Short Article - Interventional Cardiology (2012) Volume 4, Issue 2
Percutaneous pulmonary valve implantation: the Munich experience
- Corresponding Author:
- Andreas Eicken
Klinik für Kinderkardiologie und angeboren Herzfehler
Deutsches Herzzentrum München
Technische Universität München, Germany
Tel: +49 89 1218 3011
Fax: +49 89 1218 2333
E-mail: eicken@dhm.mhn.de
Abstract
Keywords
catheter intervention, percutaneous pulmonary valve implantation, right ventricular outflow tract dysfunction
In 2000, Bonhoeffer et al. introduced percutaneous pulmonary valve implantation (PPVI) as a new treatment option for right ventricular outflow tract (RVOT) dysfunction [1]. The aim of this novel catheter intervention was to reduce the total number of open heart surgeries over a patient’s lifetime. By percutaneous implantation of a stented valve into a dysfunctioning biological valve, the lifetime of the stenotic or regurgitant valved conduit can be expanded. Today, surgical replacement of a pulmonary valve can be performed with a very low mortality [2,3]. However, the morbidity of repeated cardiothoracic operations is significant. Additionally, these operations become technically more demanding with every consecutive procedure (e.g., adhesions and progressive scaring). For this reason, PPVI has emerged as the preferred treatment for selected patients with conduit dysfunction in the pulmonary position at some centers. This review summarizes the experience with PPVI at the German Heart Centre Munich (Munich, Germany).
Patient selection for PPVI
Patients are scheduled for PPVI if they fulfil the criteria for surgical valve replacement in the RVOT [4,5]. Box 1 depicts the inclusion criteria for PPVI treatment at the German Heart Centre Munich. At our center we use a combination of clinical data, serial echo-cardiographic examinations, treadmill tests with VO2 max assessment, a 24 h Holter and a cMRI examination. The cMRI plays a key role in this assessment not only for anatomical depiction to approve technical feasibility of PPVI. Determination of ventricular volumes, ventricular function, an assessment of pulmonary regurgitation (PR) and a determination of flow ratios to the pulmonary arteries [6,7] adds significant information for correct timing of this catheter intervention. In patients with predominant RVOT stenosis, the indication for treatment is relatively clear (right ventricular [RV] systolic pressure >60 mmHg, tricuspid regurgitation velocity >3.5 m/s). However, in predominant PR or in combination of stenosis and regurgitation, timing for treatment is not as simple. Most patients with RVOT dysfunction show a combination of obstruction and regurgitation. Relief of RVOT obstruction during repair of tetralogy of Fallot (TOF) in infancy often involves the placement of a transannular RVOT patch. The degree of the resultant PR is dependent on the regurgitation orifice area, the compliance of the right ventricle, the diastolic pressure difference between the main pulmonary artery and the right ventricle and the duration of diastole [8]. PR may be tolerated well for years and even decades, but leads to RV dysfunction [9], and is not a benign cardiac lesion. In adults with corrected TOF and severe PR, at 40 years of age only 50% were free of cardiac symptoms [10]. Furthermore, QRS prolongation and reduced exercise tolerance were described in these patients [11,12]. Gatzoulis et al. highlighted the association of an increased risk of malignant dysrhythmia and sudden cardiac death with QRS-complex prolongation (>180 ms) [13,14].
In contrast to the volume loaded right ventricle, the pathophysiology of the left ventricle in response to chronic volume load due to aortic regurgitation has been studied extensively [15]. After a variable period of time during which left ventricular (LV) dilatation is potentially reversible, a ‘point of no return’ is reached, which is characterized by irreversible ventricular dilatation with myocardial scaring, fibrosis and increased interstitial collagen [16]. Hence, in these patients myocardial dysfunction persists even after aortic valve replacement [17]. Although there are similarities in the pathophysiological response of the volume loaded right and left ventricle, significant differences are present in chamber geometry, myofiber architecture, myocardial contraction pattern, coronary arterial morphology and physiology, and the conduction system of both ventricles. After analyzing (RV radionuclide ventriculography) right ventricle size and function of patients with TOF, significant PR, dilated right ventricles before and after pulmonary valve replacement (PVR) Therrien et al. asked the question whether their patients were treated too late, since a significant number of their patients failed to show RV remodeling after valve replacement [18]. The same group proposed a RV end diastolic volume index of 170 ml/m2 BSA as a cut off value for decision-making for PVR [19]. However, in a study with younger patients with TOF and PR Buechel et al. demonstrated that RV remodeling after PVR was possible at even higher RV end diastolic pressure volume indices [20]. In conclusion, none of these three studies are controlled randomized trials and all three studies present rather small case series. However, there seems to be an age and volume index-dependent potential of the volume-loaded right ventricle to remodel after adequate therapy for severe PR. Oosterhof et al. reported the results of a prospective multicenter study including 71 adult patients with TOF and PR. They analyzed whether a threshold exists above which the right ventricle does not improve further after surgery and whether preoperative thresholds can be identified for normalization of RV volumes after valve replacement. They could not identify a threshold above which RV volumes did not decrease after PVR. Normalization of RV volumes could be achieved when preoperative RV end diastolic volume was <160 ml/m2 or RV end systolic volume was <82 ml/m2 [21]. Another group studied 71 consecutive patients (mean age: 22 years, 72% TOF) pre and 1 year after PVR by cMRI [22]. They concluded that treating patients with an end diastolic volume <150 ml/m2 leads to normalization of RV volumes, improvement in biventricular function and submaximal exercise capability. Normalization of ventriculatory response to carbon dioxide production was most likely to occur when surgery was performed at an age ≤17.5 years.
