Review Article - Interventional Cardiology (2012) Volume 4, Issue 2
Perioperative management after coronary stenting: role of risk assessment and the antiplatelet conundrum
- Corresponding Author:
- Christian Seiler
Department of Cardiology
University Hospital
CH-3010 Bern, Switzerland
Tel: +41 31 632 3693
Fax: +41 31 632 4299
E-mail: christian.seiler@insel.ch
Abstract
Keywords
acetylsalicylic acid, antiplatelet therapy, clopidogrel, collateral circulation, coronary artery disease, perioperative management, stent thrombosis
Percutaneous coronary intervention (PCI) including bare metal stent (BMS) or drug-eluting stent (DES) implantation is an established treatment for patients with coronary artery disease (CAD), reducing the risk of abrupt vessel closure and restenosis when compared with balloon angioplasty alone [1]. Currently, it is recommended that dual antiplatelet therapy with acetylsalicylic acid and clopidogrel is given for a period of ≥1 month after BMS and for ≥12 months after DES implantation or after PCI in the event of acute coronary syndrome regardless of stent [2,3]. The chance that a patient with CAD requires noncardiac surgery within 1 year following coronary stenting amounts to 5% [4]. The risks related to a surgical intervention include, increased platelet volume and activation, enhanced inflammatory state, arterial hypotension with impaired coronary perfusion in the context of general anaesthesia and/or blood loss, and early cessation of antiplatelet therapy due to actual or perceived bleeding risk.
In the context of temporary endothelial denudation related to PCI, the premature stop of dual or even mono antiplatelet therapy poses the patient at risk for stent thrombosis (Figure 1). Notwithstanding the relatively rare occurrence of noncardiac surgery after PCI with stenting, the consequences of stent thrombosis within 1 year of such a procedure are likely dire [5], thus rendering risk assessment for stent thrombosis at the time of PCI and perioperative antiplatelet therapy important.
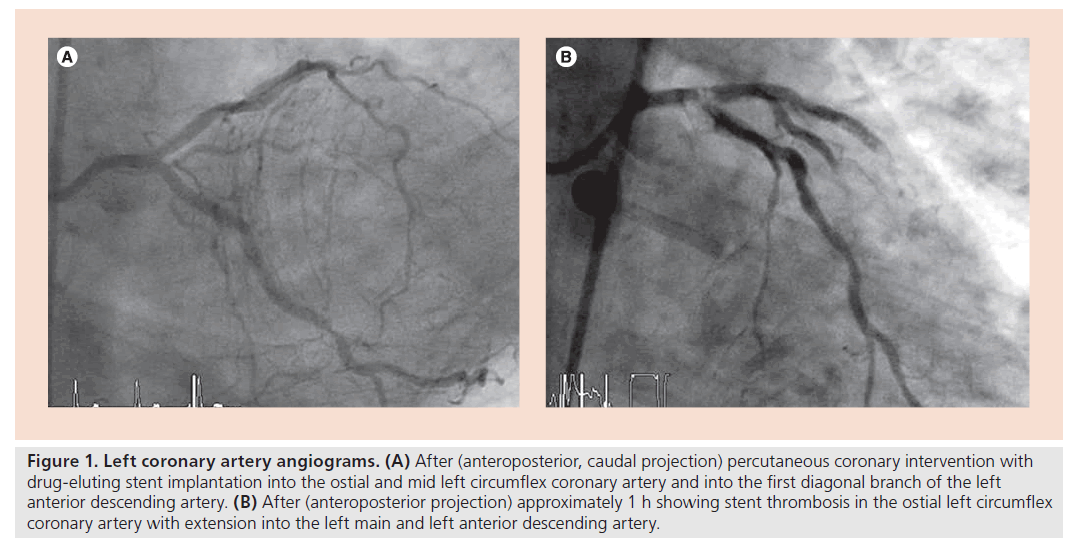
Figure 1. Left coronary artery angiograms. (A) After (anteroposterior, caudal projection) percutaneous coronary intervention with drug-eluting stent implantation into the ostial and mid left circumflex coronary artery and into the first diagonal branch of the left anterior descending artery. (B) After (anteroposterior projection) approximately 1 h showing stent thrombosis in the ostial left circumflex coronary artery with extension into the left main and left anterior descending artery.
