21), 2719–2747 (2012).
- Sick PB, Schuler G, Hauptmann KE et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J. Am. Coll. Cardiol. 49(13), 1490–1495 (2007).
- Fountain RB, Holmes DR, Chandrasekaran K et al. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial. Am. Heart J. 151(5), 956–961 (2006).
- Holmes DR, Reddy VY, Turi ZG et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 374(9689), 534–542 (2009).
- Panel F. www.fda.gov/AdvisoryCommittees/ CommitteesMeetingMaterials/MedicalDevices/ MedicalDevicesAdvisoryCommittee/ CirculatorySystemDevicesPanel/ucm152596.htm.
- Holmes DR Jr, Kar S, Price MJ et al. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL Trial. J. Am. Coll. Cardiol. 64(1), 1–12 (2014).
- Reddy VY, Doshi SK, Sievert H et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) Trial. Circulation 127(6), 720–729 (2013).
- Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman left atrial appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation 123(4), 417–424 (2011).
- Reddy VY, Sievert H, Halperin J et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 312(19), 1988–1998 (2014).
- FDA Executive Summary: Boston Scientific WATCHMAN Left Atrial Appendage Closure Therapy. Prepared for the December 11, 2013 meeting of the Circulatory System Devices Panel. www.fda.gov/downloads/ AdvisoryCommittees/CommitteesMeetingMaterials/ MedicalDevices/MedicalDevicesAdvisoryCommittee/ CirculatorySystemDevicesPanel/UCM377356.pdf
- FDA: FDA Executive Summary Prepared for the October 8, 2014 meeting of the Circulatory System Devices Panel Boston Scientific WATCHMAN® Left Atrial Appendage Closure Therapy. www.fda.gov/downloads/ AdvisoryCommittees/CommitteesMeetingMaterials/ MedicalDevices/MedicalDevicesAdvisoryCommittee/ CirculatorySystemDevicesPanel/UCM417199.pdf.
- Reddy VY, Mobius-Winkler S, Miller MA et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J. Am. Coll. Cardiol. 61(25), 2551–2556 (2013).
- Whitlock R, Healey J, Vincent J et al. Rationale and design of the Left Atrial Appendage Occlusion Study (LAAOS) III. Ann. Cardiothorac. Surg. 3(1), 45–54 (2014).
• The landmark WATCHMAN trial comparing the device to warfarin in over 700 patients, with WATCHMAN being shown to be non-inferior in efficacy to warfarin.
• Confirmatory trial of efficacy and safety of WATCHMAN versus warfarin.
• Prospective, nonrandomized registry that had improved safety margins compared with the original PROTECT AF cohort.
• Pre-specified, long-term follow-up of the PROTECT AF cohort, affirming the noninferior efficacy of WATCHMAN compared with warfarin in composite end points and significantly improved mortality.
• Non-randomized cohort of patients who are not eligible for anticoagulation, estimating a significant decrease in embolic events compared with risk estimated stroke rate.