In conclusion, we believe it is too late to wait until patients with RVOT dysfunction are clinically symptomatic. The currently used indication criteria for PPVI in Munich are depicted in Box 2. However, since long-term results of PPVI remain to be analyzed overtreatment of patients should be avoided. After the indication for PPVI is fixed the findings should be discussed in an interdisciplinary team conference.
Percutaneous pulmonary valve implantation
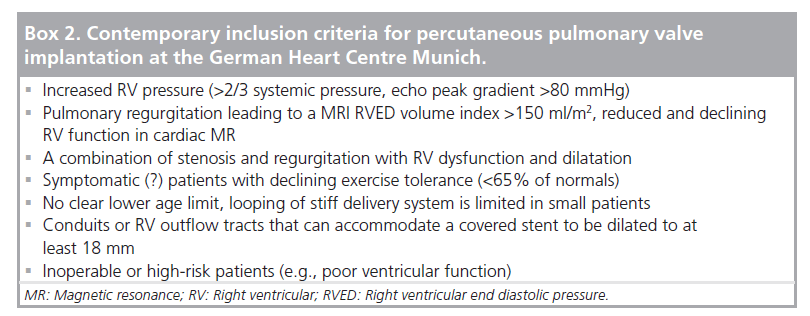
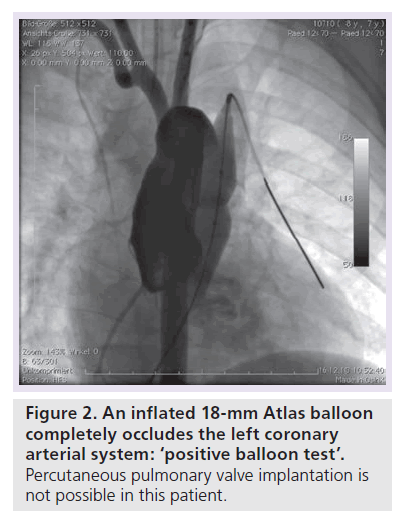
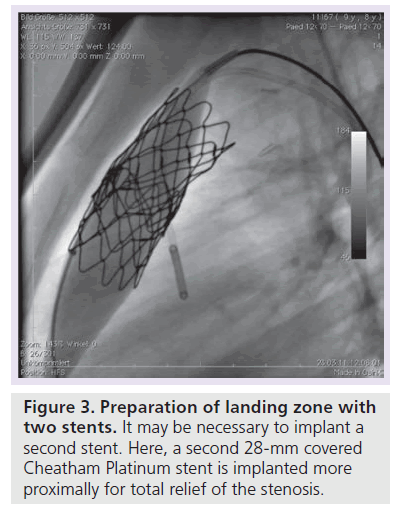
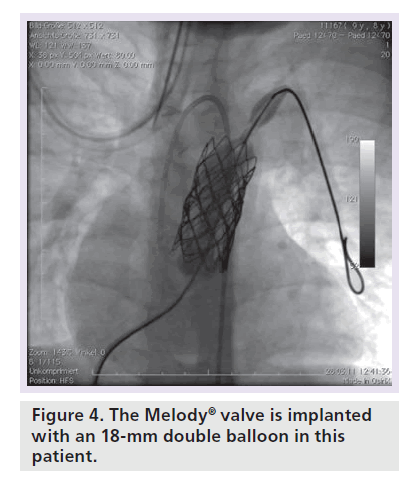
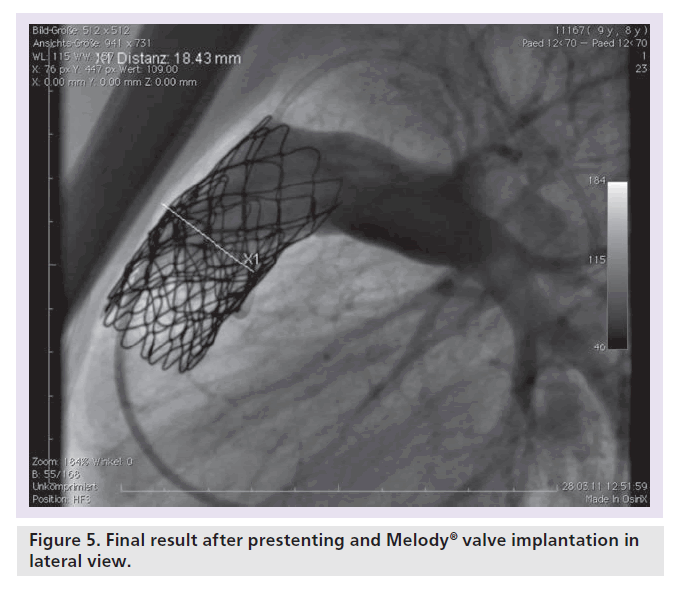
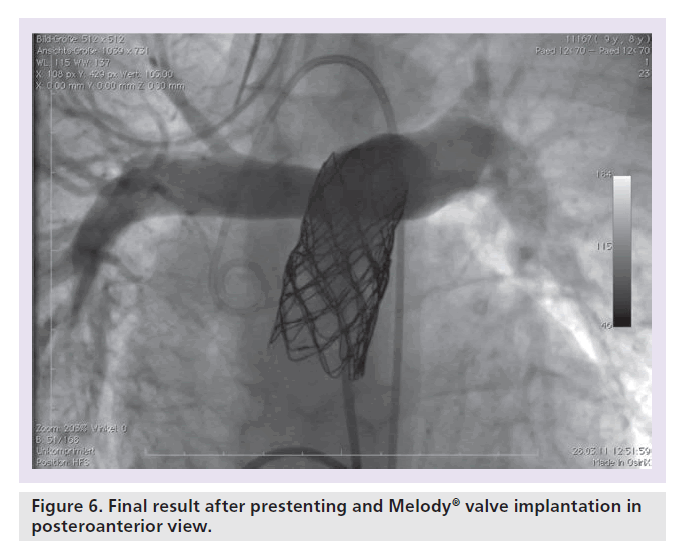
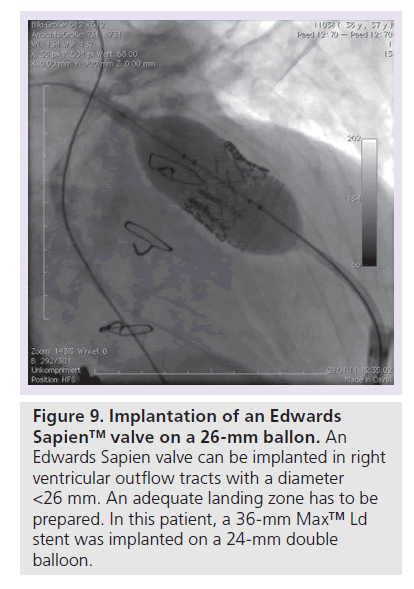
Patients with an indication for PPVI are scheduled for a complete hemodynamic and angiographic study. Figure 1 shows the lateral view of the RVOT with measurements. At our center, we use general anesthesia for PPVI. A superstiff 0.0035 inch guidewire (Lunderquist®; Cook Medical, Bjaeverskov, Denmark, or backup Meier, Boston Scientific, Maple Grove, MN, USA) is positioned distally into a pulmonary artery. To exclude potential coronary arterial compression a balloon test is performed as suggested by Sridharan et al. [23]. For the balloon test a high-pressure balloon (Atlas balloon, Bard Inc. Tempe, AZ, USA, Mullins balloon, NuMed Inc., Best, The Netherlands) with the expected end procedural RVOT diameter is inflated. At the same time an aortogram is performed, which depicts the coronary arteries, together with the inflated balloon. If the balloon compromises a coronary artery (Figure 2 positive balloon test) the procedure is abandoned. It may be necessary to repeat the balloon test with selective coronary angiograms. We prefer to start