Therefore, the aim of this article is to provide an insight into the prognostic impact of perioperative stent thrombosis, to offer clarification on the mechanism(s) related to the devastating effect of stent thrombosis, to illustrate risk factors for and prevention of stent thrombosis in the context of a case report and to review the perioperative management of antiplatelet therapy following PCI with stenting.
Prognostic impact of perioperative stent thrombosis
Surgery induces a prothrombotic state thus predisposing to stent thrombosis, but there is no convincing evidence favoring a difference between the prognostic impact of stent thrombosis with or without associated surgery. In patients undergoing a variety of elective noncardiac surgery within 1 year of coronary stenting, the rate of major adverse events has been shown to be very high (45%; 46 of 103 patients; five deaths) and mainly due to cardiac events (43 of 46) [4]. In this context, it has to be pointed out that the major adverse cardiac event is not stent thrombosis, but cardiac death, myocardial infarction, unstable angina pectoris, troponin elevation, sustained ventricular tachycardia or re-do PCI. According to the definition of stent thrombosis, it can often not be definitely confirmed (symptoms suggestive of an acute coronary syndrome and angiographic or pathologic confirmation of stent thrombosis [6]). However, cardiac death is covered by the definitions other than definite stent thrombosis probable – unexplained death within 30 days or target vessel myocardial infarction without angiographic confirmation of stent thrombosis; possible – any unexplained death after 30 days [6]. In the study by Vicenzi et al., the risk for adverse events doubled if surgery was performed less than 35 days after stenting [4]. Of note, bleeding occurred in only four out of 103 patients in the study by Vicenzi et al. and this was the case, despite the fact that patients were continued on or had only briefly interrupted antiplatelet therapy. It is undisputed in the literature that performing surgery within 3–6 weeks of stenting confers the greatest risk of adverse cardiac events, with mortality rates as high as 25% (time window <3 weeks: mortality 85%) [7]. The large variability in reported adverse event rates and mortality is mainly related to the low power of the studies on perioperative complications (n = 38–239 [7]) as opposed to investigations on stent thrombosis as a whole. In this context, the differentiation between adverse event rates among patients with BMS and DES is even more difficult in studies exclusively focusing on perioperative stent thrombosis; for example, in the study by Vicenzi et al., stent identification was impossible in 79 of 103 cases, due to insufficient documentation [4].
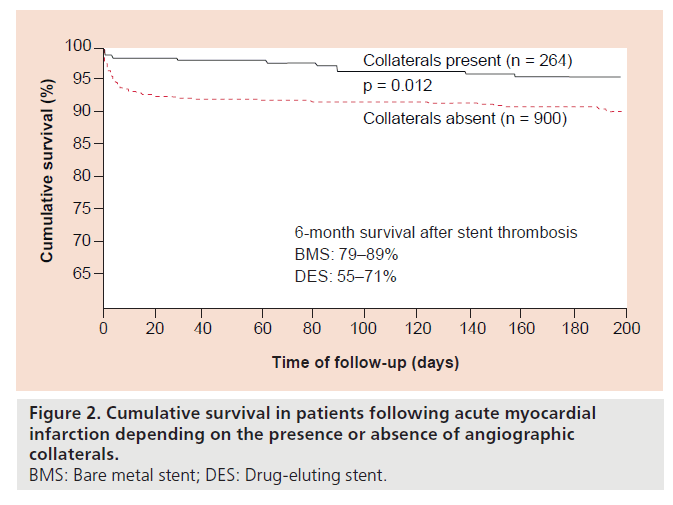
Cumulative survival rate of 6 months after acute myocardial infarction has been shown to amount to 92–97%, depending on the absence or presence of angiographic collaterals at the time of primary PCI (Figure 2) [8]. In comparison, lower survival rates after stent thrombosis leading to acute myocardial infarction has been documented: 79–89% following BMS implantation and 55–71% after treatment with DES, respectively [9–12]. Chechi et al. reported results consistent with those mentioned above, in other words, cumulative 6‑month survival rate of 79% in patients with stent thrombosis as opposed to 90% in the group with acute myocardial infarction in the absence of stent thrombosis [5]. In this study, stent thrombosis independently predicted angiographically unsuccessful primary PCI with a lower rate of normalized flow. It was reasoned that an antiplatelet regimen not aggressive enough for the increased thrombus load was responsible for the more dreadful outcome in the patients with versus those without stent thrombosis [13]. However, the deeper cause for widespread microembolization with no-reflow phenomenon, in this particular case, must be looked for elsewhere.
Figure 2. Cumulative survival in patients following acute myocardial infarction depending on the presence or absence of angiographic collaterals.
