Perspective - Imaging in Medicine (2013) Volume 5, Issue 2
Percutaneous therapy versus surgery in chronic back pain: how important is imaging in decision-making?
Alexis D Kelekis*1 & Dimitris Filippiadis11University of Athens, 2nd Radiology Department, University General Hospital ‘ATTIKON’, Athens, Greece
- Corresponding Author:
- Alexis D Kelekis
University of Athens
2nd Radiology Department
University General Hospital ‘ATTIKON’
Athens, Greece
Tel: +30 210 778 0089
Fax: +30 210 770 1547
E-mail: akelekis@med.uoa.gr
Abstract
Keywords
chronic ▪ imaging ▪ low back pain ▪ percutaneous therapy ▪ surgery
Low back pain (LBP) is a very common pathology if one considers that it has a lifetime prevalence of 70–80% and an annual incidence of 15–20% in the USA, second only to the common cold [1,2]. It is the most common disability cause in individuals aged <45 years [1–3]. LBP is usually a self-limiting procedure; however, many of the patients receive routine imaging for it. LBP can be classified according to the time frame of acute, subacute and chronic, with intervertebral disc herniation, facet syndrome and spinal stenosis accounting for the majority of cases [2]. Despite the self-limiting character of LBP, approximately onethird of patients report persistence of symptoms and activity limitations at 1-year postsymptoms appearance (chronic pain) [4].
There are continuously growing costs associated with LBP which account not only for medical imaging and therapeutic costs, but also for indirect costs due to productivity loss. Since there is widespread agreement for the use of imaging prior to surgery, it is not noteworthy that there is a simultaneous increase in the number of spine MRI and lumbar surgical operations [5]. However, despite the increase in imaging and surgery numbers, patients did not report any benefit on outcome scores (concerning physical function, working, mental health or social limitation) compared with those treated conservatively [5]. Speaking on financial terms, approximately 5% of the people with LBP account for approximately 75% of the costs associated with the pathology [6].
The majority of patients with LBP are treated conservatively [7]. The development of new surgical techniques and equipment has led to an increase in the use of surgical options for LBP. Lumbar fusion procedures have increased by 134–200% between 1993 and 2003 [8,9]. Choosing between different therapies can be difficult, especially when physical findings fail to correlate with symptoms. Furthermore, surgical indications are unclear and there is a limited consensus upon the appropriate treatment measure (anatomical defect correction vs symptoms improvement).
The advances in imaging technology and instrumentation have led to the development of other treatment techniques for LBP that avoid an open surgery through the spinal canal. Unsurprisingly, imaging plays a pivotal and unique role, not only in decision-making, but also as a guiding tool for these patients.
Low back pain
In terms of time frame, LBP can be classified into acute (lasts less than 6 weeks), subacute (duration of 6 weeks to 3 months) or chronic (lasts more than 3 months) [3,10]. According to its etiology, LBP can be classified into: nonspecific LBP; LBP associated with radiculopathy or spinal stenosis; and referent LBP due to specific spinal cause [3,11].
Furthermore, according to the type of symptoms present, LBP can be accompanied by sciatica/neuralgia, neurologic compromise or spinal stenosis. In general, symptoms can be regional (90% usually originate from musculoskeletal local/mechanic cause) or systemic (10%) [3,11]. In addition, patients should always be evaluated for evidence of psychological distress that will cause amplif ication or prolongation of symptoms, especially in chronic LBP [3].
Goals of therapy should include pain reduction, mobility improvement, fast recovery, prevention of developing a chronic or recurrent condition and restoration of physical and social comfort. The usual approach to the therapy of LBP seems to be a staircase with four steps:
▪ Conservative therapy (4–6 week course with analgesics, anti-inflammatory drugs, muscle relaxants, physiotherapy and change of dietary habit – immobilization and bed rest are controversial, since recent studies advocate preservation of a patient’s normal physical activity);
▪ Image-guided infiltrations with corticosteroid and/or local anesthetic (providing diagnostic value when used to determine and certify a potential source of pain, as well as therapeutic value when used as palliative therapies during the acute phase until therapy or natural recovery occurs);
▪ Percutaneous, image-guided minimally invasive therapies (less traumatic percutaneous techniques performed under imaging guidance with small-sized instrumentation and percutaneous approach);
▪ Surgical options.
In cases of severe neurological deficit (e.g., cauda equina syndrome), surgical options are a priority. In this article we will focus upon the value of imaging in decision-making for the most common degenerative causes of chronic pain (facet joint syndrome, intervertebral disc herniation and spinal stenosis) leaving aside other less common degenerative causes or systemic pathology (cancer and infection in which decision-making is performed during multispecialist boards) [12].
