Case Report - International Journal of Clinical Rheumatology (2022) Volume 17, Issue 4
Percutaneous vertebroplasty to treat intractable pain in Langerhans histiocytosis of the lumbar spine; a case report and review of the literature
Venetia Giannakaki*, Farooq Aziz
James Cook University Hospital, Middlesbrough, United Kingdom
- *Corresponding Author:
- Venetia Giannakaki
James Cook University Hospital, Middlesbrough, United Kingdom
E-mail: venetiagiannakaki@hotmail.com
Received: 11-Apr-2022, Manuscript No. FMIJCR-22-60312; Editor assigned: 12-Apr-2022, PreQC No. FMIJCR-22-60312(PQ); Reviewed: 26-Apr-2022, QC No. FMIJCR-22-60312; Revised: 30-Apr-2022, Manuscript No. FMIJCR-22-60312(R); Published: 07-Apr-2022, DOI: 10.37532/1758-4272.2022.17(4).080-084
Abstract
Langerhans Cell Histiocytosis (LCH) is a rare disease of the adult spine, and its clinical presentation can range from back pain to soft tissue expansion and neurological compromise. There is no specified protocol for its treatment and based on the severity of symptoms, it can involve conservative management and immobilization to en-bloc resection of the affected vertebrae. To date, there are only 4 reported cases of patients with spinal LCH that have been treated with percutaneous vertebroplasty, three of which had an associated fracture. We present a case of LCH in the lumbar spine and refractory back pain, in the absence of a fracture, where percutaneous vertebroplasty was used to successfully treat the pain and we provide a review of the literature.
Keywords: langerhans cell histiocytosis • eosinophilic granuloma • lumbar spine • vertebroplasty • fracture • back pain
Introduction
Langerhans Cell Histiocytosis (LCH) is a rare systemic disorder that is characterized by the aberrant function and differentiation or proliferation of cells of the mononuclear phagocyte system [1]. Eosinophilic granulomas can arise in virtually any organ system, but have a predilection for bone, skin, the lungs and the pituitary and they are most commonly seen in children [2]. The adult spine is very rarely affected and even more so the lumbar spine. Spinal lesions can