Review Article - Interventional Cardiology (2014) Volume 6, Issue 5
Percutaneous suture ligation of left atrial appendage using the LARIAT device: a review of technique and current data
- Corresponding Author:
- Dushad Ram
Department of Interventional Cardiology, Cavanagh Heart Center, Banner Good Samaritan Medical Center, Phoenix, AZ, 85006, USA
Tel: +1 602 839 7393
Fax: +1 602 839 7661
E-mail: ashish.pershad@bannerhealth.com
Abstract
Atrial fibrillation affects over 2.5 million people in the USA and more than 6 million people in Europe. The most devastating complication of atrial fibrillation is systemic thromboembolism, manifesting as ischemic stroke. The left atrial appendage, accounts for 90% of the atrial thrombi in atrial fibrillation. There are two strategies to prevent left atrial appendage thrombi: long term anticoagulation; and eliminating the source of the thrombus, that is, excision/exclusion of the left atrial appendage surgically or percutaneously. In this review, a detailed discussion of all aspects of the LARIAT device are presented including pre-procedural work up, intra-procedural details and post-procedural complications and follow-up, along with the evidence base in the literature for use of this device.
Atrial fibrillation affects over 2.5 million people in the USA and more than 6 million people in Europe. The most devastating complication of atrial fibrillation is systemic thromboembolism, manifesting as ischemic stroke. The left atrial appendage, accounts for 90% of the atrial thrombi in atrial fibrillation. There are two strategies to prevent left atrial appendage thrombi: long term anticoagulation; and eliminating the source of the thrombus, that is, excision/exclusion of the left atrial appendage surgically or percutaneously. In this review, a detailed discussion of all aspects of the LARIAT device are presented including pre-procedural work up, intra-procedural details and post-procedural complications and follow-up, along with the evidence base in the literature for use of this device.
Keywords
anticoagulation, CTA, LARIAT, left atrial appendage occlusion, stroke prevention, suture, technique
Background
Atrial fibrillation (AF) is the leading arrhythmia affecting over 2.5 million people in the USA and more than 6 million people in Europe [1,2]. This number is projected to increase sixfold by 2050 [3]. The most devastating complication of AF is systemic thromboembolism that manifests as ischemic stroke. Fifteen to 20% of patients with stroke have AF [4,5]. Stroke is more severe in patients with AF, causing 70% chance of death or permanent disability because of involvement of large intracranial arteries resulting in more extensive functional impairment [4]. The predominant source of thrombus is believed to be the left atrial appendage (LAA), which accounts for 90% of the atrial thrombi in AF [6].
The stroke risk for any patient with AF is calculated by using the CHA2DS2VASc scoring model, with higher scores implicating a substantial lifetime risk of stroke. The highest score for CHA2DS2VASc model confers a stroke risk of 18.2% [5]. There are two strategies to prevent LAA thrombi: long term anticoagulation; and eliminating the source of the thrombus, that is, excision/exclusion of the LAA surgically or percutaneously. The first-line approach is to initiate long-term oral anticoagulants (OACs) to prevent LAA thrombus in patients whose stroke risk score, based on above models, is >1. Although the use of warfarin with a target International Normalized Ratio (INR) of 2–3 has been shown to significantly reduce the risk of stroke, its use is affected by numerous drug/ food interactions and the need to closely monitor the INR level. Even with a good follow up, the INR remains in the therapeutic range only about half the time. Novel anticoagulants (dabigatran, rivaroxaban and apixaban) have come up in the last few years and overcome some of these disadvantages of warfarin. These agents have their limitations and are similar to warfarin in their contraindication in patients who are deemed high risk for bleeding. Ironically, stroke risk increases with age and so does the risk of serious bleeding and falls. Moreover, many of the patients have coexisting coronary artery disease requiring the concomitant use of antiplatelet agents that further aggravate the bleeding risk. The HAS-BLED scoring system assesses the risk of bleeding in patients receiving OAC and a score of 3 and above requires caution with the use of OAC [7]. About 60% of patients with AF have CHA2DS2VASc score of more than one, warranting the need for anticoagulation, but an overwhelming 45% of these patients are ineligible for OAC. This leaves a large number of patients unprotected and susceptible to thrombus formation and subsequent stroke [7].
Surgical methods to eliminate thrombus from LAA include excision of the LAA or its exclusion using endocardial or epicardial sutures, staples or clips. Surgical methods have been traditionally used along with valve surgery, coronary artery bypass graft, or as part of a MAZE procedure [10]. Surgical exclusion of the LAA is fraught with low overall success. In a transesophageal echocardiography (TEE)-based assessment of the LAA post-surgery, only about 73% successful closure with excision was reported and a dismal 23% success rate with stapler or suture occlusion was observed [8]. This has been attributed primarily to flaccidity of the heart during cardiopulmonary bypass, difficult access to the relatively posterior location of the LAA base, difficulty in capturing the base of the appendage with sutures/staples in a beating heart and the inability to verify completeness of closure until the patient is off cardiopulmonary bypass [9,10].
Percutaneous approach to LAA exclusion is promising because it is minimally invasive, eliminates the morbidity associated with surgery and can be performed as a standalone procedure. A few novel devices have been developed in the last decade and the first to be commercially tested was the PLAATO device. This device is no longer available.