BMS: Bare metal stent; DES: Drug-eluting stent.
Possible mechanism(s) related to the prognosis of stent thrombosis
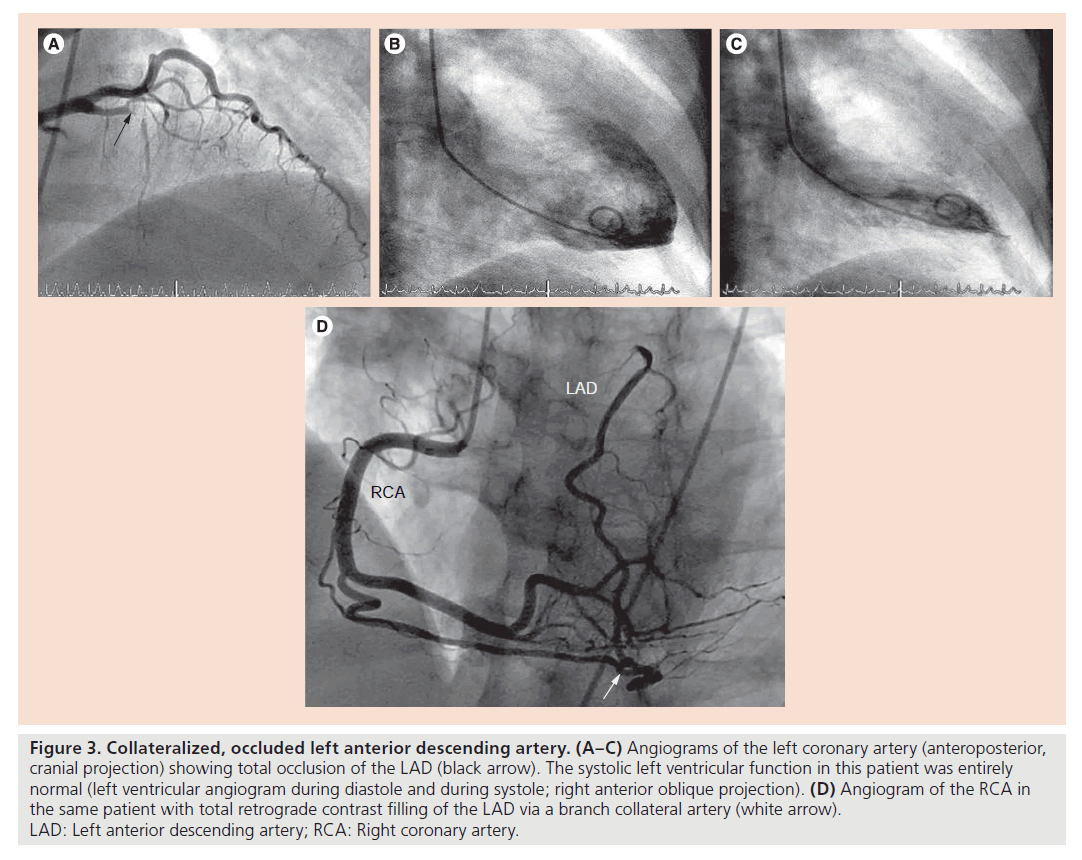
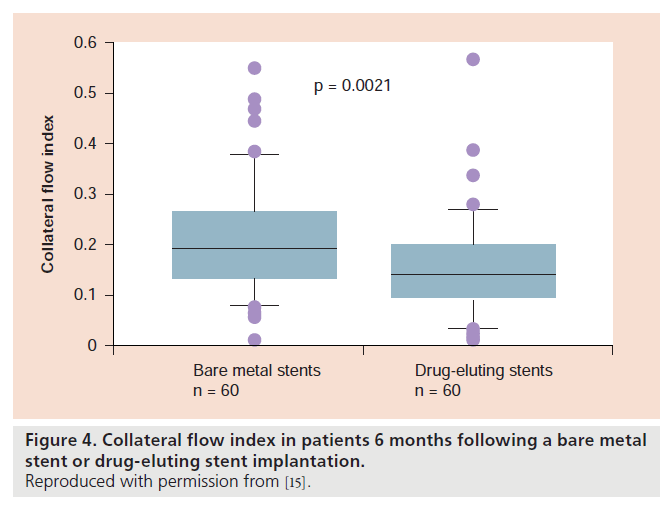
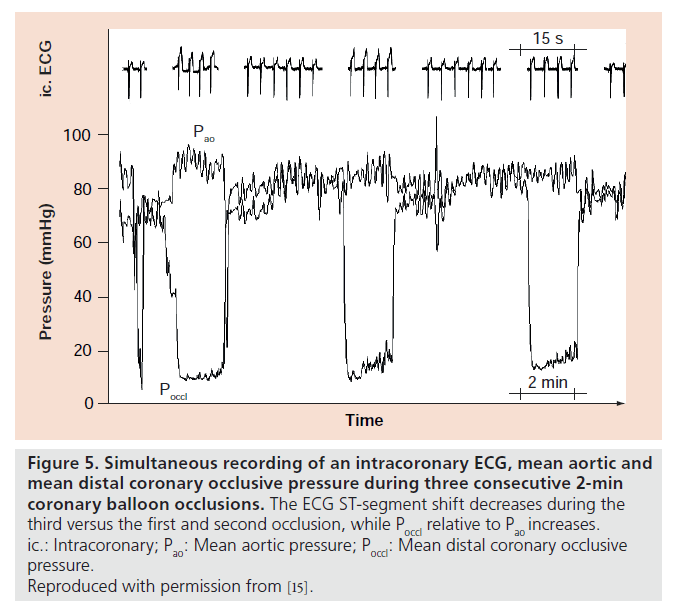
No-reflow in the context of extensive microembolization is dependent on coronary thrombus load, which itself is prevented from build-up by the presence of residual flow to the occluded artery. Is the probability of residual flow different in the context of a gradual chronic total occlusion (CTO) in comparison with the sudden total occlusion occurring with stent thrombosis? There are several arguments in favor of a systematically diminished residual, that is, collateral blood supply to the area at risk for infarction in the presence of a stent thrombosis as opposed to CTO, which may occur entirely unnoticed (Figure 3). Coronary collateral function in the presence of CTO without or with myocardial infarction is significantly higher than in the context of a less severe coronary atherosclerotic lesion (approximately 35% of normal flow during vessel patency versus 20%, respectively); in fact, there is a direct correlation between the degree of stenosis and collateral function [14,15]. As a consequence, treatment of a stenotic lesion by PCI (i.e., the event preceding that of stent thrombosis) leads to a decrease, although incomplete elimination of collateral function from the mentioned average value of 20–15% of collateral, relative to normal flow during vessel patency [16]. The amount of post- PCI collateral function reduction is greater following DES than BMS implantation (Figure 4) [17], the fact of which has been interpreted related to the anti-inf lammatory action of DES, which counteracts monocyte function, which is crucial in the pathogenesis of collateral growth. This collateral function pruning following PCI together with the fact that collateral artery