the balloon test with an aortogram since compression of a coronary arterial ostium may be missed, if a coronary catheter ‘intubated’ the coronary artery. Originally, prestenting of the RVOT was not suggested [24]. However, Nordmeyer et al. reported a significant incidence of stent fractures (21%) in patients with PPVI without prestenting [25]. We therefore adopted a policy of prestenting and recently demonstrated a reduced incidence of stent fractures of 5% [26]. Currently, a stent is positioned into the RVOT via a long sheath of Cheatham Platinum™ covered or uncovered stent, NuMed Inc., Best, The Netherlands; MaxLD EV3, Plymouth, MN, USA, or Andra Stents®, Andramed, Reutlingen, Germany). If the RVOT is narrow, a covered stent is preferrably placed to prevent RVOT rupture during PPVI. The stented ‘landing zone’ for the transcutaneous valve is dilated to the previously tested RVOT diameter, if necessary with highpressure balloons. In some patients it may be necessary to implant additional stents until the complete outflow tract is wide enough (Figure 3). The balloon deflation is then recorded and reviewed. In case of a significant stent-recoil during balloon deflation further stents are delivered into the RVOT. The Melody® valve can then be delivered into the RVOT (Figure 4). If the prepared landing zone in the RVOT has a diameter <22 mm a Melody valve is chosen, if the diameter is >22 mm up to 26 mm the Sapien™ valve (Edwards Lifesciences; Irvine, CA, USA) is implanted at our center [27]. The final result is depicted in Figures 5 & 6. During prestenting in preparation of implantation of the Sapien valve a fluoroscopic image in the en face view should be obtained to ensure that the prestented conduit landing zone appears circular prior to implantation of the valve.