The WATCHMAN device (Boston Scientific, Plymouth, MN, USA) has a self-expanding nitinol frame with a porous polyester covering and fixation barbs for attachment to the endocardium. The device is delivered percutaneously via trans-septal puncture. The device is secured close to the LAA ostium to trap any thrombus before it exits the LAA. Usually it takes 45 days for the device to be endothelialized and requires the patient to be anticoagulated with warfarin for at least 45 days and with DAPT for 6 months thereafter. The Watchman device was found to be noninferior to warfarin for stroke prevention in the PROTECT- AF trial wherein, 707 patients with AF and CHADS2 score ≥1 were randomized to receive warfarin or this device [11,12]. The primary efficacy (stroke, systemic embolism, and cardiovascular or unexplained death) event rate per 100 patient-years was lower with the Watchman device compared with controls (2.3 vs 3.8%), demonstrating a 40% relative risk reduction (RR 0.60; 95% CI: 0.41–1.05). Subgroup analysis maintained these results but showed slight differences based on gender, CHADS2 score and A-fib pattern. Notably, efficacy was not diminished among patients with a history of transient ischaemic attack/stroke. In the ASAP trial that enrolled patients that were not eligible to take warfarin, the use of the WATCHMAN device was associated with lower rates of stroke and transient ischemic attacks [13]. The results were reproduced in the PREVAIL trial [14] that included patients with a higher CHADS score of >2. The need for shortterm anticoagulation post procedure is a major limitation to its use in patients who have significant bleeding risk. The Amplatzer Cardiac Plug (ACP) (St Jude, Plymouth, MN, USA) device is a further development and consists of a lobe (or distal plug) and a disc connected by a central waist. There are six pairs of barbs on the lobe that stabilize the device within the appendage while the disc seals the LAA orifice. Preliminary studies have shown encouraging results [15–17] and a multicenter prospective randomized trial to compare this device to OAC or potentially the WATCHMAN device once it is commercially available in the USA, in patients with CHADS2 score ≥2 is being planned [18]. The next-generation Amplatzer Cardiac Plug (ACP2) also known as the Amulet is now available in Canada and Europe. Potential advantages of the Amulet device include ease of device preparation, better fit to different LAA anatomy, facilitation of recapture and repositioning, more stable anchoring in the LAA and minimized risk of thrombus.
A newer approach to exclude the LAA is the LARIAT Suture Delivery Device (SentreHEART Inc, Redwood City, CA, USA) that aims at snaring and ligating the LAA without leaving a permanent intracardiac implant. The LARIAT device has been shown to be safe and effective in reliably excluding the LAA. It may also confer some advantages over currently available implantable devices that not only require continuation of anticoagulation until the device is endothelialized, but also result in complications such as device erosion, embolization/migration and infection. In this review, we present a detailed discussion of the various procedural aspects of this novel device and a review of current data for its use.
Pre-procedure details
Clinical characteristics of patients screened for LAA occlusion with SentreHEART device
The ideal patients for LAA closure have non-valvular atrial fibrillation, a sufficiently high risk of stroke (CHA2DS2VASc >2), are ineligible for warfarin/OAC (labile INRs, non-compliance, contraindications to OAC and/or considered OAC failure, i.e., cerebrovascular event while on warfarin/OAC therapy) and have a life expectancy of at least 1 year [19].
A standard pre-procedure TEE to rule out an atrial thrombus and a cardiac 3D computed tomography (CT) to define anatomical features of the LAA and the surrounding structures is a prerequisite.
Pre-procedure cardiac 3D CT
Cardiac 3D CT is used to determine the size, shape and anatomic orientation of the LAA. The anteroposterior (AP) and lateral projections of the 3D CT help guide the approximate position of pericardial access. As most LAAs are positioned anteriorly, pericardial access is obtained in the anterior and inferior aspect of the pericardial space to enable a direct approach to the LAA. The LARIAT suture device has a slight bend distally that allows it to start in the anterior position first encircling the apex of the LAA and then with advancement it curves posteriorly and inferiorly to encircle the base of the LAA for placement of a snare over it. Less frequently, the 3D CT demonstrates a more posteriorly positioned LAA, in which case the pericardial access is angled more laterally.
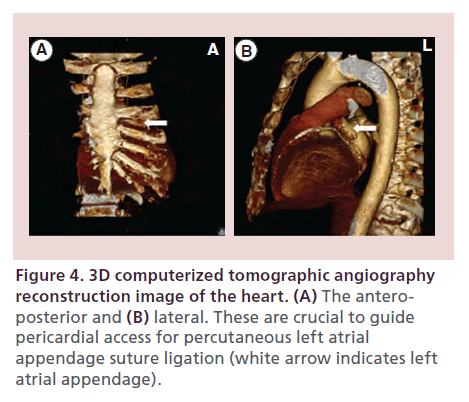
Also, the lateral view of the CT helps determine the actual distance between the sternum and anterior myocardium. Thus, the operator can adjust the angle of entry of the pericardial needle. The CT also helps determine the inferior border of the pericardium in relation to the inferior edge of the sternum to determine how far the needle would be expected to travel for access into the pericardium.
Clinical & anatomical exclusion criteria
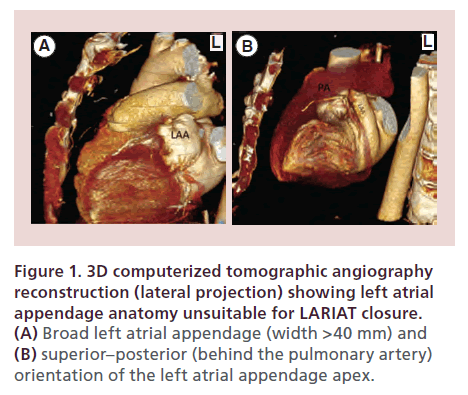
Clinical exclusion criteria for use of the LARIAT device include: history of pericarditis, any history of cardiac surgery, pectus excavatum, recent myocardial infarction within 3 months, prior embolic event within the last 30 days, recent New York Heart Association class IV heart failure symptoms, left ventricular function <30%, and history of thoracic radiation. Anatomic exclusion criteria are as follows: an LAA width >40 mm (Figure 1A), a superiorly oriented LAA with the LAA apex directed behind the pulmonary trunk (Figure 1B), and a bi-lobed LAA or multi-lobed LAA in which lobes are oriented in different planes exceeding 40 mm.