typically present with back pain, compression fractures and subsequent vertebral collapse, as well as soft tissue expansion and neurological compromise [3]. Management of such lesions can vary between conservative methods with analgesia and immobilization to decompressive surgery and fusion when patients present with neurological deficit [4]. In our paper we present a case of LCH of the L5 vertebra, where the patient presented with refractory back pain and was treated successfully with percutaneous vertebroplasty (PVP).
Case Report
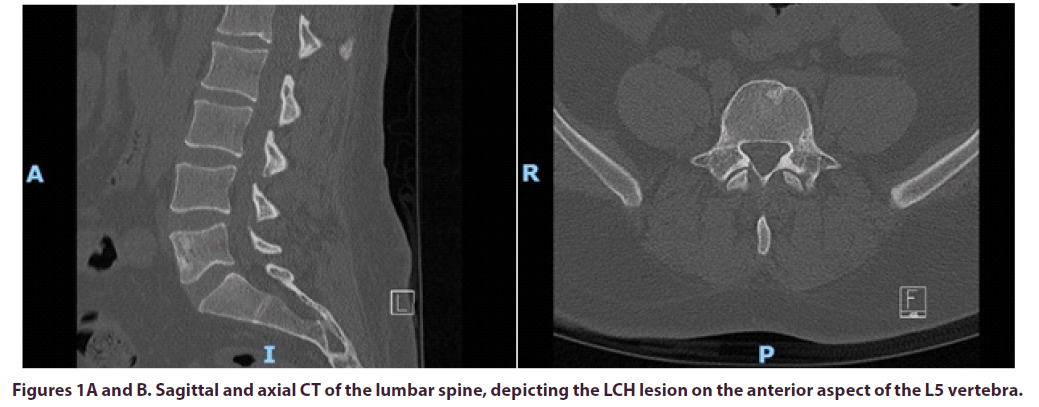
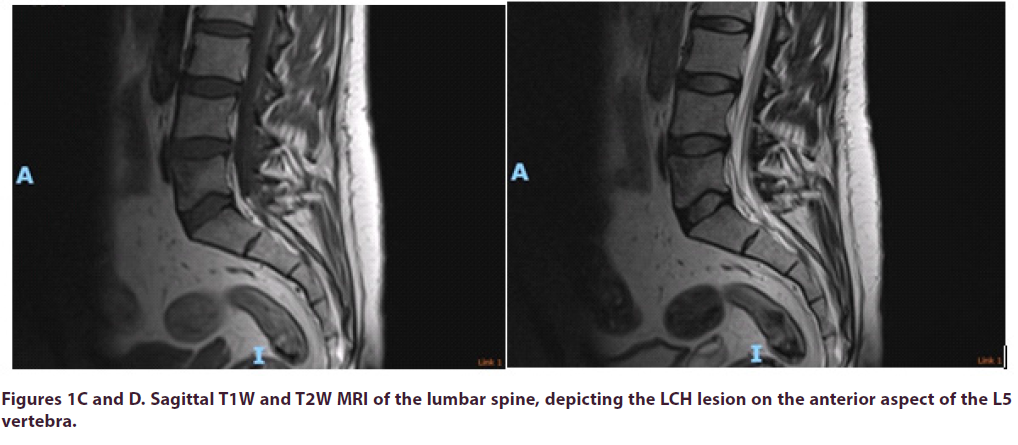
A 36-year-old male patient was referred by his Haematologist to the pain MDT (Pain Anesthetist, Pain Psychologist, Pain Nurse, Physiotherapist and Neurosurgeon), for consideration of an intrathecal morphine infusion pump for intractable lower back pain radiating into both hips. He had been suffering from lower back pain for many years and it was progressively getting worse. This gentleman was found to have a solitary L5 bony lesion for which he underwent biopsy three years ago and was diagnosed with Langerhans Cell Histiocytosis, Figures 1AD. He was on nearly 0.5g of morphine daily, ketamine and intermittent epidurals. Notably, the epidural was quite effective in managing his pain, but it only had a very short duration. As a last resort, and even though his PET-CT scan had always been negative, he was given high dose empirical and low dose maintenance chemotherapy, but this did not manage to control his pain. Despite treatment, he rated his pain as 9/10 in the Numeric Rating Scale (NRS).
During the MDT proceedings, the senior author of this paper suggested that since this is a solitary lesion in the lumbar spine, percutaneous vertebroplasty (PVP) could be attempted instead of an intrathecal morphine pump. There have been only 4 cases reported in the literature so far that suggested this to be an effective treatment. We explained to the patient that there was a level of uncertainty on whether this treatment would work, and after explaining the risks and benefits he decided to go ahead with the procedure.