Facet joint syndrome
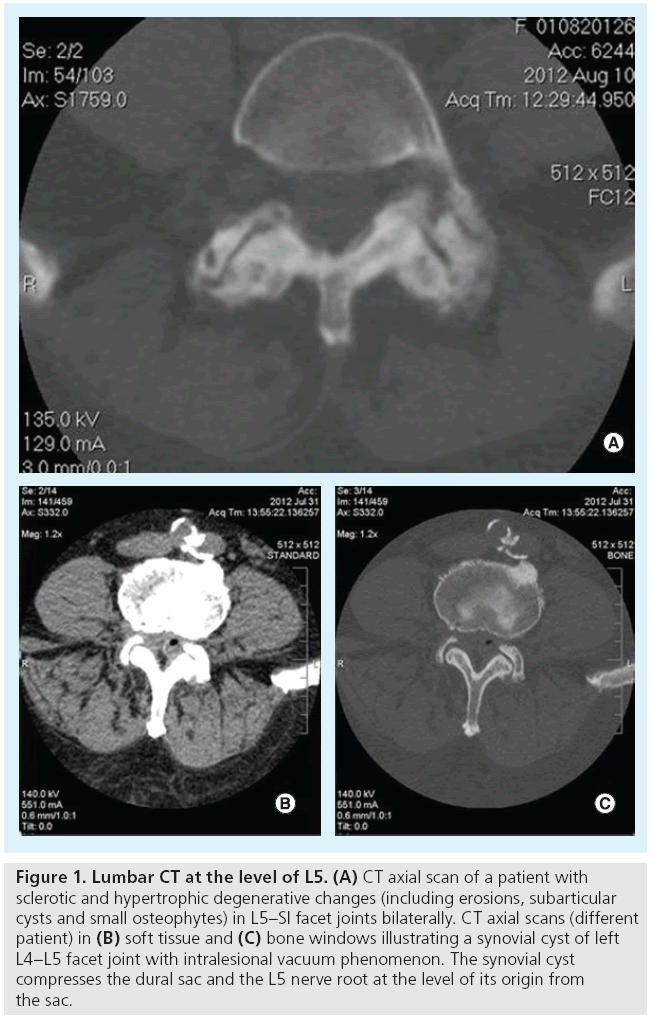
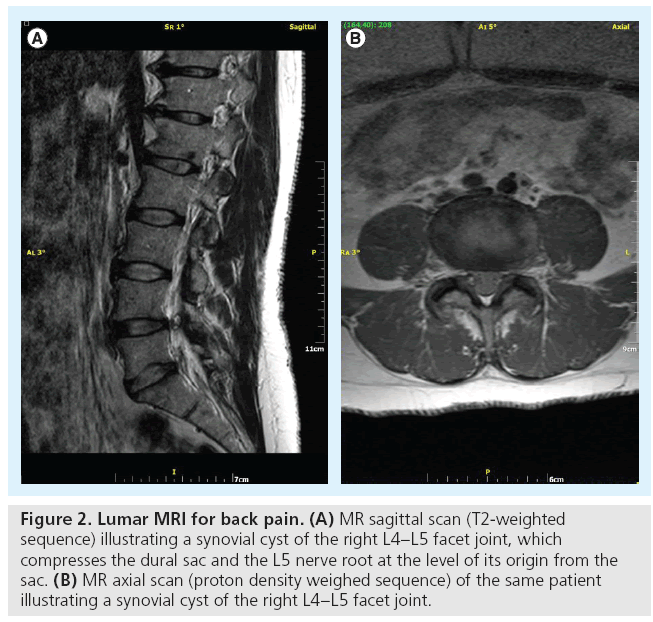
Osteoarthritis frequently affects facet joints. Imaging studies illustrate joint space narrowing and/or intra-articular vacuum phenomenon or f luid, osteophytes (usually involving the upper articular surface of the lower vertebra) and hypertrophy of flaval ligaments (Figure 1) [13]. Although x-rays can illustrate the osseous degenerative changes, axial imaging with CT or MRI completely evaluates not only the inflammatory and degenerative process, but also its consequences (stenosis usually of the lateral recess or of the neural foramen and rarely of the central canal) [13]. The degenerative process can be further complicated by synovial cyst formation (which either remains connected to the joint or not) with subsequent pressure upon neural structures (Figure 2).
Figure 1: Lumbar CT at the level of L5. (A) CT axial scan of a patient with sclerotic and hypertrophic degenerative changes (including erosions, subarticular cysts and small osteophytes) in L5–SI facet joints bilaterally. CT axial scans (different patient) in (B) soft tissue and (C) bone windows illustrating a synovial cyst of left L4–L5 facet joint with intralesional vacuum phenomenon. The synovial cyst compresses the dural sac and the L5 nerve root at the level of its origin from the sac.
Figure 2: Lumar MRI for back pain. (A) MR sagittal scan (T2-weighted sequence) illustrating a synovial cyst of the right L4–L5 facet joint, which compresses the dural sac and the L5 nerve root at the level of its origin from the sac. (B) MR axial scan (proton density weighed sequence) of the same patient illustrating a synovial cyst of the right L4–L5 facet joint.
During clinical examination, there are no specific signs of facet joint syndrome. However, suspicion is raised in patients with pain at the facet joint level exacerbated with pressure, hyperextension, torsion and lateral bending, pain usually worse when waking up from bed or trying to stand after prolonged sitting. This kind of pain might radiate to thigh, iliac crest and, rarely, to the groin.
In case of equivocal findings, contrast enhancement of the facet during MRI is an indicator of inflammation. In order to correlate to the symptoms, percutaneous image-guided infiltration of the joint with local anesthetic will produce temporary pain reduction or relief verifying the particulate facet joint as the pain source [14]. Patients with symptomatic facet joint syndrome can initially be treated conservatively with a course of drugs (e.g., analgesics, muscle relaxants and NSAIDs), immobilization, bed rest and physical therapy [14]. As a second step, image-guided infiltrations can be performed (during or after medical treatment). Although there is moderate evidence-based medicine for this technique, it is commonly practiced and governed by a high success rate when applied to proper patients [14]. When infiltrations do not provide the expected results, radiofrequency ablation of the medial branch nerves of the dorsal rami innervating each facet may act as an attractive alternative, either by image-guided neuromodulation or neurolysis [14,15].
Surgical options include bone spur removal. Furthermore, in the cases of degenerative spondylolisthesis, a direct repair of pars interarticularis defect (pars repair) and slip reduction in conjunction to arthrodesis and spinal fusion can be performed.
Intervertebral disc herniation
Herniation of the intervertebral disc usually affects patients who are 30–40 years old [15]. The most commonly affected levels are L4–L5 and L5–S1 [16].
Following the previous scheme, patients with symptomatic intervertebral disc herniation can initially be treated conservatively with a 4–6 week course of analgesics, muscle relaxants, NSAIDs, immobilization, bed rest and physical therapy [14,17]. Image-guided infiltrations can either be performed in combination to this course or solely performed.