Figure 1. 3D computerized tomographic angiography reconstruction (lateral projection) showing left atrial appendage anatomy unsuitable for LARIAT closure. (A) Broad left atrial appendage (width >40 mm) and (B) superior–posterior (behind the pulmonary artery) orientation of the left atrial appendage apex.
LARIAT device components
The LARIAT device (SentreHEART, Redwood City, CA, USA) for exclusion of the LAA consists of the following components:
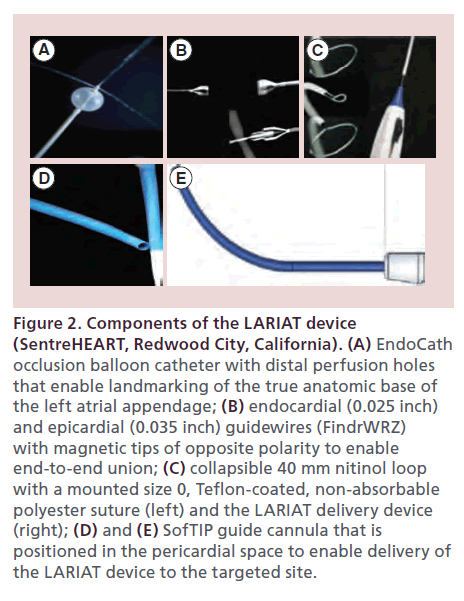
• Occlusion balloon catheter (EndoCATH): This is compliant, echogenic balloon, 15 mm in diameter and 12 mm in length, made of a non-latex (polyisoprene) material that is compatible with 8.5 Fr transseptal guide catheters. It has a 0.038-inch guidewire lumen and distal perfusion holes for contrast diffusion. It enables identification and landmarking of the true anatomic base of the LAA and acts as a template for LAA exclusion (Figure 2A);
Figure 2. Components of the LARIAT device (SentreHEART, Redwood City, California). (A) EndoCath occlusion balloon catheter with distal perfusion holes that enable landmarking of the true anatomic base of the left atrial appendage; (B) endocardial (0.025 inch) and epicardial (0.035 inch) guidewires (FindrWRZ) with magnetic tips of opposite polarity to enable end-to-end union; (C) collapsible 40 mm nitinol loop with a mounted size 0, Teflon-coated, non-absorbable polyester suture (left) and the LARIAT delivery device (right); (D) and (E) SofTIP guide cannula that is positioned in the pericardial space to enable delivery of the LARIAT device to the targeted site.
• Magnet-tipped guidewire system (FindrWIRZ): The FindrWIRZ guidewire system (Figure 2B) is composed of a 0.025-inch endocardial and a 0.035-inch epicardial guidewire with magnet tips of opposite polarity. The magnetic tips establish confluence easily when they are within 4–5 mm of each other and provide a controlled pathway for delivery of the LARIAT snare to the base of the LAA without the need for traction or grasping of the friable LAA tissue;
• Suture delivery device (LARIAT): The LARIAT suture delivery catheter (Figure 2C) delivers a 40-mm diameter, pre-tied suture loop contained on a closure snare composed of size 0 Teflon-coated braided polyester suture. The unique design features of the LARIAT catheter provide radiopaque identification of the position of the pre-tied suture at all times during delivery and deployment. The LARIAT can be opened and closed as desired for ideal positioning without risk of suture deployment. The operator may therefore confirm the completeness and quality of exclusion of the LAA before deployment of the suture. Also, the unique method for suture release eliminates the risk of tearing or bleeding at the LAA base, adjacent structures, or vessels such as the left circumflex artery;
• SofTIP (Guide cannula): The 14 FrSofTIP guide cannula (Figure 2D & E) uses braided catheter technology integrated with an atraumatic tip design to enable positioning of the LARIAT at the targeted closure site;
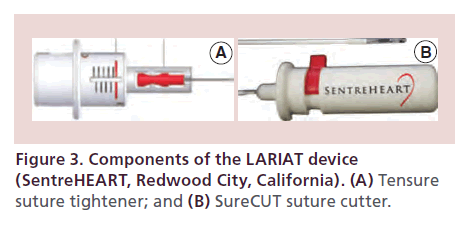
• Tensure (suture tightener): The TenSURE Suture Tightener is designed to be used with the LARIAT Suture Delivery Device to optimize tactile feedback during tightening and minimize operator variability (Figure 3A);
• SureCUT (suture cutter): The SureCUT Suture Cutter is designed to cut the multifilament, size ‘0’ suture included in the LARIAT Suture Delivery Device in order to minimize the amount of remnant suture upon closure (Figure 3B).
Description of procedure
Most centers in the USA perform the LARIAT procedure with general anesthesia, as prolonged intubation with the TEE probe is required. This is however variable and center dependent in other countries. Surgical backup is essential in event of complications, especially right ventricular or atrial rupture related to pericardial/transseptal access or the device per se. Intraoperative TEE plays an important role in every step of the procedure. TEE is utilized to: guide transseptal access; ensure that the SoftTIP guide canula is not impinging on the right ventricle; confirm positioning of the EndoCATH balloon at the os of the LAA to optimize device placement; identify adjacent anatomic structures that may be injured by the device such as the left circumflex artery, which runs close to the base of the LAA; and, finally, to confirm appendage closure with the snare prior to deployment of the suture. Patients are prepped and draped in the subxiphoid region and in both groins. A 5 Fr radial arterial line is placed for real-time blood pressure monitoring. An 8 Fr short sheath is placed in the right femoral vein for transseptal catheterization.
The procedure involves four steps [19,20]:
• Pericardial access;
• Transseptal access;
• Connection of the epicardial and endocardial magnet- tipped guidewires followed by snare capture of the LAA os;
• Closure confirmation and release of the pre-tied suture for LAA ligation.