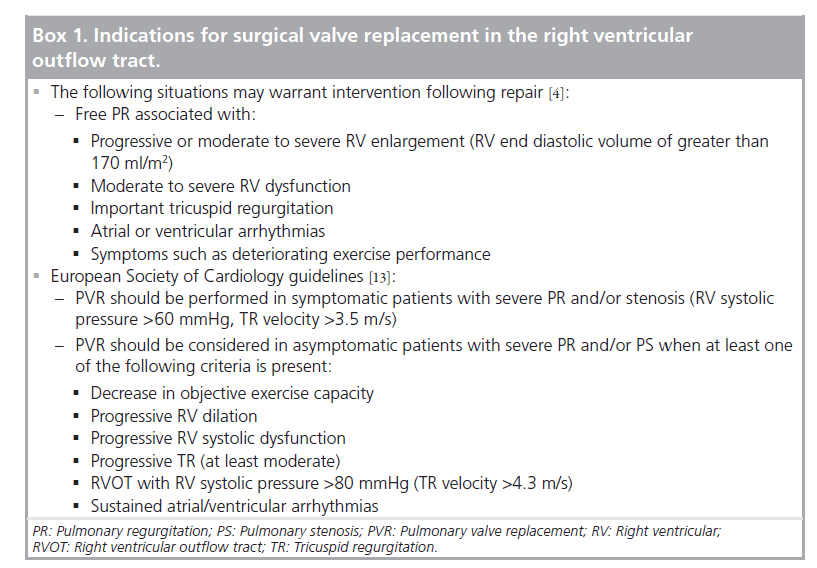
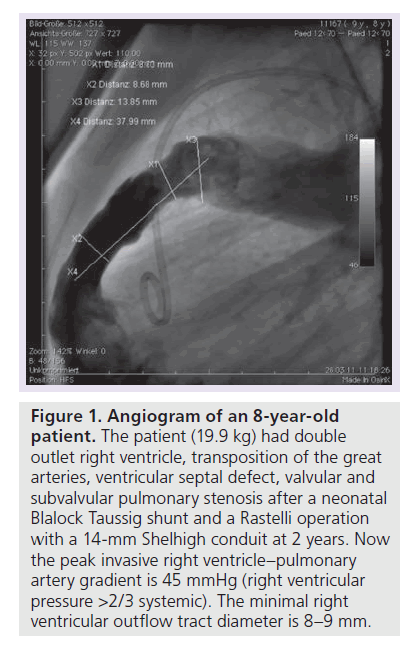
Figure 1. Angiogram of an 8-year-old patient.
The patient (19.9 kg) had double outlet right ventricle, transposition of the great arteries, ventricular septal defect, valvular and subvalvular pulmonary stenosis after a neonatal Blalock Taussig shunt and a Rastelli operation with a 14-mm Shelhigh conduit at 2 years. Now the peak invasive right ventricle–pulmonary artery gradient is 45 mmHg (right ventricular pressure >2/3 systemic). The minimal right ventricular outflow tract diameter is 8–9 mm.
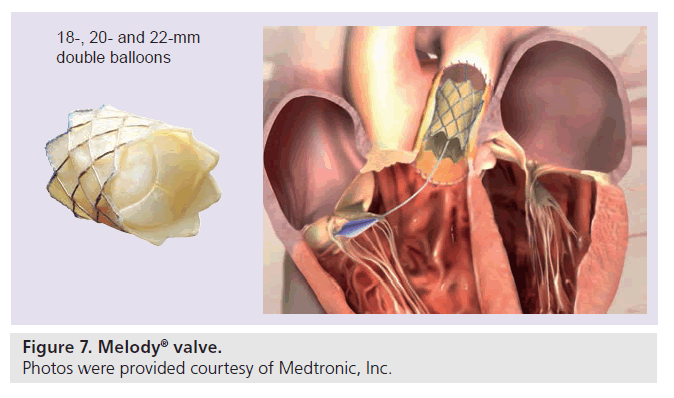
The currently available Melody valve (Medtronic, Minneapolis, MN, USA) consists of a 34-mm bare metal Cheatham Platinum stent (8zig NuMED; Cornwell, ON, Canada) into which a ContegraTM bovine jugular venous valve has been sawn into (Figure 7). The valve is crimped on an 18-, 20- or 22-mm double balloon delivery system, which has an external diameter of 22 F. Over a guidewire the system is advanced into the target position, where it can be inflated. After valve delivery, the hemodynamics are reassessed and if necessary the RVOT is postdilated with high-pressure balloons. The residual RVOT gradient should be less than 20 mmHg. A gradient >20 mmHg has been shown to be a risk factor for early valve dysfunction [28].