growth in response to reocclusion takes several weeks to fully occur [15], renders the sudden total occlusion during stent thrombosis prone to devastating effects in the sense of large infarcts despite often only short occlusion times. In addition, the sudden total coronary occlusion in stent thrombosis is unique, because ischemic preconditioning with repetitive episodes of ischemia before total occlusion and coronary collateral recruitment does not take place as in chronic CAD (Figure 5) [18]. Considering the relatively low overall incidence of stent thrombosis of 0.5–1% per year [19] and the prospect that newer generation DES may tend to have a lower incidence than earlier DES and than BMS (0.8 vs 1.7% per 5 years; p = 0.21 [20]), clinical practice of DES and BMS implantation should not be altered based on the abovementioned mechanisms related to the prognostic impact of stent thrombosis.
Figure 3. Collateralized, occluded left anterior descending artery. (A–C) Angiograms of the left coronary artery (anteroposterior, cranial projection) showing total occlusion of the LAD (black arrow). The systolic left ventricular function in this patient was entirely normal (left ventricular angiogram during diastole and during systole; right anterior oblique projection). (D) Angiogram of the RCA in the same patient with total retrograde contrast filling of the LAD via a branch collateral artery (white arrow). LAD: Left anterior descending artery; RCA: Right coronary artery.
Figure 5. Simultaneous recording of an intracoronary ECG, mean aortic and mean distal coronary occlusive pressure during three consecutive 2- min coronary balloon occlusions.
The ECG ST-segment shift decreases during the third versus the first and second occlusion, while Poccl relative to Pao increases. ic.: Intracoronary; Pao: Mean aortic pressure; Poccl: Mean distal coronary occlusive pressure. Reproduced with permission from [15].