Pericardial access
An optimally placed needle access to the pericardial space is crucial to the success of a LARIAT procedure. The majority of LAAs are directed anteriorly in the pericardial space and situated next to the superior lateral aspect of the main pulmonary artery and superior to the left ventricular free wall. The goal is therefore to achieve access to the anterior pericardial space. The subxiphoid anterior approach, originally described by Sosa et al. [21], provides access to the anterior pericardial space and facilitates approach to the LAA with the LARIAT snare from its apex to base. Cardiac 3D CT reconstruction can assist with preoperative planning of the pericardial access. The AP view helps in deciding the lateral trajectory of the pericardial needle, while the lateral view helps with the steepness of the direction of the pericardial needle (Figure 4A & B). Fluoroscopically, the needle is most commonly directed at 1.30 or 2 o’clock position in the AP projection (Figure 5A & B). A 17 G blunt-tipped Tuohy epidural needle, which is designed to enter virtual spaces, is routinely employed. Alternatively, a micropuncture needle may be used to mitigate the risk of inadvertent right ventricle puncture. The preferred skin entry point is 2–3 cm below the line that joins the xiphoid process and the costal margin, left of the midline. A skin incision is usually made to facilitate needle and sheath entry and to enhance tactile sensation of various structures encountered on the way, especially the contracting myocardium. The needle is advanced in AP projection in a direction guided by 3D CT imaging and fluoroscopy as described above. Once the needle is under the rib cage, fluoroscopy is performed in the left lateral projection (Figure 5C). As the needle approaches the heart border, cardiac contractions can be felt through the needle and injection of small amounts of contrast can be made to visualize tenting of the pericardium (Figure 5D). Layering of contrast within pericardial space confirms needle entry into the pericardial space (Figure 5E). Transition of the needle into the pericardial space is usually accompanied by a sensation of ‘give’. Once access is achieved, a 0.035 inch guide wire is generously advanced and wrapped around the left ventricle in left anterior oblique (LAO) position to confirm access of pericardial space (Figure 5F). The access tract is dilated using a series of dilators (Figure 5G) prior to placement of the 14 Fr SoftTIP guide canula. Transesophageal visualization for right ventricle compression and pericardial effusion is performed during the passage of the dilators and SoftTIP guide canula. A ‘buddy’ wire can be placed for rapid placement of a pericardial drain in case a pericardial effusion develops.
Figure 4. 3D computerized tomographic angiography reconstruction image of the heart. (A) The anteroposterior and (B) lateral. These are crucial to guide pericardial access for percutaneous left atrial appendage suture ligation (white arrow indicates left atrial appendage).
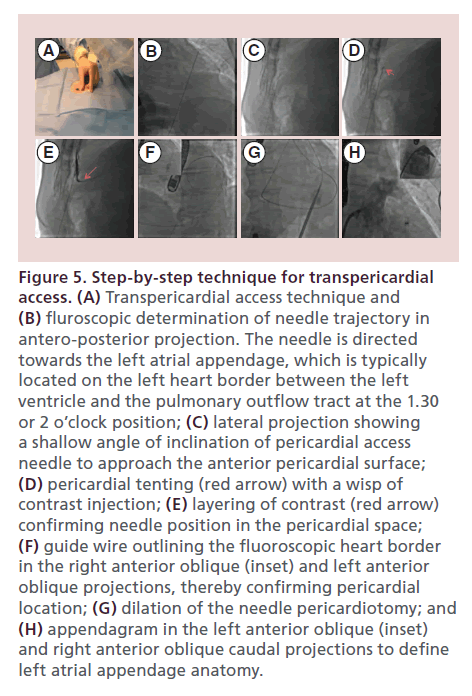
Figure 5. Step-by-step technique for transpericardial access. (A) Transpericardial access technique and (B) fluroscopic determination of needle trajectory in antero-posterior projection. The needle is directed towards the left atrial appendage, which is typically located on the left heart border between the left ventricle and the pulmonary outflow tract at the 1.30 or 2 o’clock position; (C) lateral projection showing a shallow angle of inclination of pericardial access needle to approach the anterior pericardial surface; (D) pericardial tenting (red arrow) with a wisp of contrast injection; (E) layering of contrast (red arrow) confirming needle position in the pericardial space; (F) guide wire outlining the fluoroscopic heart border in the right anterior oblique (inset) and left anterior oblique projections, thereby confirming pericardial location; (G) dilation of the needle pericardiotomy; and (H) appendagram in the left anterior oblique (inset) and right anterior oblique caudal projections to define left atrial appendage anatomy.
Transseptal access
Transseptal access is gained using standard technique with an 8.5 Fr transseptal sheath (e.g., SL1-SL4 or Mullins sheath) and the Brockenborough needle under TEE and fluoroscopic guidance. An inferior and posterior position on the fossa ovalis provides a direct approach to the more anterior and superior LAA os and is the preferred location for transseptal access. TEE views of the septum including the aortic short axis, and bicaval views are important to position the transeptal access in the antero-posterior and superoinferior axes of the fossa ovalis, respectively. Once transseptal access is obtained, the transseptal sheath is advanced to the LAA os over a pigtail catheter that is placed in the appendage. Angiograms of the LAA are obtained in the RAO caudal and LAO projections to delineate the os and anterior/posterior lobes of the appendage respectively (Figure 5H).
Connection of the epicardial & endocardial magnet-tipped guidewires followed by snare capture of the LAA
The EndoCATH balloon catheter is back-loaded with the 0.025 inch magnet-tipped endocardial guidewire (FindrWIRZ). The endocardial guidewire tip is given a mild curve to enable steerability and is positioned in the desirable lobe (mostly superior and anterior lobe) of the LAA. The 0.035 inch magnet-tipped epicardial guidewire is then advanced through the epicardial SoftTIP guide cannula to achieve end-to-end union of the magnet tips of the epicardial and endocardial guidewires (FindrWIRZ)(Figure 6A). The rail created by the magnetic union of the epicardial and endocardial guidewires is used to advance the LARIAT snare (suture delivery catheter) over the LAA without the need for traction or grasping of the highly friable LAA tissue. Initially, the LAO projection is used to ensure that the LARIAT snare loop encircles the anterior and posterior lobes of the LAA (Figure 6C), followed by the RAO caudal projection to visualize the LARIAT snare advance to the neck of the appendage (Figure 6D). At this point, the EndoCATH balloon is advanced over the endowire and positioned at the os of the LAA, which is confirmed by TEE (Figure 6D). Fluoroscopically the proximal marker of the balloon should lie at the LAA os. The LARIAT snare is carefully positioned at the LAA os using above markers and then tightened to approximate the LAA tissue (Figure 6D & E).