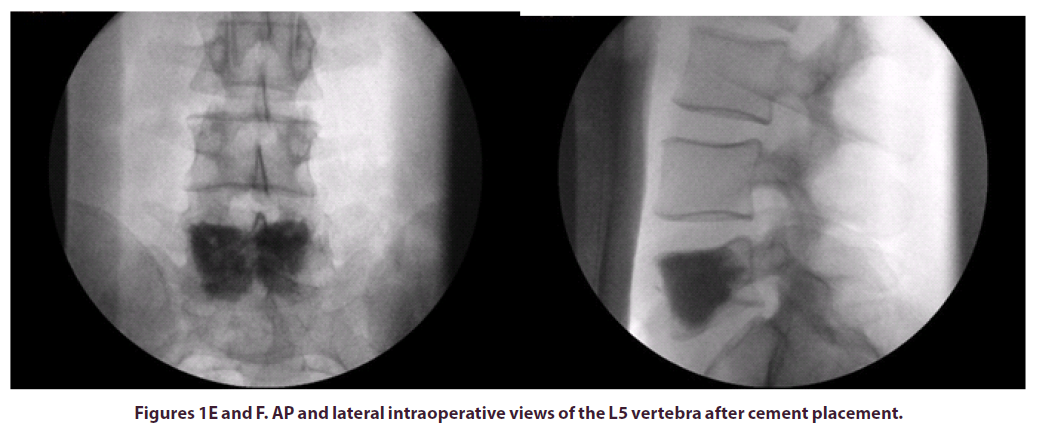
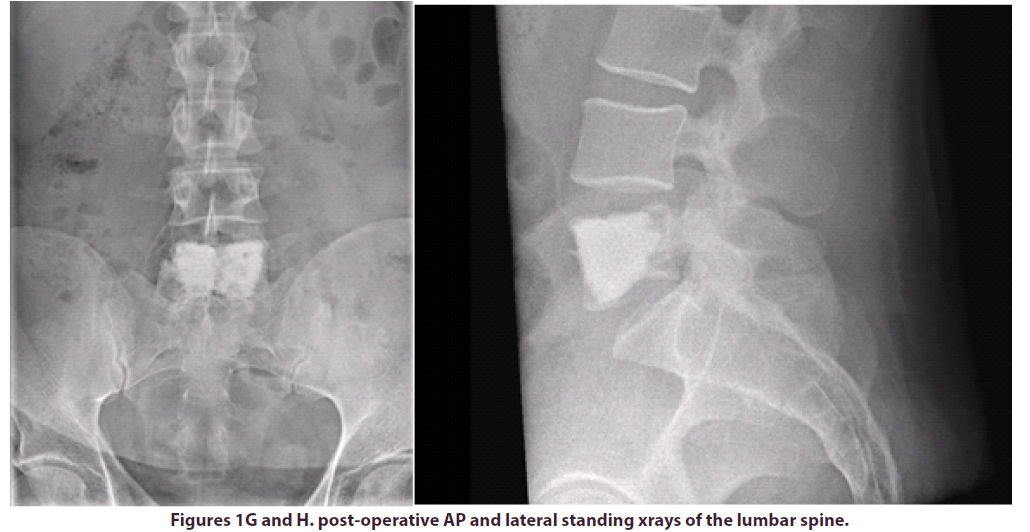
The patient was positioned prone on a Montreal mattress and the procedure was performed under spinal epidural. The skin was prepped with betadine solution and infiltrated with local anaesthetic (bupivacaine and adrenaline 0.25% w/v, 1 in 200,000). Two stab incisions were made and two access needles were inserted through pedicles in the L5 vertebral body under x-ray guidance. In total, 15ml of polymethylmethacrylate (PMMA)- based acrylic bone cement (ABC) was inserted in the vertebral body, to ensure that cement would cover the largest part of the vertebral body, including the anterior part where the LCH lesion was observed. The intraoperative and post-operative x-rays can be seen in the images below, Figures E-H.
We reviewed the patient in clinic 12 weeks postoperatively and at this time he rated his pain as 1-2/10 in the NRS. He claimed that the pain relief was almost immediate after surgery and reported that he was able to return to his daily activities after many years.
Discussion
Langerhans cells are myeloid cells that can be found in the epidermis and stratified epithelia of the corneal, buccal, gingival and genital mucosae [5]. Langerhans cell histiocytosis (LCH) is a rare systemic disorder that is characterized by aberrant function and differentiation or proliferation of cells of the mononuclear phagocyte system [1]. Langerin-positive (CD207+) histiocytes can form granulomas that can arise in virtually any organ system, but have a particular affinity for bone, skin, the lungs and the pituitary [2].
Its aetiology remains unknown but it is now characterized as an inflammatory myeloid neoplasm in the revised 2016 Histiocyte Society classification [6]. LCH is most commonly encountered in children and can have a variable clinical presentation, ranging from single asymptomatic lesions to explosive multisystem disease [7]. Solitary bone lesions are most often encountered in the skull (particularly the calvarium and temporal bones), the mandible, the femur, the pelvis and spine [8]. In the spine, they most commonly arise in the thoracic vertebrae (54%), followed by the lumbar (35%) and the cervical (11%) vertebrae [9].