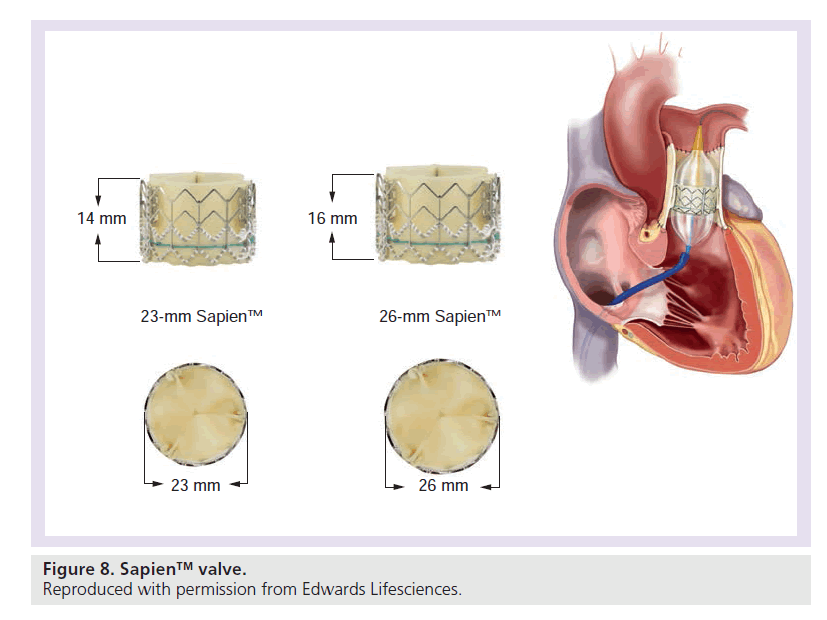
The Edwards Sapien transcatheter heart valve is made of three bovine pericardial leaflets sewn inside a stainless steel stent (Figure 8) and the valve implantation is very similar to the procedure mentioned above. First an adequate landing zone has to be prepared by stent implantation. The valve is subsequently pushed over the stiff guidewire into the correct position. The Sapien valve (dimensions: 23 × 14.3 mm and 26 × 16.1 mm) is mounted on a high-pressure balloon, which is inflated once the valve is in position (Figure 9). The valve is crimped on the balloon with a defined fluid volume in a special crimper and was originally designed for aortic valve replacement via an antegrade or retrograde approach. To facilitate delivery of the valve in a retrograde fashion, a specially designed stearable delivery catheter (retroflex) is used [29]. Prestenting is mandatory for the Sapien valve. The Sapien valve can be used in larger diameter RVOTs, however, the large introducer assembly limits the use in small patients.
The experience with the Melody valve is documented in three larger clinical studies [26,28,30], but the experience with the Sapien valve is limited so far [31,32].
Complications during PPVI
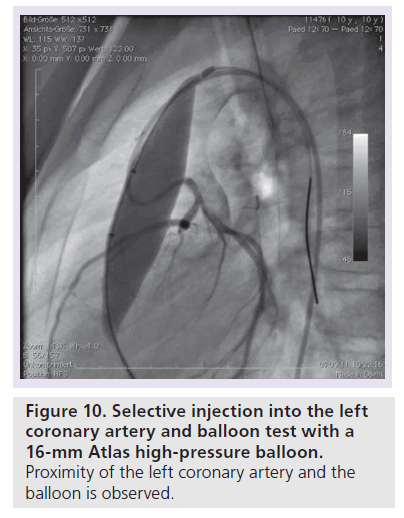
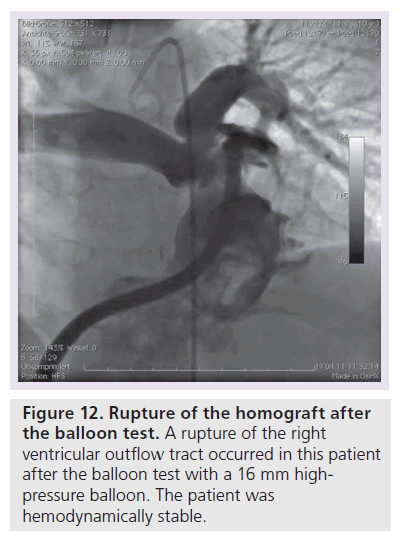
There are two severe periprocedural complications which occurred during PPVI – coronary compression and RVOT perforation/rupture. Coronary arterial compression occurred and has contributed to the reported death in at least two patients so far (one patient in Munich and one patient in Berlin). Figure 10 shows the selective coronary angiogram with a balloon test (Atlas 16 mm) in a 10‑year-old patient after a Ross operation with systemic pressure in the right ventricle. A 34-mm covered stent was implanted along with a Melody valve on a 18-mm double balloon. After repetitive dilatation with an 18-mm Atlas balloon the origin of the left coronary artery was completely occluded (Figure 11). The patient was put on a extracorporal membrane oxygenation in the catheterization laboratory and received a LV assist device 2 