Case report of acute stent thrombosis
Figure 1 illustrates the case of acute stent thrombosis in a 75‑year-old female patient who had suffered from angina pectoris and dyspnea for 3 months prior to hospital admission for elective coronary angiography. The peri-PCI occurrence of the event renders it obvious that it is not a case related to a surgical intervention.
However, its swift temporal dynamics and the details of the antiplatelet therapy exemplify the potentially even riskier, because slowed-down situation of perisurgery stent thrombosis. Cardiovascular risk factors of the patient included systemic hypertension, smoking, obesity and diabetes mellitus. Her medication at the start of the invasive exam included acetyl-salicylic acid, amlodipin, insulin, pantoprazol and citalopram.
Renal function was normal. Left ventricular ejection fraction was 62% and without regional wall motion abnormalities. Coronary angiography (start time 8:54 AM) revealed a completely collateralized (angiographic degree 3 of 0–3) ostial CTO of the right coronary artery; there was a 75% diameter stenosis of the first diagonal branch of the left anterior descending artery and a 50% stenosis of the ostial as well as an 80% stenosis of the mid left circumflex coronary artery. Fractional flow reserve in the left circumflex coronary artery with the pressure sensor located downstream of the more distal stenosis was 0.80. Before PCI, the patient received 5000 U of heparin. PCI (6F AL1 guiding catheter) of the first diagonal branch and of both stenotic lesions of the left circumflex coronary artery was performed (Figure 1A); three everolimus-eluting stents were implanted (2.5 × 28 mm in the diagonal branch, 2.5 × 28 mm in the ostial and 2.5 × 12 mm in the mid-left circumflex coronary artery, overlapping); end of PCI: 10:47 AM. At 10:57 AM, 600 mg of clopidogrel per os was given (treatment with clopidogrel following PCI is standard at our institution). Right femoral artery closure was performed by compression. The patient developed a vasovagal reaction (nausea, sweating, bradycardia and hypotension) 30 min later, which was successfully treated with 1 mg intravenous atropine. At 11:45 AM, the patient complained about chest pain and lost consciousness with development of shock immediately thereafter.
Cardiac resuscitation was initiated right away and the patient was intubated within 10 min. At 12:05 PM, the invasive re-examination commenced and the left coronary angiogram revealed stent thrombosis involving the left main stem (Figure 1B), which was treated by balloon angioplasty. At 12:45 PM and under continuous resuscitation, a left ventricular assist device (TandemHeart®) was implanted. At 1:32 PM, reanimation was terminated due to insufficient left ventricular mechanical action and asystole.
In summary, long and small-sized ostial, overlapping stents were implanted in an elderly, diabetic female patient with diabetes mellitus, three-vessel CAD with a well collateralized ostial CTO of the right coronary artery. The patient developed vasovagal reaction in the context of groin compression and went into shock while still being in the catheterization laboratory and was not resuscitable despite the use of a left ventricular assist device.
▪ Interpretation
The initiating event leading to ostial left circumflex coronary stent thrombosis with extension into the left main stem was the vasovagal reaction in the context of groin compression. At the time when the vasovagal reaction occurred, the patient was not under full dual antiplatelet therapy, because clopidogrel was given only 30 min earlier; peak plasma levels of the main metabolite of clopidogrel occur approximately 1 h after dosing (i.e., in this regard analogous to early stop of clopidogrel before surgery). All conditions of Virchov’s triad explaining the pathophysiology of thrombosis were fulfilled: there was diminished coronary perfusion, endothelial denudation due to stent implantation and enhanced blood coagulation because of insufficient platelet inhibition. Due to the complete collateral supply of the entire right coronary artery territory, all of the myocardium, including that of the right ventricle, was cut short of any blood supply in the context of the left main coronary occlusion. In the presence of right ventricular infarction, left ventricular assist device was ineffective due to insufficient hemoglobin oxygenation.
Risk factors for & prevention of stent thrombosis
The above case history provides information about the majority of risk factors for stent thrombosis, and in this context, it could have been prevented by not performing PCI. Seven out of 12 risk factors were present in the patient described: she was an elderly patient, who suffered from diabetes mellitus and antiplatelet therapy was started late after stent implantation (equivalent to early stop); risk factors in regard to PCI were: stenting of an ostial lesion, long stents, overlapping stents and small-sized stents ≤2.5 mm [7]. Risk factors for stent thrombosis, in addition to those described in the patient are: insufficient response to antiplatelet therapy, renal insufficiency, low left ventricular ejection fraction, acute coronary syndrome and a suboptimal PCI result [7].
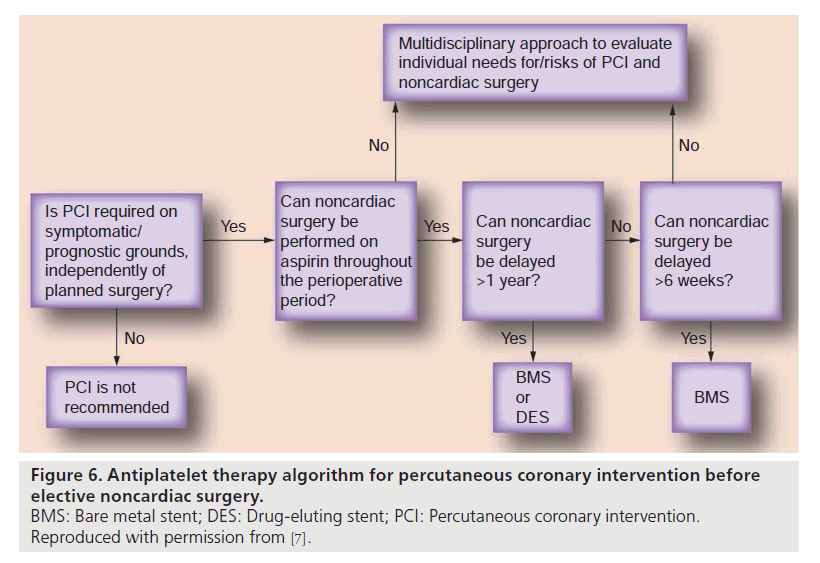
Preventive actions against stent thrombosis are mainly effective among patients undergoing elective as opposed to urgent, unexpected surgery. If they concern the antiplatelet management, prevention of stent thrombosis has to be balanced against the risk of bleeding, the latter of which is obviously dependent on the type of surgery (see below). In the context of elective surgery, the most effective way of preventing stent thrombosis is not to perform PCI if possible (Figure 6). The widespread use of PCI before noncardiac surgery attempting to reduce the rate of perioperative cardiac complications has decreased, because it has not been demonstrated to be effective when performed solely for this purpose [21]. Preoperative PCI is, however, indicated in the following circumstances [22]: acute ST-elevation myocardial infarction, non- ST-elevation myocardial infarction or high-risk unstable angina pectoris, stable angina pectoris with left main stem stenosis, stable angina pectoris with three-vessel CAD, stable angina pectoris with two-vessel CAD involving proximal left anterior descending artery and left ventricular ejection fraction <50% or demonstrable ischemia. Considering the above case presentation, the guidelines for preoperative PCI give room for interpretation: the absence of a significant stenosis in the left anterior descending artery of the patient could have allowed the definition of a two-vessel CAD. In this case, the obtained fractional flow reserve value of 0.80 in the left circumflex coronary artery would have been taken for absent ischemia and the decision not to perform PCI would have been justifiable. Conversely, the 75% stenosis of the first diagonal branch was sufficient to allow the definition of a three-vessel CAD.
As an alternative to omit PCI entirely for preventing perioperative stent thrombosis, mere balloon angioplasty can be attempted. The primary risk factor for perioperative stent thrombosis, aside from PCI, is the early deviation from the recommended dual antiplatelet therapy: ≥1 month acetylsalicylic acid plus clopidogrel in case of BMS implantation, ≥12 months acetylsalicylic acid plus clopidogrel in case of DES implantation, ≥12 months acetylsalicylic acid plus clopidogrel in case of an acute coronary syndrome irrespective of the type of stent used [2]. However, very recent data suggest that 6 months of dual antiplatelet treatment following DES implantation is likely equivalent to 24 months [23].
The antiplatelet conundrum
The puzzle of perioperative antiplatelet therapy is caused by the multitude of variables influencing the outcome of either excess bleeding during surgery or stent thrombosis. In the context of elective surgery, the number of variables is primarily related to the three questions, whether dual antiplatelet therapy (i.e., the gold standard for preventing stent thrombosis) can be fully maintained during surgery, whether it has to be replaced by mono antiplatelet therapy (aspirin 100 mg daily), or whether it has to be entirely stopped (Figure 6). To answer these questions, the different types of surgery have to be taken into account. The perioperative bleeding risk is usually not increased in minor surgical interventions including dental procedures, cataract surgery, dermatologic operations, angiographic and endoscopic diagnostic procedures, which all can be performed under dual antiplatelet therapy [24].
Conversely, surgical procedures in closed space such as intracranial surgery, posterior eye chamber surgery or medullary canal surgery or when major bleeding complications are to be expected and even require antiplatelet monotherapy to be stopped 5–7 days preoperatively [24]. In the context of vascular, visceral and transbronchial surgery, an increase in hemorrhagic risk has been consistently reported [25,26], the fact of which requires a change from dual to mono antiplatelet therapy with clopidogrel administration ceased 5 days before surgery. Of course, altering antiplatelet therapy is necessary only when the abovementioned surgical procedures are elective, but cannot be delayed >6 weeks after BMS implantation or >12 months after DES implantation (Figure 6). Therefore, one of the crucial questions to be answered in the context of stent thrombosis prevention, aside from the indication of PCI, is whether surgery can be deferred.
In patients undergoing unexpected, urgent or emergent surgery after stent implantation, stopping antiplatelet therapy will not diminish platelet inhibition in a timely fashion and therefore, not mitigate the bleeding risk [24]. Therefore, the management focus is primarily directed towards treatment of hemorrhagic complications using platelet transfusion and/or other procoagulant interventions than on the prevention of stent thrombosis. If unexpected surgery is not urgent, the question of its deferral beyond the time of necessary dual antiplatelet therapy has to be evaluated analogous to elective surgery: >6 weeks from BMS implantation and >12 months from DES implantation.
If surgery cannot be deferred beyond the mentioned time lines (exception: minor noncardiac surgery; see above), full antiplatelet therapy is required perioperatively, but acetylsalicylic acid and/or clopidogrel cannot be employed and a ‘bridging’ therapy should be considered using the short-acting platelet antagonists eptifibatitide or tirofiban [24]. However, these protocols have been evaluated only in small patient populations and there is currently no clear evidence to support their broad application. In the same context, neither unfractionated heparin nor low-molecular-weight heparin can provide the antiplatelet effects of acetylsalicylic acid and/or clopidogrel.
The role of new antiplatelet drugs, such as the irreversible adenosine diphosphate receptor blocker prasugrel or the reversible P2Y12 antagonist ticagrelor with regard to the risk of perioperative stent thrombosis versus bleeding has not been defined yet [24].
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Surgery induces a prothrombotic state, thus predisposing to coronary stent thrombosis.
▪ The chance that a patient with coronary artery disease requires noncardiac surgery within 1 year after stenting is 5%.
▪ In patients undergoing elective noncardiac surgery within 1 year of coronary stenting, the rate of major adverse events mainly due to stent thrombosis is high.
▪ The poor prognosis of stent thrombosis is related to its sudden onset leaving no time for collateral development to the ischemic area.
▪ In the context of elective surgery, the most effective way of preventing stent thrombosis is not to perform percutaneous coronary intervention if possible.
▪ For prevention of stent thrombosis, dual antiplatelet therapy with acetylsalicylic acid and clopidogrel is given for ≥1 month after bare metal stent and for ≥12 months after drug-eluting stent implantation or after percutaneous coronary intervention in the event of acute coronary syndrome.
▪ Perioperative bleeding risk is usually not increased in minor surgical interventions such as dental procedures, cataract surgery, dermatologic operations, angiographic and endoscopic diagnostic procedures, which all can be performed under dual antiplatelet therapy.
▪ Surgical procedures in a closed space (intracranial surgery, posterior eye chamber surgery or medullary canal surgery) even require antiplatelet monotherapy to be stopped.
▪ Vascular, visceral and transbronchial surgery require a change from dual to mono antiplatelet therapy with a stop of clopidogrel 5 days before surgery.
▪ In patients undergoing unexpected surgery after stent implantation, stopping antiplatelet therapy will not diminish the bleeding risk.
References
- Serruys PW, de Jaegere P, Kiemeneij F et al. A comparison of balloon expandable- stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 331, 489–495 (1994).
- Silber S, Albertsson P, Avilés F et al. Guidelines for percutaneous coronary interventions. The Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur. Heart J. 26, 804–847 (2005).
- Gaglia MA, Waksman R. Systematic review of thienopyridine discontinuation and its impact upon clinical outcomes. Eur. Heart J. 32, 2358–2364 (2011).