Adults with LCH lesions in their spine commonly present with pain, restricted movements and very rarely with neurological deficit, which can be caused by soft tissue expansion [10]. The bony lesions can be osteoblastic or osteolytic and it is due to the latter that vertebral bodies can collapse and cause back pain, partly due to vertebral instability [11].
There are many treatment modalities for LCH lesions in the spine, ranging from conservative management and immobilization to radio/chemotherapy and surgery [12]. Xu et al. recommend pain management and immobilization as the first line treatment and reserve chemotherapy for multifocal lesions, while low dose radiotherapy (20-30 Gy) is recommended for spinal lesions with marked bony erosion, especially where there is soft tissue expansion that can threaten neural structures [13]. Surgical excision should be considered in patients with pathological fractures and refractory back pain, in patients with neurological deficit and patients with spinal instability [14]. Chen et al also describe a rare case with LCH on the L5 vertebra that was causing nerve root compression, which was treated with en-bloc The first time PVP was used to treat LHC of the spine was in 1994 by Cardon et al on a 25-year-old patient with recurrent eosinophilic granulomas post radiotherapy and progressive spinal instability [16]. Following this, Tan et al performed the same procedure in a 10-year-old girl with a C4 lesion with a compression fracture and mild collapse [17]. Kevane et al published in 2009 a series of patients where they used PVP to treat severe refractory pain associated with vertebral fractures, secondary to osteoporosis, myeloma and a case of LCH [18]. Feng et al reported in 2013 a case of a 51-yearold male patient with a LCH osteolytic lesion on the right lateral mass of L4 vertebra with paravertebral and intraspinal soft tissue mass. The patient presented with localized tenderness over the L4 vertebra, restricted waist motion and numbness of the right leg. Despite the presence of soft tissue expansion and neural compression, the patient was successfully treated with PVP only, rather than decompressive surgery, and after injecting 3.6ml of bone cement in the vertebral body and pedicle, the patient’s pain completely resolved, and the neurological symptoms started to improve. Notably the patient further received chemotherapy following PVP that controlled the soft tissue expansion [19].
The mechanism of pain relief in vertebroplasty is not yet fully understood. There are different theories including, thermal necrosis of nerve endings during the exothermic polymerization of MMA, direct chemotoxicity of MMA and restoration of mechanical stability as well as stopping the micromovements of bony fragments by the cured cement [20]. PVP was first described by Galibert et al [21] and was originally used to treat vertebral angiomas. Since then, it has been found to offer significant pain relief in certain vertebral body tumours, such as osteolytic metastases and myelomas, but also in painful compression osteoporotic fractures that fail conservative management [22-24]. PVP has the advantages of being minimally invasive, it does not require implants, it can offer immediate pain relief and the patients are able to mobilize quickly after the procedure.
Our patient did not present with a pathological fracture and instability pain, we believe that his pain was rather attributed to the lesion itself and it was significantly restricting his ability to carry out his daily activities. All previous medical treatments failed to cure his pain longterm, including chemotherapy.
Even though successful use of PVP for LCH has only been reported in 4 cases in the literature, only one of these cases did not involve a pathological fracture and instability pain. Our case is the second reported case of spinal LCH without a pathological fracture where refractory back pain is successfully treated with PVP.
Conclusion
Langerhans cell Histiocytosis is a rare disease of the adult spine and can present with a variety of symptoms. There is no specified protocol for its treatment, and this is usually tailored to the severity of clinical presentation. We present a case where refractory back pain due to LCH in the lumbar spine, is successfully treated with percutaneous vertebroplasty.
Conflict of interest
The authors report no conflict of interest.
References
- Kobayashi M, Tojo A. Langerhans cell histiocytosis in adults: Advances in pathophysiology and treatment. Cancer Sci. 109, 3707–3713 (2018).
- Allen CE, Merad M, McClain KL. Langerhans-Cell Histiocytosis. N Engl J Med. 379, 856–868 (2018).