Percutaneous, image-guided decompression techniques are therapeutic treatments for intervertebral disc herniation that can be performed under imaging guidance as outpatient procedures. Using a percutaneous, posterolateral approach, a trocar is inserted inside the disc thus gaining access for a thermal, chemical or mechanical decompression, with the least disruption of surrounding tissues. According to the theory of the Hijikata principle, by removing a small nuclear volume, the result is a significant intradiscal pressure decrease [14,17,18]. Other theories support that there is acceleration of disc degeneration, and thus, size reduction and potential inwards imploding of the herniated fragment. A single center, prospective, randomized trial has shown that these treatments have better and more sustained results than medical therapy [17].
Surgical treatments offer removal of the affected disc. This can require partial or complete laminae removal [18]. Nowadays, this can be performed with less invasive techniques (microdiscectomy) requiring no bone removal and/or combined with implants positioning at the disc level [14,19].
Spinal stenosis
Spinal stenosis is caused by degeneration of the intervertebral disc, flaval ligaments and facet joints leading to hypertrophic and sclerotic changes. These changes reduce the surface area of the spinal canal and put pressure upon neural elements and their blood supply. Imaging studies that illustrate the reduced surface area and the subsequent narrowing of the spinal canal provide diagnosis of spinal stenosis. Grossly, any antero–posterior diameter measurement <12 mm is an indication of the spinal canal’s pathologic narrowing [20]. Symptoms usually start at the age of 50–60 years [21]. The most commonly affected levels are L3–L4 and L4–L5 [21].
The initial therapeutic steps of spinal stenosis include conservative therapy and infiltrations, although response to these treatment is moderate [22].
Percutaneous, image-guided decompression techniques are therapeutic treatments for spinal stenosis which can be performed under imaging guidance as outpatient procedures. Under percutaneous approach, fixation devices that raise the height of the posterior elements, achieve decompression [23].
Decompression is also the goal of surgical therapy and it is achieved by the removal of bony and/or soft tissue elements that put pressure upon the neural elements (dural sac and/or nerve roots) [22,24]. Extensive decompression results in spinal instability that requires either arthrodesis (bone graft) and/or instrumentation (fixation devices) [22,24].
Imaging
Imaging techniques for LBP can be classified in noninvasive imaging and minimally invasive diagnostic techniques (myelography, discography/quantitative discomanometry [QD] and image guided infiltrations).
■ Radiography
Imaging planning begins with plain films of the spinal column, which are promptly available and not expensive [13]. These imaging techniques of the spinal column are mostly obtained in order to provide information about spinal bony elements and possible vertebral misalignment, thus excluding other potential sites and causes of pain origin [25].
Although in the past, radiography was widely used for evaluation of the musculoskeletal system, nowadays CT and MRI have systematically replaced standard x-rays. Nevertheless, even today x-rays play a central role in the evaluation of musculoskeletal pathology and are the first exam to be performed from the diagnostic armamentarium [26]. This is due to the low cost and widespread availability of x-rays, as well as the dynamic, load-bearing information obtained. In every case, patient positioning and knowledge of the technologist and modern equipment still constitute a requisite. Modern equipment with pulsed fluoroscopy and reconstructed CT-like images provides valuable information faster and at lower radiation doses. In addition, it can serve as a guiding method for other diagnostic procedures.
■ CT
Advances in CT technology, including both the hardware and software (e.g., multidetector CT, 3D software and high-speed computers, among others), have greatly altered the use of the modality for musculoskeletal imaging. One great advantage is the widespread availability of this modality and its superiority (concerning resolution) over x-rays in bone tissue. In postoperative patients, sagittal and coronal reconstructions evaluate bony bridging and integrity of the fusion [27]. In spines, although indications for surgery are still debatable, 75% of the fusions performed are due to spinal stenosis, disc pathology or other degenerative causes [27–29]. The fixation devices used are either from stainless steel (producing substantial artifacts) or titanium (artifacts are less profound in these implants).
CT is the modality of choice for the evaluation of failure or complication in postoperative spinal patients [27]. Multiplanar reformatting images are crucial for evaluating the placement of the screw in a pedicle. Furthermore, with correct windowing, nerve impingement due to screw malpositioning can be illustrated. In any case, imaging findings must be associated with those of clinical examination [27]. It is characteristic that the majority of the literature evaluates surgical results based on CT findings, demonstrating the key role of the modality postsurgical treatment of any kind.
■ MRI
MRI provides both anatomical (concerning hypertrophic changes and stenosis of the spinal canal) and functional (concerning disc hydration) information. The standard imaging MR protocol for LBP patients (in the absence of absolute contraindications concerning MR performance) includes T1- and T2-weighted sequences with or without fat saturation and short TI inversion recovery sequences [30]. Degenerated intervertebral discs are characterized by hypoxia, inflammation, innervations, accelerated catabolism and reduced water and glucosaminoglycan content [31]. Nowadays, these pathophysiologic/degenerative phenomena can be measured with ‘functional’ MR sequences and techniques such as MR spectroscopy, T1r calculation, T2 relaxation time measurement and diffusion quantitative imaging [31]. Further clinical studies are necessary in order to prove whether these novel techniques can aid in proper patient selection or evaluate treatment efficacy.
MR scans in T2-weighted sequences may show a regional signal of increased intensity in the annulus fibrosus (high intensity zone), which is considered as an annular fissure and is closely related to discogenic pain, thus being an additional tool to patient selection [32,33].
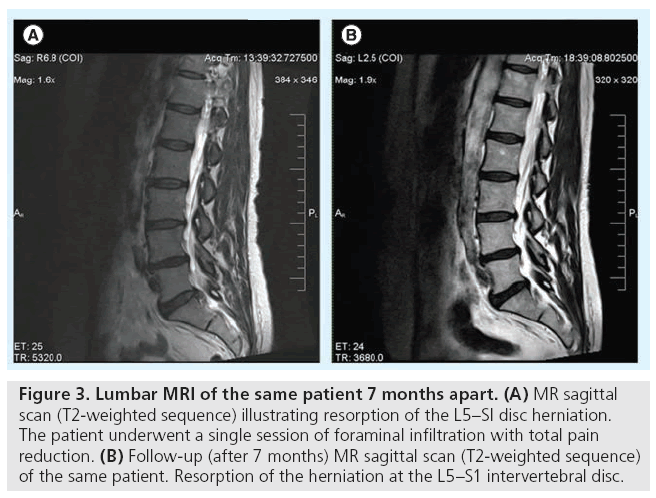
Although MRI is the modality of choice concerning the evaluation of back pain, it lacks the specificity to associate the imaging to the clinical findings (Figure 3). In addition, it is not as sensitive as provocative discography in the detection of annular fissures and internal disc disruption in general. In addition, 13–14% of high-intensity zones and annular fissures discovered on MR scans do not correlate to the patient’s clinical complaints or are reported on individuals without LBP [33,34]. Furthermore, studies upon asymptomatic volunteers report that 38% of the control asymptomatic group had MR scans with abnormal findings in more than one disc level [34].
Figure 3: Lumbar MRI of the same patient 7 months apart. (A) MR sagittal scan (T2-weighted sequence) illustrating resorption of the L5–SI disc herniation. The patient underwent a single session of foraminal infiltration with total pain reduction. (B) Follow-up (after 7 months) MR sagittal scan (T2-weighted sequence) of the same patient. Resorption of the herniation at the L5–S1 intervertebral disc.
■ Myelography
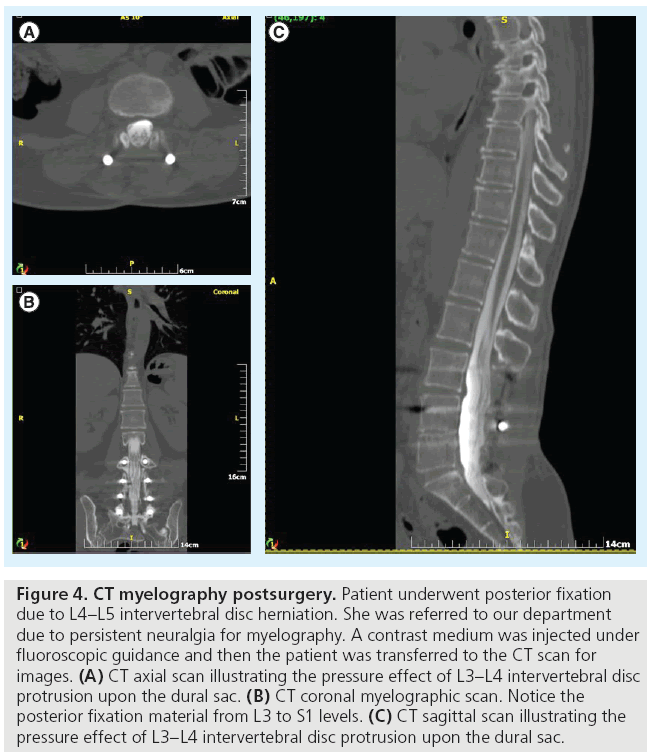
Although the increase in performed MR scans has reduced the request for myelography, it has not eliminated it, particularly for patients who are not compatible with MRI, in cases where MRI is not available or in postoperative patients (whenever metal artifacts reduce the scan’s diagnostic quality) (Figure 4). Clinical indications of myelography include:
Figure 4: CT myelography postsurgery. Patient underwent posterior fixation due to L4–L5 intervertebral disc herniation. She was referred to our department due to persistent neuralgia for myelography. A contrast medium was injected under fluoroscopic guidance and then the patient was transferred to the CT scan for images. (A) CT axial scan illustrating the pressure effect of L3–L4 intervertebral disc protrusion upon the dural sac. (B) CT coronal myelographic scan. Notice the posterior fixation material from L3 to S1 levels. (C) CT sagittal scan illustrating the pressure effect of L3–L4 intervertebral disc protrusion upon the dural sac.
▪ Factors contraindicating MRI (e.g., pacemaker, neurostimulator and claustrophobia, among others);
▪ Spinal surgical hardware that, due to extensive artifacts, result in scans of low diagnostic quality;
▪ Patients with equivocal MR findings that require further evaluation;
▪ Cases where dynamic or tilting MRI is not possible and there is a need for dynamically assessing instability or stenosis.
Contraindications of myelography include allergies to iodinated contrast agents, seizures or drugs lowering the seizure threshold, blood modifiers, increased intracranial pressure, uncooperative patients and pregnancy. The most common complication is headache postpuncture, the incidence of which decreases in correlation to the reduced diameter of the needle used [35]. Rarer complications include seizures, nausea and/or vomiting, bleeding, infection, arachnoiditis and nerve root injury.
Myelography reports include a description of all intramedullary, intradural extramedullary and extradural abnormalities, as well as specific details of the pathologic levels. Nowadays, classic myelography is combined with subsequent scans of multislice CT and flat panel detector-based and volumetric CT, providing multiplanal spinal images at low radiation doses and 3D reconstructions adequately depicting the disc space and potential postsurgical implants, as well as high-resolution images of osseous structures [36,37]. Recent studies report complete matching and observer agreement between high-resolution images of noninvasive 3T MR myelography and CT scans immediately postdiscography for the diagnosis of far lateral disc herniation [38].
■ Discography
Provocative discography is a diagnostic imageguided procedure, during which a needle is percutaneously placed inside the intervertebral disc for contrast medium injection to the nucleus pulposus (Figure 5). The technique’s diagnostic value lies upon the correlation of the symptoms (e.g., the patient’s usual pain) to the intervertebral disc’s morphology and is evidenced first by the pain triggering, which may be induced through intradiscal volume and pressure increase; and second by the assessment of the disc’s morphology with the contrast medium, as evidenced in fluoroscopy and/or CT [39].