weeks later. He died on the waiting list for cardiac transplantation whilst on LV assist device support. During a Ross operation, the coronary arteries are repositioned and this in combination with the extra-anatomic position of the conduit in the RVOT may impose an increased risk for potential coronary arterial compression. The second potential severe complication is RVOT rupture (Figure 12). This occurred in four of our cases and was controlled by implantation of a covered stent (Figure 13). In the initial cohort of Bonhoeffer, emergency rescue surgery was necessary in six of 152 (3.9%) patients (homograft rupture two, valve dislodgement two, ‘jailing’ of the right pulmonary artery one, coronary compression one) [33]. All patients survived this operation. If proximity of the coronary arteries to the landing zone is suspected, at our unit we abandon the procedure, or at least perform the balloon test with a high-pressure balloon that equals the maximal anticipated RVOT diameter. If severe complications (coronary compression, RVOT rupture with hemodynamic compromise) occur, the surgical team needs to be involved in further decision-making for therapeutic options.
Figure 10. Selective injection into the left coronary artery and balloon test with a 16-mm Atlas high-pressure balloon.
Proximity of the left coronary artery and the balloon is observed.
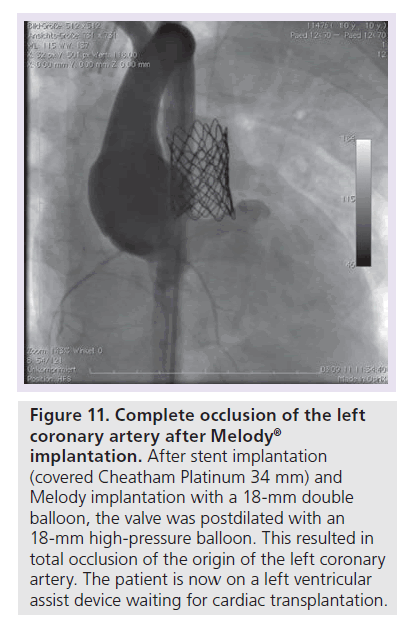
Figure 11. Complete occlusion of the left coronary artery after Melody R implantation.
After stent implantation (covered Cheatham Platinum 34 mm) and Melody implantation with a 18-mm double balloon, the valve was postdilated with an 18-mm high-pressure balloon. This resulted in total occlusion of the origin of the left coronary artery. The patient is now on a left ventricular assist device waiting for cardiac transplantation.
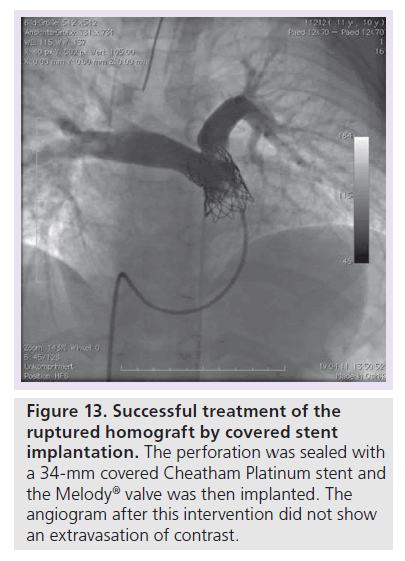
Figure 13. Successful treatment of the ruptured homograft by covered stent implantation.
The perforation was sealed with a 34-mm covered Cheatham Platinum stent and the Melody® valve was then implanted. The angiogram after this intervention did not show an extravasation of contrast.