Figure 6. Procedural steps of the LARIAT procedure. (A) Magnetic union of the endocardial and epicardial guide wires (FindrWRZ) that provides a rail for advancing the LARIAT snare over the appendage; (B) inflation of the EndoCATH balloon (arrow) to position it at the anatomical base of the appendage using echocardiographic guidance; (C) left anterior oblique projection to ensure encirclement of anterior and posterior lobes of the left atrial appendage (LAA) by the LARIAT snare; (D) LARIAT snare encircling the EndoCATH balloon (arrow), which is positioned at the LAA os using echocardiographic guidance; (E) fluroscopically the proximal balloon marker is at the LAA os and the snare (nitinol loop) is seen encircling the true anatomic base of the LAA; and (F) angiographic preconfirmation of complete LAA exclusion with the nitinol loop prior to final deployment of the LARIAT suture.
Closure confirmation & release of the pre-tied suture for LAA ligation
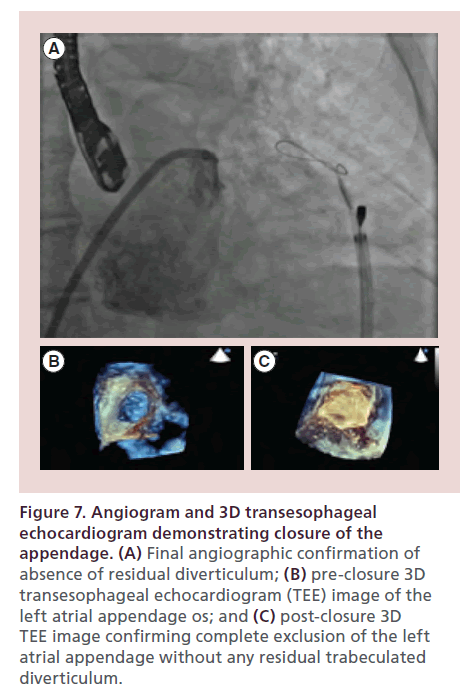
Complete occlusion and absence of remnant-trabeculated LAA is first confirmed by TEE followed by a left atriagram (Figure 6F). The EndoCATH balloon and endowire are then withdrawn from the appendage and the preloaded suture is released from the snare and tightened using the TenSURE suture-tightening device. The suture is tightened again after waiting 5 minutes. Following deployment of the suture, final angiographic and echocardiographic images are obtained to document complete closure (Figure 7A–C). The LARIAT delivery catheter is removed and the suture is cut using the SutureCUT device. The SofTIP sheath is removed and a pigtail catheter is left in the pericardial space for a minimum of 4 to 6 h. If drainage is minimal (<50 cc) then the pigtail catheter is removed in 4–6 h. If drainage is greater than 50 cc then it is kept overnight and pulled the following morning.
Figure 7. Angiogram and 3D transesophageal echocardiogram demonstrating closure of the appendage. (A) Final angiographic confirmation of absence of residual diverticulum; (B) pre-closure 3D transesophageal echocardiogram (TEE) image of the left atrial appendage os; and (C) post-closure 3D TEE image confirming complete exclusion of the left atrial appendage without any residual trabeculated diverticulum.
Post-procedure care
Post-procedure care is primarily centered around issues related to pericardial access. Patients are observed in the intensive care unit overnight and typically discharged within 24 to 48 h if post-procedure course remains without complications. A transthoracic echocardiogram can be performed to rule out a pericardial effusion before the pericardial drain is removed.
Pain and pericardial effusion is common after the procedure [19,22,23] and is probably due to inflammation of the pericardium from pericardial access, manipulation within the pericardial space and ligation of the LAA. A recent study [24] enrolled 58 subjects undergoing LARIAT procedure to compare a regimen of colchicine 0.6 mg two-times a day plus standard therapy (combination of anti-inflammatory drugs, narcotics and pericardial drain care) with standard therapy alone for 1 week. Use of colchicine decreased the pericardial drain output from 269 ± 114 to 156 ± 78 cc (p < 0.01), drain duration from 23 ± 19 to 16 ± 4 h (p < 0.04), days of pericarditic chest pain from 7.8 ± 2.8 to 3.5 ± 1.4 days (p < 0.01) and an audible rub suggestive of pericarditis from 42 to 25% (p < 0.01). Combination therapy with scheduled colchicine and an NSAID is therefore recommended for 1–2 weeks post procedure.
Clinical follow up within 1 month, at 6 months and 1-year post ligation is recommended. A weekly follow-up evaluation by phone until the first clinical follow up can be important to screen for late pericardial and or pleural effusion documented in some studies [19,23,25]. Accordingly, the patient should be asked about chest pain, dyspnea or any changes in respiratory status.
Although there is no established protocol, a followup TEE examination by 90 days post-procedure, to exclude a residual post-closure diverticulum, development of significant color Doppler leak (>5 mm in diameter) or thrombus at the site of suture ligation, is highly recommended.
One advantage of the LARIAT device over other endocardial implants is that warfarin therapy is not warranted to prevent thrombus formation as the implant surface endothelializes. However, recently reported instances of thrombus at the ligation site [25–27] warrant further studies before recommendations regarding discontinuing oral anticoagulants post-LAA ligation can be made with certainty. Our institutional practice is to discontinue OAC in all patients with complete LAA closure (<5 mm residual jet by TEE) after the LARIAT procedure and continue aspirin indefinitely. Dual anti-platelet therapy, if taken for other indications, is continued.
Data on LARIAT device
Preclinical feasibility of percutaneous LAA suture ligation using the SentreHEART LARIAT device was initially established in two animal studies [28,29]. Bartus et al. [20] from Poland reported the first human experience with LAA suture ligation in thirteen patients undergoing either mitral valve surgery (n = 2) or radiofrequency catheter ablation for atrial fibrillation (n = 11). All but one patient, belonging to the AF ablation group, had complete ligation of the LAA. There were no significant complications except for one patient with pectus excavatum that required thoracoscopy for snare removal. The same group [19], in a single-center observational study (PLACE II registry) of 89 patients at high risk or ineligible for warfarin therapy and/or failed warfarin therapy, demonstrated reliable LAA occlusion with the LARIAT suture delivery device with acceptably low access-related complications and adverse events. Eighty-five of 89 patients (96%) underwent successful LAA ligation in this registry. Eighty-one of 85 patients (95.5%) had complete closure (≤1 mm jet by TEE color Doppler) immediately, whereas three patients had a small residual leak (≤2 mm) and one had a ≤3 mm leak. Medium-term follow up showed durability of the closure with 95% (77/81) complete closure by TEE at 6 months and 98% (63/65) complete closure at 1 year, including patients with previous leaks. No device-related complications were noted. There were three access-related complications (during pericardial access [n = 2]; and transseptal access [n = 1]). Adverse events included severe pericarditis post-operatively (n = 2), late pericardial effusion 2 weeks post procedure (n = 1), unexplained sudden death (n = 2), and late strokes thought to be non-embolic (n = 2). There was one instance of a left atrial thrombus at a site distant from the ligation site noted on a 1-year follow-up TEE, which was treated with warfarin inconsequentially.
Massumi et al. [22] reported their initial clinical experience in 21 patients at high risk or ineligible for oral anticoagulation. The mean CHADS2 score was 3.2 ± 1.2, the CHA2DS2-Vasc score was 4.8 ± 1.3 and the HASBLED score was 3.5 ± 1. Twenty patients underwent percutaneous suture ligation of the LAA. One patient was excluded due to presence of LAA thrombus on intraoperative TEE. Complete occlusion was achieved in all patients with no Doppler flow in 19 and 1 mm Doppler flow in one patient. Complete closure was maintained at 90-day follow-up TEE Doppler. There was one right ventricular perforation resulting in pericardial tamponade and requiring surgery. There were two prolonged hospitalizations: one due to pericarditis requiring pericardiocentesis and the other for non-cardiac comorbidities. Three patients had pericarditis within 1 month of the procedure, one of which needed a pericardiocentesis for pericardial effusion. No stroke events were reported during an average follow up of 354 ± 143 days.
Stone et al. [23] reported their experience evaluating early outcomes in 27 patients who were at high risk or ineligible to take anticoagulant therapy. The mean CHADS2 score was 3.5 ± 1.6, CHA2DS2-Vasc score was 5.1 ± 1.5, and the HAS-BLED score was 4.6 ± 0.9. The procedural success rate was 92.6%, and TEE at 4 months confirmed complete closure in 22 of 25 patients. One patient developed a procedural perforation of the LAA that was managed conservatively, ultimately undergoing a surgical atrial fibrillation ablation and LAA ligation 1 day later. Three patients developed pericarditis, and one patient developed a remote cerebrovascular accident thought to be due to aortic atheroembolism.
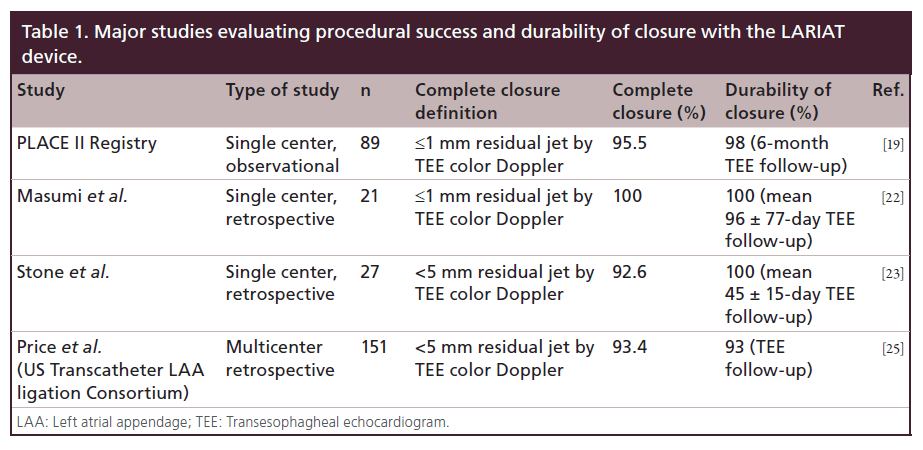
Early efficacy and safety results from the US Transcatheter LAA Ligation Consortium were presented at Transcatheter Cardiovascular Therapeutics 2013. This was a multicenter (eight participating US sites), retrospective study of 151 consecutive patients undergoing LARIAT procedure. Technical success (deployment of LARIAT with <5 mm residual leak by postprocedure TEE) was achieved in 141 of 151 (93.4%) patients. Procedural success, defined as <5 mm residual shunt, no major complication (death, stroke, Bleeding Academic Research Consortium type 3 or greater bleeding or emergent cardiac surgery) at hospital discharge, was reported in 130 of 151 (86%) patients. In-hospital safety events were driven by significant pericardial effusion, n = 15 (10%) and major bleeding, n = 13 (8.6%). Out-of-hospital events at median follow- up of 133 days (interquartile range: 56–274 days) included a composite of death, myocardial infarction and cerebrovascular accident in five out of 129 (3.9%), pericardial effusion in three (2.3%), pleural effusion in three (2.3%) and cardiac surgery in one (0.8%). Overall incidence of thrombus and residual Doppler leak is hard to quantify, nevertheless three of 60 (5%) with TEE follow-up had atrial thrombus and one additional thrombus was noted on a follow up CT scan. TEE Doppler leak of >5 mm was noted in four of 60 (7%) patients with TEE follow-up. Table 1 provides a summary of current data for technical success and durability of LAA closure by the LARIAT device.
Complications
Major complications associated with the LARIAT device procedure include pericarditic pain, pericardial effusion/tamponade, and right ventricular and LAA/ left atrial perforation [19,22,23,25,30]. Pericardial effusions most commonly result from pericardial irritation related to pericardial access and catheter/sheath placement. The pericarditic chest pain and effusions are managed, as discussed earlier, with NSAIDs, colchicine and a pericardial drain. Rarely, hemorrhagic pericardial effusion can result from right ventricular perforation related to needle access of the pericardial space. Needle perforations of the right ventricle without passage of a catheter/sheath are typically self-limiting and do not require surgery. LAA perforation resulting from FindrWIRZ magnet-tipped guide wires is rare and is best managed by completing LARIAT suture closure and ensuring that the closure is complete. Alternatively, an implantable device (Watchman or ACP) closure or surgical closure may be required. Left atrial perforation related to the device or transseptal access may require surgical management.
Conclusion & future perspective
The LARIAT device is a reasonable alternative in the management of thromboembolic risks in patients with atrial fibrillation and high risk for bleeding with OAC. The device provides for percutaneous exclusion of the atrial appendage without leaving a permanent implant. The LARIAT device therefore eliminates the potential complications related to implantable devices such as device erosion, embolization/migration and infection. Current non-randomized data from single-center experiences and a US-based multicenter registry provide compelling evidence for reliable LAA exclusion with acceptably low access-related complications and adverse events. However, successful exclusion of the LAA is not a validated surrogate for stroke prevention and there is currently no randomized clinical trial proving stroke prevention with the use of the LARIAT device. The device nevertheless satisfies an unmet clinical need in patients who have an absolute contraindication for post-procedure OAC. The rationale for its use primarily hinges on data from complete surgical LAA exclusion and the results of the PROTECT-AF trial, which confirmed efficacy of LAA device closure versus OAC in preventing thromboembolic events. A well-conducted randomized clinical trial demonstrating stroke risk reduction with the LARIAT device is needed to broaden the indications for its use. Also, a trial in a patient population not treated with anticoagulation in the immediate post-procedure phase would be an important consideration.
Post-procedure pericarditis and pericardial effusions are the major adverse outcomes impacting hospital stay. Safer techniques to access the pericardium, improved steerable guiding catheters and lower profile sheaths need to be developed. A larger loop (>40 mm) to accommodate larger appendages for exclusion may be a consideration in future iterations of the LARIAT device. Furthermore, a small study recently demonstrated acute reduction in LAA voltage and capture inhibition of the left atrium with LAA pacing thereby raising a possibility of atrial arrhythmia burden reduction after LARIAT procedure [31]. Lakkireddy et al. [32] evaluated this concept in 18 patients who underwent percutaneous LAA ligation with the LARIAT device. Three months post ligation there was reduction in atrial arrhythmia burden from 81 to 47%. A registry evaluating the possibility of improved RF ablation outcomes with concomitant LARIAT LAA ligation is currently enrolling patients.
It is possible that LAA ligation with LARIAT device in conjunction with AF ablation will improve ablation success and further widen the scope of its use. It is also important to note that LAA is an important source of atrial natriuretic peptide (ANP) with potential hemodynamic consequence if ligated. Until recently, effects of LAA ligation had not been studied. A small study [33] of 54 consecutive patients who underwent the LARIAT procedure showed a statistically significant drop in serum sodium levels and blood pressures immediately post procedure. The effects were however not sustained on long-term (4 to 8 month) follow-up, suggesting attainment of a steady state. A larger study to evaluate the long-term hemodynamic effects and possibility of volume overload and hyponatremia related to LAA ligation is needed.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
• Atrial fibrillation (AF) is the leading arrhythmia affecting over 2.5 million people in the USA and more than 6 million people in Europe. The most devastating complication of AF is systemic thromboembolism. The predominant source of thrombus is believed to be the left atrial appendage (LAA), which accounts for 90% of the atrial thrombi in AF.
• About 60% of patients with AF have CHA2DS2 Vasc score >1, warranting anticoagulation, but 45% of these patients are ineligible for oral anticoagulants (OAC). This leaves these patients unprotected and susceptible to stroke.
• Percutaneous approach to LAA exclusion is promising because it is minimally invasive, eliminates the morbidity associated with surgery and can be performed as a standalone procedure. Amongst the percutaneous LAA occlusion devices, the LARIAT is appealing because no foreign body is left behind in the left atrium and it does not require any period of OAC after appendage occlusion.
• The ideal patients for LAA closure include those with non-valvular atrial fibrillation that possess a CHA2DS2VASc score of ≥2 who are ineligible for OAC.
• Pre-procedure work up includes careful clinical screening and a pre-procedure cardiac 3D CT and a transesophageal echocardiogram (TEE). A common clinical exclusion is patients having had prior cardiac surgery and a common anatomic exclusion is a LAA apex located behind the pulmonary artery.
• Pericardial access is the most challenging aspect of the procedure and has a learning curve of 5–10 cases associated with it. The rest of the procedure is intuitive.
• Post-procedure care involves administration of Colchicine and NSAIDS for 1–2 weeks after the procedure.
• Although there is no established protocol, a follow-up TEE examination by 90 days post procedure to exclude a residual post-closure diverticulum, development of significant color Doppler leak (>5 mm in diameter) or thrombus at the site of suture ligation, is highly recommended.
• Current non-randomized data from single-center experiences and a US-based multi-center registry provide compelling evidence for reliable LAA exclusion with acceptably low access related complications and adverse events.
References
- Camm AJ, Kirchhof P, Lip GY et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 31(19), 2369–2429 (2010).
- Fuster V, Rydén LE, Cannom DS et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114(7), e257–e354 (2006).
- Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114(2), 119–125 (2006).
- Holmes DR. Atrial fibrillation and stroke management: present and future. Semin. Neurol. 30(5), 528–536 (2010).
- Tu HT, Campbell BC, Christensen S et al. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc. Dis. 30(4), 389–395 (2010).
- Johnson WD, Ganjoo AK, Stone CD, Srivyas RC, Howard M. The left atrial appendage: our most lethal human attachment! Surgical implications. Eur. J. Cardiothorac. Surg. 17(6), 718–722 (2000).
- Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation 126(7), 860–865 (2012).
- Kanderian AS, Gillinov AM, Pettersson GB, Blackstone E, Klein AL. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J. Am. Coll. Cardiol. 52(11), 924–929 (2008).
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 61(2), 755–759 (1996).
- Katz ES, Tsiamtsiouris T, Applebaum RM, Schwartzbard A, Tunick PA, Kronzon I. Surgical left atrial appendage ligation is frequently incomplete: a transesophageal echocardiograhic study. J. Am. Coll. Cardiol. 36(2), 468–471 (2000).
- Viles-Gonzalez JF, Kar S, Douglas P et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J. Am. Coll. Cardiol. 59(10), 923–929 (2012).
- Holmes DR, Reddy VY, Turi ZG et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 374(9689), 534–542 (2009).
- Reddy VY, Mobius-Winkler S, Miller MA et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study With Watchman Left Atrial Appendage Closure Technology). J. Am. Coll. Cardiol. 61(25), 2551–2556 (2013).
- FDA review of P130013. www.fda.gov/downloads/advisorycommittees/ committeesmeetingmaterials/medicaldevices/ medicaldevicesadvisorycommittee/ circulatorysystemdevicespanel/ucm378235.pdf
- Park JW, Bethencourt A, Sievert H et al. Left atrial appendage closure with Amplatzer cardiac plug in atrial fibrillation: initial European experience. Catheter. Cardiovasc. Interv. 77(5), 700–706 (2011).
- Lam YY, Yip GW, Yu CM et al. Left atrial appendage closure with AMPLATZER cardiac plug for stroke prevention in atrial fibrillation: initial Asia-Pacific experience. Catheter. Cardiovasc. Interv. 79(5), 794–800 (2012).
- Urena M, Rodes-Cabau J, Freixa X et al. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J. Am. Coll. Cardiol. 62(2), 96–102 (2013).
- Landmesser U, Holmes DR Jr. Left atrial appendage closure: a percutaneous transcatheter approach for stroke prevention in atrial fibrillation. Eur. Heart J. 33(6), 698–704 (2012).
- Bartus K, Han FT, Bednarek J et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J. Am. Coll. Cardiol. 62(2), 108–118 (2013).
- Bartus K, Bednarek J, Myc J et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm 8(2), 188–193 (2011).
- Sosa E, Scanavacca M, d’Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J. Cardiovasc. Electrophysiol. 7(6), 531–536 (1996).
- Massumi A, Chelu MG, Nazeri A et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am. J. Cardiol. 111(6), 869–873 (2013).
- Stone D, Byrne T, Pershad A. Early results with the LARIAT device for left atrial appendage exclusion in patients with atrial fibrillation at high risk for stroke and anticoagulation. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.25065 (2013) (Epub ahead of print).
- Vallakati A, Janga P, Reddy M, Nath J, Ferrell R, Freeman S, Swarup V, Baqdunes MW, Badhwar N, Lee R, DiBiase L, Mansour M, Ruskin J, Natale A, Earnest M, Lakkireddy D. Impact of Colchicine on Post Procedural Pericardial Inflammation and Pain after LARIAT Left Atrial Appendage Ligation. Presented at: 34th annual scientific sessions, Denver, CO, USA, May 8–11 2013. (Poster VI PO06–75).
- Price MJ, Burkhardt JD, Biase LD et al. Safety and early efficacy of the lariat procedure: results from the U.S. left atrial appendage ligation consortium. J. Am. Coll. Cardiol. 64(6), 565–572 (2014).
- Briceno DF, Fernando RR, Laing ST. Left atrial appendage thrombus post LARIAT closure device. Heart Rhythm (13), 1284–1288 (2013).
- Giedrimas E, Lin AC, Knight BP. Left atrial thrombus after appendage closure using LARIAT. Circ. Arrhythm. Electrophysiol. 6(4), e52–e53 (2013).
- Singh SM, Dukkipati SR, d’Avila A, Doshi SK, Reddy VY. Percutaneous left atrial appendage closure with an epicardial suture ligation approach: a prospective randomized pre-clinical feasibility study. Heart Rhythm 7(3), 370–376 (2010).
- Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: initial experience in a canine model. Circ. Cardiovasc. Interv. 3(3), 224–229 (2010).
- Claessen BE, Mehran R, Mintz GS et al. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. JACC Cardiovasc. Interv. 4(9), 974–981 (2011).
- Han FT, Bartus K, Lakkireddy D et al. The effects of LAA ligation on LAA electrical activity. Heart Rhythm 11(5), 864–870 (2014).
- Lakkireddy DR, Earnest M, Janga P et al. Effect of endoepicardial percutaneous left atrial appendage ligation (LARIAT) on arrhythmia burden in patients with atrial fibrillation. J. Am. Coll. Cardiol. 61(Supple. 10), e385 (2013).
- Maybrook R, Pillarisetti J, Yarlagadda V et al. Hyponatremia and hemodynamic changes following percutaneous left atrial appendage ligation with the lariat device. J. Am. Coll. Cardiol. 63, 12 (2014).