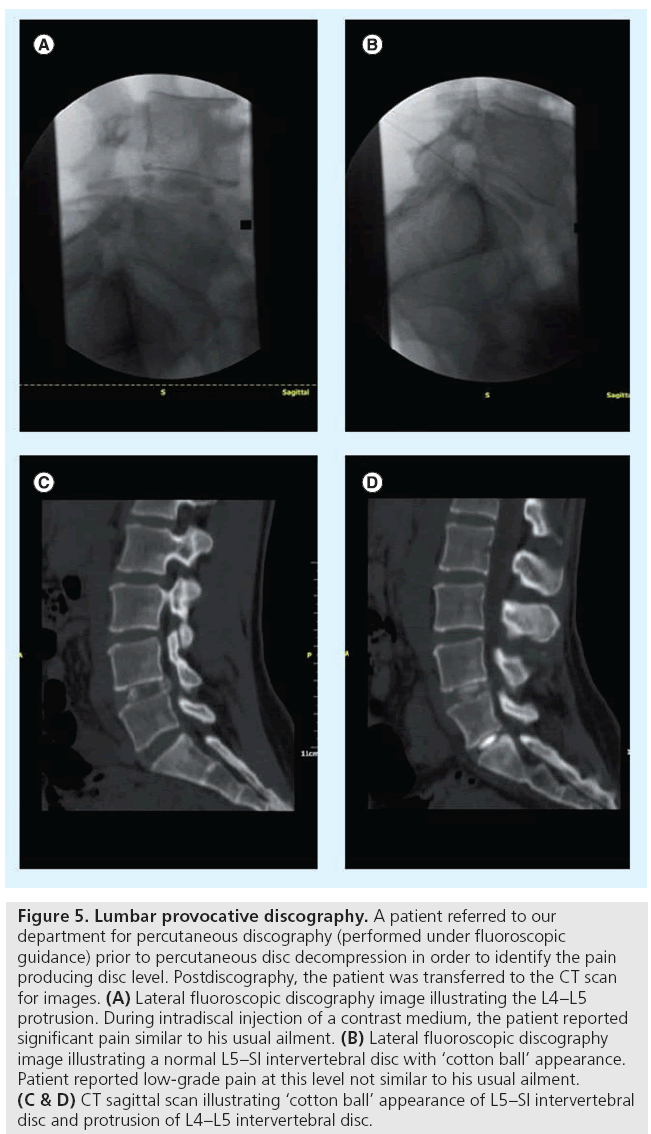
Figure 5: Lumbar provocative discography. A patient referred to our department for percutaneous discography (performed under fluoroscopic guidance) prior to percutaneous disc decompression in order to identify the pain producing disc level. Postdiscography, the patient was transferred to the CT scan for images. (A) Lateral fluoroscopic discography image illustrating the L4–L5 protrusion. During intradiscal injection of a contrast medium, the patient reported significant pain similar to his usual ailment. (B) Lateral fluoroscopic discography image illustrating a normal L5–SI intervertebral disc with ‘cotton ball’ appearance. Patient reported low-grade pain at this level not similar to his usual ailment. (C & D) CT sagittal scan illustrating ‘cotton ball’ appearance of L5–SI intervertebral disc and protrusion of L4–L5 intervertebral disc.
Although spine x-rays, myelography, CT and MRI illustrate the morphologic changes of intervertebral discs associated with discogenic pain, they cannot reproduce the patient’s clinical syndrome and thus cannot characterize a disc as symptomatic or not, as stated above.
A great amount of controversy was raised concerning the discography’s diagnostic value, advantages, disadvantages and possible complications [40–42]. Despite its minimal invasive nature, provocative discography is the only diagnostic procedure used for the evaluation of LBP that directly correlates internal disc morphology with the patient’s triggered pain response [43]. In 1995, the North American Spine Society published an article by Guyer et al., reaching a consensus on specific patient indications for this test [44].
Currently, refined provocative discography is considered a valuable diagnostic tool for the evaluation of LBP, which should ideally be performed as a complementary procedure, mainly after MRI and prior to percutaneous treatments or surgery. In addition, instrumentation development allows us to perform the injection constantly measuring intradiscal pressures and injectate’s volume at the same time and performing correlations of these values (QD). Preliminary studies have shown that this technique may contribute to proper patient selection by setting disc pressure thresholds that are governed by statistically significant higher chances of improved outcome post-treatment [45]. Furthermore, recent studies have shown that, in cases where diagnostic uncertainty remained after clinical examination and diagnostic MRI, discography altered the initial decisions of the spine specialists [46].
■ Diagnostic infiltrations
Occasionally, differentiating a symptomatic from a nonsymptomatic imaging abnormality might be difficult. Image-guided percutaneous infiltration with local anesthetic may help in defining whether a certain imaging abnormality is the source of pain [47,48]. Facet joint infiltrations can be used to differentiate symptomatic from asymptomatic levels and sides or to verify/exclude the facet joint as source of pain. The same is valid for sacroiliac joint infiltrations or injections for piriformis syndrome [49]. Transforaminal nerve root infiltrations assist in verifying the presence and the causative level of a certain radiculopathy.
■ Value of imaging in decision-making
Imaging is expected to contribute in diagnosis confirmation or establishment, to exclude other suspected abnormalities, to assess location and extent of pathology and to aid in treatment planning. The clinician’s experience plays a significant role in the perception of the diagnosis from imaging studies. Furthermore, sometimes certain clinicians, despite the imaging finding, seem to stay attached to their initial treatment plan. Moreover, patient symptoms might differ from the clinical assessment to imaging, reflecting the pathology’s natural history.
Most importantly, imaging will steer the physician towards the possible treatment options for a defined pathology. Specifically, concerning disc herniation, percutaneous decompression techniques are contraindicated (and therefore, surgical options should be considered) in the presence of a free, noncontained or sequestered fragment and in the presence of herniation occupying more than one-third of the spinal canal’s diameter. There are also situations where equivocal results of tests, such as provocative and analgesic discograms, are considered to be a contraindication for operation [18]. Findings of decreased disc height do not favor percutaneous techniques, as they exhibit moderate improvement in these patients [17]. In addition, isthmic lysis is considered to be a contraindication in all percutaneous decompression techniques except intradiscal alcohol gel injection [18].
Sometimes clinical evaluation will guide the treatment, despite the imaging findings, as in the case of cauda equina syndrome or signs of neurological deficit, or whenever general contraindications of any minimal invasive procedure are encountered [18]. Active infection is considered to be a contraindication for all therapeutic arms [18].
There is no doubt that clinical examination should be the first step when seeking diagnosis for the patient with LBP. Imaging is a valuable additive that follows clinical evaluation either immediately when there is a need to identify underlying systemic disease (MRI is an excellent tool for that), or when the initial course of conservative therapy has failed in LBP patients due to degenerative causes [49,50]. Following this rule, one can succeed in decreasing the radiation exposure, reducing the financial cost and minimizing the provocation of unnecessary percutaneous or surgical intervention. Beyond doubt, imaging (particularly MRI) clearly illustrates disc degeneration and herniation; however, there is poor correlation between clinical and imaging (especially from noninvasive diagnostic techniques) findings [43]. On the other hand, percutaneous, minimally invasive, imageguided techniques (infiltrations and discography) can correlate the imaging finding to the patient’s pain pattern or neurologic deficit, thus verifying a certain abnormality as the pain source.
The source of pain seems to be the most important factor determining the therapeutic (surgical or percutaneous) outcome. However, this source of pain is often not accurately identified from a patient’s record and clinical evaluation. Furthermore, sometimes there are more than one pain generators that must be addressed in order to achieve a successful treatment. Occasionally, when psychological factors are masking or exacerbating symptoms, the combination of clinical and imaging examinations may conclude in inaccurate diagnosis. On the other hand, many studies in the literature evaluating the effect of imaging upon decision-making seem to be susceptible to selection bias. This results from the keenness of the surgeon to apply a certain treatment [51]. In addition, spine surgery rates seem to be governed by geographic variation, which clearly reflects a lack of consensus among indications governing these therapies. It has been demonstrated that the level of clinical agreement for most appropriate diagnostic and therapeutic techniques is grouped by geographic location [46]. Doctors in the same area tend to ask for the same imaging and apply similar surgical treatments.
There is an absence of strong evidence favoring percutaneous or surgical therapeutic techniques for the treatment of disc herniation or spinal stenosis; therefore, a high variation degree in practicing is reported to be further affected by patient’s preference as well [46]. Spine specialists may differently define the success of a certain therapy, set their thresholds for imaging definitions of certain pathology and interpret signs, symptoms and imaging findings, even on the same diagnostic imaging report.
Conclusion
LBP is a debilitating condition that can be treated with conservative, percutaneous/minimally invasive or surgical therapies. A clinical decision for a certain therapy depends on the specialization of the doctor. The absence of high level evidencebased medicine, according to the literature, promotes this tendency. No doubt, imaging (not lacking its own specific limitations) can guide the spine specialist to take the best therapeutic decision, as long as indications for imaging are respected and findings of imaging are properly described, evaluated and interpreted by the spine specialist. At the end of the day, success of a certain therapy is defined by its effect upon the patient’s wellbeing. It is a fact that higher evidence (large, prospective, randomized blind studies) will result in more confident clinical decisions.
Future perspective: where do we go from here
Up until now, all noninvasive diagnostic techniques were static. During the last two decades, efforts of performing MRI in dynamic positions were performed (flexion–extension). Nowadays, tilting MRI, which performs sequences at both supine and standing positions, provides additional diagnostic information. With tilting MRI, examination is performed with the spine at its natural weight-bearing position, thus revealing all the biomechanical changes that exist between standing and supine positions. The true position of tilting MRI within the diagnostic armamentarium still remains to be defined. A possible option is to reserve it for patients with LBP and negative MRI in supine position, in patients whose symptoms appear only during standing or in those patients who do not improve after an initial course of conservative therapy [52,53].
Currently, new therapies for disc degeneration are being developed focusing on functional characteristics and on the cellular level. These new therapies, instead of removing the disc or accelerating disc degeneration, actually aim in repair and regeneration. Under this scope, new imaging techniques will be required, which will reveal the source of pain and/or quantify the therapeutic result. These new techniques include the measurement of T2 relaxation time and T1r time constant, diffusion of solute into the disc, chemical spectra and radionucleotides [31]. This functional imaging will illustrate biochemical and inf lammatory processes that produce discogenic pain and, at the same time, will improve proper patient selection for minimally invasive techniques. The use of reparative therapeutic techniques, especially the ones which aim in repairing or reversing degeneration, will probably augment the number of image-guided procedures, as those are less invasive.
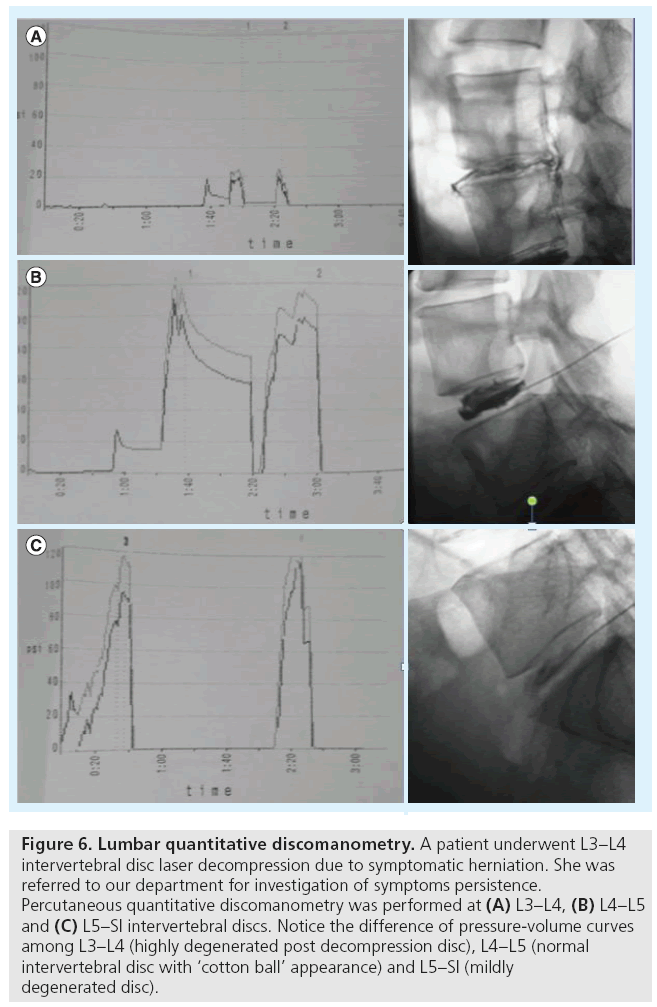
Another technique which integrates imaging findings to objective clinical measurement is percutaneous QD (Figure 6). It is a variation of discography’s standard technique, during which the intradiscal injection is performed at a constant rate, and at the same time there is recording of both the injected volume as well as the intradiscal pressures, thus producing a pressure–volume curve [45]. This pressure– volume curve provides valuable information concerning the biomechanical integrity of the endplate–intervertebral disc complex. With QD, the recording of intradiscal pressure and injected volume evaluates both the physical status and the degree of degeneration of the intervertebral disc. The successful outcome of minimally invasive therapeutic techniques is highly dependent upon these two parameters, since they establish proper patient selection. Recently, a study has shown that the maximum intradiscal pressure seems to be the most important parameter, which could act as an indicator of degeneration, strength of the endplate–disc complex and successful outcome of therapeutic techniques [45].
Figure 6: Lumbar quantitative discomanometry. A patient underwent L3–L4 intervertebral disc laser decompression due to symptomatic herniation. She was referred to our department for investigation of symptoms persistence. Percutaneous quantitative discomanometry was performed at (A) L3–L4, (B) L4–L5 and (C) L5–SI intervertebral discs. Notice the difference of pressure-volume curves among L3–L4 (highly degenerated post decompression disc), L4–L5 (normal intervertebral disc with ‘cotton ball’ appearance) and L5–SI (mildly degenerated disc).
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.

References
- Gillan MG, Gilbert FJ, Andrew JE et al.; Scottish Back Trial Group. Influence of imaging on clinical decision making in the treatment of lower back pain. Radiology 220, 393–399 (2001).
- Abdi S, Datta S, Trescot AM et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician 10, 185–212 (2007).
- Bhangle SD, Sapru S, Panush RS. Back pain made simple: an approach based on principles and evidence. Cleve. Clin. J. Med. 76(7), 393–399 (2009).
- Von Korff M, Saunders K. The course of back pain in primary care. Spine 21, 2833–2837; discussion 2838–2839 (1996).
- Martin BI, Deyo RA, Mirza SK et al. Expenditures and health status among adults with back and neck problems. JAMA 299, 656–664 (2008).
- Frymoyer JW, Cats-Baril WL. An overview of the incidences and costs of low back pain. Orthop. Clin. North Am. 22, 263–271 (1991).
- Maroon JC, Abla A. The microlumbar discectomy. Clin. Neurosurg. 33, 407–417 (1986).
- Brox JI, Nygaard OP, Holm I, Keller A, Ingebrigsten T, Reikeras O. Four-year followup of surgical versus non-surgical therapy for chronic low back pain. Ann. Rheum. Dis. 69, 1643–1648 (2010).
- Cowan JA Jr, Dimick JB, Wainess R, Upchurch GR Jr, Chandler WF, La Marca F. Changes in the utilization of spinal fusion in the United States. Neurosurgery 59, 15–20 (2006).
- Merskey H, Bogduk N. Classification of chronic pain. In: Descriptions of Chronic Pain, Syndromes and Definition of Pain Terms (2nd Edition). International Assosiation for the Study of Pain Press, Seattle, WA, USA, 180–181 (1994).
- Last AR, Hulbert K. Chronic low back pain: evaluation and management. Am. Fam. Physician 79(12), 1067–1074 (2009).
- Varlotta GP, Lefkowitz TR, Schweitzer M et al. The lumbar facet joint: a review of current knowledge: part II: diagnosis and management. Skeletal Radiol. 40(2), 149–157 (2011).
- Gallucci M, Limbucci N, Paonessa A, Splendiani A. Degenerative disease of the spine. Neuroimag. Clin. N. Am. 17(1), 87–103 (2007).
- Kelekis AD, Somon T, Yilmaz H et al. Interventional spine procedures. Eur. J. Radiol. 55(3), 362–383 (2005).
- Gallucci M, Conchiglia A, Lanni G, Conti L, Limbucci N. Treatments for sciatica mimics: facets and sacroiliac joints. Neuroradio. J. 22(1), 154–160 (2009).
- Frymoyer JW. Lumbar disc disease: epidemiology. Instr. Course Lect. 41, 217–223 (1992).
- Erginousakis D, Filippiadis DK, Malagari A et al. Comparative prospective randomized study comparing conservative treatment and percutaneous disk decompression for treatment of intervertebral disk herniation. Radiology 260(2), 487–493 (2011).
- Kelekis AD, Filippiadis DK, Martin JB, Brountzos E. Standards of practice: quality assurance guidelines for percutaneous treatments of intervertebral discs. Cardiovasc. Intervent. Radiol. 33(5), 909–913 (2010).
- Frymoyer JW. Back pain and sciatica. N. Engl. J. Med. 318, 291–300 (1988).
- Mirkovic S, Garfin SR, Rydevik B, Lipson SJ. Pathophysiology of spinal stenosis. Instr. Course Lect. 41, 165–177 (1992).
- Herkowitz HN. Spinal stenosis: clinical evaluation. Instr. Course Lect. 41, 183–185 (1992).
- Daffner SD, Wang JC. The pathophysiology and nonsurgical treatment of lumbar spinal stenosis. Instr. Course Lect. 58, 657–668 (2009).
- Kotani Y, Abumi K, Ito M, Sudo H, Abe Y, Minami A. Mid-term clinical results of minimally invasive decompression and posterolateral fusion with percutaneous pedicle screws versus conventional approach for degenerative spondylolisthesis with spinal stenosis. Eur. Spine J. 21(6), 1171–1177 (2012).