General considerations on PPVI
In our personal experience in Munich after 94 successful PPVI procedures to date, only one emergency operation has been necessary due to coronary compression. If valvular function dete-riorates, the valve-in-valve concept proved to be feasible [34]. Initially, PPVI was only suggested if a biological conduit was present in the RVOT [28]. However, in selected patients PPVI is also possible in patients with a ‘native’ RVOT [26,35]. In a recent study, a total of 65 patients (pulmonary stenosis [PS] 35, PR 30) with sustained effects of PPVI were studied before, 1 month and 12 months after the intervention with cMRI and cardiopulmonary exercise testing [36]. There was a significant decrease in RV end diastolic pressure volume early after PPVI. RV ejection fraction only improved in patients with PS (p < 0.001). In patients with PR, no difference in ejection fraction was seen. Late after the intervention, there were no further changes in the MRI parameters [36]. In the PS group at cardiopulmonary exercise testing, there was a significant improvement in peak oxygen uptake early (p = 0.008), with no further significant change late. In the PR group, no significant changes in peak oxygen uptake from early to late could be demonstrated [36]. The authors concluded that in patients with sustained hemodynamic result 1 year after PPVI, a prolonged phase of maintained cardiac function is observed. However, there is no evidence for further positive functional remodeling beyond the acute effects of PPVI. Another study documented electrical remodeling following PPVI [37]. In patients with predominant PR, QRS duration decreased significantly (p < 0.007). Yet long-term results are missing after this intervention.
Comparison of PPVI & surgery: periprocedural complications
Finally, the results of patients with RVOT dysfunction from our institution treated surgically (n = 56) and by PPVI (n = 53) were compared [38]. Whereas the leading lesion in the surgical collective was PR, stenosis was the leading lesion in the PPVI group. The median postinterventional hospital stay was significantly shorter after PPVI (2 vs 17 days, p < 0.001). Peri-interventional complications occurred in 38% of the surgically treated patients (urgent reoperations two patients, pacemaker one, fever of unknown reason 13, severe arrhythmia with medical treatment four and seizures one) and only in 4% of the catheter interventionally treated patients (fever of unknown reason one patient and temporary complete AV-block). The peak systolic Doppler gradient was 30 mmHg after PPVI versus 19 mmHg in the surgical group (p < 0.0001) 1 year after the intervention.
Conclusion
In conclusion, PPVI is feasible and relatively safe after adequate patient selection, if performed by an experienced interventionalist. Good and sustained hemodynamic results at mid term follow- up are documented so far.
Future perspective
Until now only patients with RVOT dysfunction and a RVOT diameter <26 mm were amenable for catheter based interventional treatment. Usually in prevailing PR the RVOT is significantly enlarged (often >26 mm). The advent of a self expanding stent in combination with an inserted valve (‘outflow tract reducer’) may overcome some of this limitation [39]. This device may be used in compliant RVOTs, when prestenting is not an option. Alternatively, transcutaneous valves with a larger internal valve diameter need to be developed in order to meet the demands of patients with larger RVOTs. At the other end of the spectrum, smaller trancutaneous valves and delivery systems may enable treatment in younger children.
Financial & competing interests disclosure
A Eicken and J Hess work as proctors for Medtronic Melody valve implantations. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Percutaneous pulmonary valve implantation is one of the most significant innovations in the catheter interventional treatment of patients with congenital heart disease within the last 10 years.
▪ After adequate patient selection the intervention leads to significant improvement in hemodynamics.
▪ Coronary arterial compression and rupture of the right ventricular outflow tract are the major hazards.
▪ Long-term studies are needed to demonstrate a persistent effect of the excellent mid-term results.
References
- Bonhoeffer P, Boudjemline Y, Saliba Z et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 356, 1403–1405 (2000).
- Lange R, Weipert J, Homann M et al. Performance of allografts and xenografts for right ventricular outflow tract reconstruction. Ann. Thorac. Surg. 71, S365–S367 (2001).
- Bielefeld MR, Bishop DA, Campbell DN, Mitchell MB, Grover FL, Clarke DR. Reoperative homograft right ventricular outflow tract reconstruction. Ann. Thorac. Surg. 71, 482–487; discussion 487–488 (2001).
- Silversides CK, Kiess M, Beauchesne L et al. Canadian Cardiovascular Society 2009 consensus conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan’s syndrome. Can. J. Cardiol. 26, e80–e97 (2009).
- Baumgartner H, Bonhoeffer P, De Groot NM et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010). Eur. Heart J. 31, 2915–2957 (2010).
- Eicken A, Fratz S, Gutfried C et al. Hearts late after fontan operation have normal mass, normal volume, and reduced systolic function: a magnetic resonance imaging study. J. Am. Coll. Cardiol. 42, 1061–1065 (2003).
- Fratz S, Hess J, Schwaiger M, Martinoff S, Stern HC. More accurate quantification of pulmonary blood flow by magnetic resonance imaging than by lung perfusion scintigraphy in patients with fontan circulation. Circulation 106, 1510–1513 (2002).
- Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J. Cardiovasc. Magn. Reson. 13, 9 (2011).
- Redington AN, Oldershaw PJ, Shinebourne EA, Rigby ML. A new technique for the assessment of pulmonary regurgitation and its application to the assessment of right ventricular function before and after repair of tetralogy of Fallot. Br. Heart J. 60, 57–65 (1988).
- Shimazaki Y, Blackstone EH, Kirklin JW. The natural history of isolated congenital pulmonary valve incompetence: surgical implications. Thorac. Cardiovasc. Surg. 32, 257–259 (1984).