- Vicenzi MN, Meislitzer T, Heitzinger B, Halaj M, Fleisher LA, Metzler H. Coronary artery stenting and non-cardiac surgery – a prospective outcome study. Br. J. Anaesth. 96, 686–693 (2006).
- Chechi T, Vecchio S, Vittori G et al. ST-segment elevation myocardial infarction due to early and late stent thrombosis a new group of high-risk patients. J. Am. Coll. Cardiol. 51, 2396–2402 (2008).
- Cutlip DE, Windecker S, Mehran R et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115, 2344–2351 (2007).
- Luckie M, Khattar RS, Fraser D. Non-cardiac surgery and antiplatelet therapy following coronary artery stenting. Heart 95, 1303–1308 (2009).
- Antoniucci D, Valenti R, Moschi G et al. Relation between preintervention angiographic evidence of coronary collateral circulation and clinical and angiographic outcomes after primary angioplasty or stenting for acute myocardial infarction. Am. J. Cardiol. 89, 121–125 (2002).
- Cutlip DE, Baim DS, Ho KK et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 103, 1967–1971 (2001).
- Wenaweser P, Rey C, Eberli FR et al. Stent thrombosis following bare metal stent implantation: success of emergency percutaneous coronary intervention and predictors of adverse outcome. Eur. Heart J. 26, 1180–1187 (2005).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293, 2126–2130 (2005).
- Ong AT, Hoye A, Aoki J et al. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after baremetal, sirolimus, or paclitaxel stent implantation. J. Am. Coll. Cardiol. 45, 947–953 (2005).
- Alfonso F. The ‘vulnerable’ stent. Why so dreadful? J. Am. Coll. Cardiol. 51, 2403–2406 (2008).
- Pohl T, Seiler C, Billinger M et al. Frequency distribution of collateral flow and factors influencing collateral channel development. Functional collateral channel measurement in 450 patients with coronary artery disease. J. Am. Coll. Cardiol. 38, 1872–1878 (2001).
- Seiler C. Collateral Circulation of the Heart (1st Edition). Springer, London, UK (2009).
- Perera D, Kanaganayagam GS, Saha M, Rashid R, Marber MS, Redwood SR. Coronary collaterals remain recruitable after percutaneous intervention. Circulation 115, 2015–2021 (2007).
- Meier P, Zbinden R, Togni M et al. Coronary collateral function long after drug-eluting stent implantation. J. Am. Coll. Cardiol. 49, 15–20 (2007).
- Billinger M, Fleisch M, Eberli FR, Garachemani A, Meier B, Seiler C. Is the development of myocardial tolerance to repeated ischemia in humans due to preconditioning or to collateral recruitment? J. Am. Coll. Cardiol. 33, 1027–1035 (1999).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4‑year results from a large 2‑institutional cohort study. J. Am. Coll. Cardiol. 52, 1134–1140 (2008).
- Mauri L, Massaro JM, Jiang S et al. Long-term clinical outcomes with zotarolimus-eluting versus bare-metal coronary stents. J. Am. Coll. Cardiol. Cardiovasc. Interv. 3, 1240–1249 (2010).
- Poldermans D, Schouten O, Vidakovic R et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE‑V pilot study. J. Am. Coll. Cardiol. 49, 1763–1769 (2007).
- Fleisher LA, Beckman JA, Brown KA et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. J. Am. Coll. Cardiol. 50, 1707–1732 (2007).
- Valgimigli M, Campo G, Percoco G et al. Randomized comparison of 6‑ versus 24‑month clopidogrel therapy after balancing anti-intimal hyperplasia stent potency in all-comer patients undergoing percutaneous coronary intervention design and rationale for the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia study (PRODIGY). Am. Heart J. 160, 804–811 (2010).
- Korte W, Cattaneo M, Chassot PG et al. Peri-operative management of antiplatelet therapy in patients with coronary artery disease: joint position paper by members of the working group on Perioperative Haemostasis of the Society on Thrombosis and Haemostasis Research (GTH), the working group on Perioperative Coagulation of the Austrian Society for Anesthesiology, Resuscitation and Intensive Care (ÖGARI) and the Working Group Thrombosis of the European Society for Cardiology (ESC). Thromb. Haemost. 105, 743–749 (2011).
- Moore M, Power M. Perioperative hemorrhage and combined clopidogrel and aspirin therapy. Anaesthesiology 101, 792–794 (2004).
- Ernst A, Eberhardt R, Wahidi M, Becker HD, Herth FJ. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest 129, 734–737 (2006).