- Smorgick Y, Park DK, Baker KC et al. Single versus multilevel fusion, for single level degenerative spondylolisthesis and multilevel lumbar stenosis: four-year results of the spine patient outcomes research trial. Spine doi:10.1097/BRS.0b013e31827db30f (2012) (Epub ahead of print).
- Almen A, Tingberg A, Besjakov J et al. The use of reference image criteria in x-ray diagnostics: can application for the optimisation of lumbar spine radiographs. Eur. Radiol. 14, 1651–1567 (2004).
- Renner JB. Conventional radiography in musculoskeletal imaging. Radiol. Clin. North Am. 47(3), 357–372 (2009).
- Ohashi K, El-Khoury GY. Musculoskeletal CT: recent advances and current clinical applications. Radiol. Clin. North Am. 47(3), 387–409 (2009).
- Bjarke Christensen F, Stender Hansen E, Laursen M, Thomsen K, Bünger CE. Longterm functional outcome of pedicle screw instrumentation as a support for posterolateral spinal fusion: randomized clinical study with a 5-year follow-up. Spine 27(12), 1269–1277 (2002).
- Fritzell P, Hägg O, Nordwall A; Swedish Lumbar Spine Study Group. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur. Spine J. 12(2), 178–189 (2003).
- Tall MA, Thompson AK, Vertinsky T, Palka PS. MR imaging of the spinal bone marrow. Magn. Reson. Imaging Clin. N. Am. 15, 175–198 (2007).
- Lotz JC, Haughton V, Boden SD et al. New treatments and imaging strategies in degenerative disease of the intervertebral disks. Radiology 264(1), 6–19 (2012).
- Aprill CN, Bogduk N. High intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br. J. Radiol. 65, 361–369 (1992).
- Schellhas KP, Pollei SR, Gundry CR et al. Lumbar disc high intensity zone. Spine 21, 79–86 (1996).
- Jensen MC, Brant-Zawadski MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. MRI of the lumbar spine in people without low back pain. N. Engl. J. Med. 331, 69–73 (1994).
- Williams AL. Myelography. In: Handbook of Diagnostic and Therapeutic Spine Procedures. Williams AL, Murtagh FR (Eds). CV Mosby, St Louis, MO, USA, 107–129 (2002).
- Kau T, Rabitsch E, Celedin S et al. Feasibility and potential value of flat-panel detectorbased computed tomography in myelography after spinal surgery. J. Neurosurg. Spine 10(1), 66–72 (2009).
- Engelhorn T, Rennert J, Richter G, Struffert T, Ganslandt O, Doerfler A. Myelography using flat panel volumetric computed tomography: a comparative study in patients with lumbar spinal stenosis. Spine 32(18), E523–E527 (2007).
- Kim DG, Eun JP, Park JS. New diagnostic tool for far lateral lumbar disc herniation: the clinical usefulness of 3-Tesla magnetic resonance myelography comparing with the discography CT. J. Korean Neurosurg. Soc. 52(2), 103–106 (2012).
- Fenton DS, Czervionke LF. Discography. In: Image Guided Spine Intervention. Fenton DS, Czervionke LF (Eds). Saunders, PA, USA, 227–256 (2003).
- Clifford JR. Lumbar discography: an outdated procedure. J. Neurosurg. 64, 686 (1986).
- Shapiro R. Current status of lumbar discography. Radiology 159(3), 815 (1986).
- Schullin DR. Lumbar discography. Radiology 162, 284 (1987).
- Fenton DS, Czervionke LF. Discography. In: Handbook of Diagnostic and Therapeutic Spine Procedures. Williams AL, Murtagh FR (Eds). CV Mosby, St Louis, MO, USA, 167–199 (2002).
- Guyer RD, Ohnmeiss DD. Lumbar discography. Position statement from the North American Spine Society diagnostic and therapeutic committee. Spine 20(18), 2048–2059 (1995).
- Filippiadis DK, Mazioti A, Papakonstantinou O et al. Quantitative discomanometry: correlation of intradiscal pressure values to pain reduction in patients with intervertebral disc herniation treated with percutaneous, minimally invasive, image-guided techniques. Cardiovasc. Intervent. Radiol. 5(5), 1145–1153 (2012).
- Berg S, Isberg B, Josephson A, Fällman M. The impact of discography on the surgical decision in patients with chronic low back pain. Spine J. 12(4), 283–291 (2012).
- Datta S, Lee M, Falco FJ, Bryce DA, Hayek SM. Systematic assessment of diagnostic accuracy and therapeutic utility of lumbar facet joint interventions. Pain Physician 12(2), 437–460 (2009).
- Falco FJ, Manchikanti L, Datta S et al. An update of the systematic assessment of the diagnostic accuracy of lumbar facet joint nerve blocks. Pain Physician 15(6), E869–E907 (2012).
- Masala S, Crusco S, Meschini A, Taglieri A, Calabria E, Simonetti G. Piriformis syndrome: long-term follow-up in patients treated with percutaneous injection of anesthetic and corticosteroid under CT guidance. Cardiovasc. Intervent. Radiol. 35(2), 375–382 (2012).
- Maus T. Imaging the back pain patient. Phys. Med. Rehabil. Clin. N. Am. 21(4), 725–766 (2010).
- Birkmeyer NJ, Weinstein JN. Medical versus surgical treatment for low back pain: evidence and clinical practice. Eff. Clin. Prac. 218–227 (1999).
- Tarantino U, Fanucci E, Iundusi R et al. Lumbar spine MRI in upright position for diagnosing acute and chronic low back pain: statistical analysis of morphological changes. J. Orthop. Traumatol. doi:10.1007/s10195- 012-0213-z (2012) (Epub ahead of print).
- Gilbert JW, Wheeler GR, Kreft MP et al. Repeat upright positional magnetic resonance imaging for diagnosis of disorders underlying chronic noncancer lumbar pain. J. Manipulative Physiol. Ther. 31(8), 627–631 (2008).