- Carvalho JS, Shinebourne EA, Busst C, Rigby ML, Redington AN. Exercise capacity after complete repair of tetralogy of Fallot: deleterious effects of residual pulmonary regurgitation. Br. Heart J. 67, 470–473 (1992).
- Wessel HU, Paul MH. Exercise studies in tetralogy of Fallot: a review. Pediatr. Cardiol. 20, 39–47; discussion 48 (1999).
- Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 92, 231–237 (1995).
- Gatzoulis MA, Balaji S, Webber SA et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 356, 975–981 (2000).
- Chaliki HP, Mohty D, Avierinos JF et al. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 106, 2687–2693 (2002).
- Sparrow P, Messroghli DR, Reid S, Ridgway JP, Bainbridge G, Sivananthan MU. Myocardial T1 mapping for detection of left ventricular myocardial fibrosis in chronic aortic regurgitation: pilot study. AJR Am. J. Roentgenol. 187, W630–W635 (2006).
- Borer JS, Truter S, Herrold EM et al. Myocardial fibrosis in chronic aortic regurgitation: molecular and cellular responses to volume overload. Circulation 105, 1837–1842 (2002).
- Therrien J, Siu SC, McLaughlin PR, Liu PP, Williams WG, Webb GD. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J. Am. Coll. Cardiol. 36, 1670–1675 (2000).
- Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am. J. Cardiol. 95, 779–782 (2005).
- Buechel ER, Dave HH, Kellenberger CJ et al. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur. Heart J. 26, 2721–2727 (2005).
- Oosterhof T, van Straten A, Vliegen HW et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation 116, 545–551 (2007).
- Frigiola A, Tsang V, Bull C et al. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation 118, S182–S190 (2008).
- Sridharan S, Coats L, Khambadkone S, Taylor AM, Bonhoeffer P. Images in cardiovascular medicine. Transcatheter right ventricular outflow tract intervention: the risk to the coronary circulation. Circulation 113, e934–e935 (2006).
- Khambadkone S, Coats L, Taylor A et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation 112, 1189–1197 (2005).
- Nordmeyer J, Khambadkone S, Coats L et al. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation 115, 1392–1397 (2007).
- Eicken A, Ewert P, Hager A et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. Eur. Heart J. 32, 1260–1265 (2011).
- Garay F, Webb J, Hijazi ZM. Percutaneous replacement of pulmonary valve using the edwards-cribier percutaneous heart valve: first report in a human patient. Catheter. Cardiovasc. Interv. 67, 659–662 (2006).
- Lurz P, Coats L, Khambadkone S et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation 117, 1964–1972 (2008).
- Webb JG, Chandavimol M, Thompson CR et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 113, 842–850 (2006).
- McElhinney DB, Hellenbrand WE, Zahn EM et al. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation 122, 507–516 (2010).
- Boone RH, Webb JG, Horlick E et al. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheter. Cardiovasc. Interv. 75, 286–294 (2010).
- Kenny D HZ, Rhodes J, Mullen M et al. Percutaneous implantation of the Edwards Sapien transcatheter heart valve for conduit failure in the pulmonic position: early Phase 1 results from international multicenter clinical trial. J. Am. Coll. Cardiol. 58(21), 2248–2256 (2011).
- Kostolny M, Tsang V, Nordmeyer J et al. Rescue surgery following percutaneous pulmonary valve implantation. Eur. J. Cardiothorac. Surg. 33, 607–612 (2008).
- Nordmeyer J, Coats L, Lurz P et al. Percutaneous pulmonary valve-in-valve implantation: a successful treatment concept for early device failure. Eur. Heart J. 29, 810–815 (2008).
- Momenah TS, El Oakley R, Al Najashi K et al. Extended application of percutaneous pulmonary valve implantation. J. Am. Coll. Cardiol. 53, 1859–1863 (2009).
- Lurz P NJ, Giardini A, Khambadkone S et al. Early versus late functional outcome after successful percutaneous pulmonary valve implantation – are the acute effects of altered right ventricular loading all we can expect? J. Am. Coll. Cardiol. 57, 724–731 (2011).
- Plymen CM, Bolger AP, Lurz P et al. Electrical remodeling following percutaneous pulmonary valve implantation. Am. J. Cardiol. 107(2), 309–314 (2011).
- Dilber D, Hager A, Fratz S, Hoerer J, Malcic I, Hess J. Percutaneous pulmonary valve implantation versus surgical implantation/replacement in patients with right ventricular outflow tract dysfunction. Cardiol. Young (Abstract) 21(Suppl. 1), S9 (2011).
- Schievano S, Taylor AM, Capelli C et al. First-in-man implantation of a novel percutaneous valve: a new approach to medical device development. EuroIntervention 5, 745–750 (2010).