- Jiang L. Langerhans cell histiocytosis of the cervical spine: a single Chinese institution experience with thirty cases. Spine. 35, 8-15 (2010).
- Lü GH, Li J, Wang XB et al. Surgical treatment based on pedicle screw instrumentation for thoracic or lumbar spinal Langerhans cell histiocytosis complicated with neurologic deficit in children. Spine J Off J North Am Spine Soc. 14, 768–776 (2014).
- Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. J Invest Dermatol. 132, 872–881 (2012).
- Emile JF. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 127, 2672–2681 (2016).
- Gadner H. Improved outcome in multisystem Langerhans cell histiocytosis is associated with therapy intensification. Blood. 111, 2556–2562 (2008).
- Zhong WQ. Langerhans cell histiocytosis of the atlas in an adult. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 19, 19–22 (2010).
- Azouz EM, Saigal G, Rodriguez MM et al. ‘Langerhans’ cell histiocytosis: pathology, imaging and treatment of skeletal involvement. Pediatr Radiol. 35, 103–115 (2005).
- Tanaka N. Langerhans cell histiocytosis of the atlas: a report of three cases. J Bone Joint Surg Am. 87, 2313-2317 (2005).
- Hassan BW, Moon BJ, Kim YJ et al. Langerhans cell histiocytosis in the adult lumbar spine: case report. SpringerPlus. 5, 1398 (2016).
- Yeom JS, Lee CK, Shin HY et al. ‘Langerhans’ cell histiocytosis of the spine. Analysis of twenty-three cases’. Spine. 24, 1740–1749 (1999).
- Xu X. Clinical features and treatment outcomes of Langerhans cell histiocytosis of the spine. Spine J Off J North Am Spine Soc. 18, 1755–1762 (2018).
- Dhillon CS, Tantry R, Ega SR et al. Langerhans Cell Histiocytosis in the Adult Lumbar Spine - A Case Report and Literature Review. J Orthop Case Rep. 10, 28–32 (2020).
- Chen L, Chen Z, Wang Y. Langerhans cell histiocytosis at L5 vertebra treated with en bloc vertebral resection: a case report. World J Surg Oncol. 16, 96 (2018).
- Cardon T. Percutaneous vertebroplasty with acrylic cement in the treatment of a Langerhans cell vertebral histiocytosis. Clin Rheumatol. 13, 518–521 (1994).
- Tan HQ, Li MH, Wu CG et al. Percutaneous vertebroplasty for eosinophilic granuloma of the cervical spine in a child. Pediatr Radiol. 37, 1053–1057 (2007).
- Kevane B, Ryder DQ, Gilligan O. Percutaneous vertebroplasty in osteoporosis, myeloma and Langerhans’ cell histiocytosis. Ir Med J. 102, 212–215 (2009).
- Feng F, Tang H, Chen H et al. Percutaneous vertebroplasty for Langerhans cell histiocytosis of the lumbar spine in an adult: Case report and review of the literature. Exp Ther Med. 5, 128–132 (2013).
- Yimin Y, Zhiwei R, Wei M et al. Current status of percutaneous vertebroplasty and percutaneous kyphoplasty – a review. Med Sci Monit Int Med J Exp Clin Res. 19, 826–836 (2013).
- Galibert P, Deramond H, Rosat P et al. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie. 33, 166–168 (1987).
- Gangi A, Guth S, Imbert JP et al. Percutaneous vertebroplasty: indications, technique, and results. Radiogr Rev Publ Radiol Soc N Am Inc. 23, e10 (2003).
- Shimony JS, Gilula LA, Zeller AJ et al. Percutaneous vertebroplasty for malignant compression fractures with epidural involvement. Radiology. 232, 846–853 (2004).
- Kobayashi K, Shimoyama K, Nakamura K et al. Percutaneous vertebroplasty immediately relieves pain of osteoporotic vertebral compression fractures and prevents prolonged immobilization of patients. Eur Radiol. 15, 360–367 (2005).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref