Case Report - Interventional Cardiology (2021) Volume 13, Issue 2
PFO, push-ups and heavy lifting; valsalva provocation before cryptogenic stroke
- Corresponding Author:
- A. Salerian
Department of Clinical Neurosciences, Tulane University School of Medicine, New Orleans, Louisiana,USA,
E-mail: jsaleria@tulane.edu
Received date: January 18, 2021 Accepted date: February 01, 2021 Published date: February 08, 2021
Abstract
Background: Patent Foramen Ovale (PFO) can serve as a conduit for paradoxical embolus or as a nidus itself for thrombus formation in Cryptogenic Stroke (CS). Debate for PFO closure is ongoing but favors closure in well-selected patients. Four randomized-control trials demonstrated a reduction in stroke recurrence with PFO closure. Patients were selected with high-risk PFO features in DEFENSE-PFO and CLOSE trials.
Cases: This case series describes four patients with CS immediately following or during exercise- induced-Valsalva: Push-ups, bench pressing, and lifting heavy boxes. PFOs with severe shunting during Valsalva were demonstrated with bubble echocardiograms in all cases.
Conclusion: Recent clinical trials have shown benefit of percutaneous PFO closure, especially in younger patients with high-risk PFO features. Valsalva-like straining may provide the hemodynamic terminal-step for embolism to occur in CS patients with PFO. Future PFO-closure screening tools should consider Valsalva-like straining as a criterion towards closure.
Keywords
Stroke • Patent foramen ovale • RoPE score • PFO closure
Introduction
Stroke is a leading cause of morbidity and mortality in adults in the U.S. Approximately 25%-30% of ischemic strokes are classified as “cryptogenic” when no definitive etiology or identifiable source is found. Cryptogenic Stroke (CS) is a diagnosis of exclusion; a complete work-up must include evaluation for large vessel artery disease, small vessel artery disease, cardiac abnormalities, and dysrhythmias. Particularly in younger patients, evaluation of other causes such as thrombophilias or rheumatological disorders may be appropriate.
One proposed mechanism of CS is via a Patent Foramen Ovale (PFO), a cardiac defect arising from a persistent component of fetal circulation serving as a conduit for paradoxical embolization of small venous thrombi, or thrombus formation within the conduit itself. While 25% of the general adult population has a PFO [1,2], that prevalence increases up to 60% in patients with CS [3,4].
Attributing causation of CS to a PFO can be a challenge. The Risk of Paradoxical Embolism (RoPE) score is an objective estimate of the probability that a PFO in a CS patient is incidental or pathogenic [5]. Younger patients with fewer cardiovascular risk factors have a higher score. With a maximum score of 10, the index estimates a 90% chance that the stroke is attributable to the PFO. Nearly 80% of CS patients with a RoPE score greater than 6 had the presence of a PFO [5]. However, the score has limitations and should not be used in isolation. Lower scores have been associated with higher stroke recurrence, likely influenced by traditional cardiovascular risk factors [6,7]. Additionally, the score does not take into account high-risk anatomic features such as large shunt size, Atrial Septal Aneurysm (ASA), or hypermobility of the septum that may be better markers for pathogenicity [8,9].
The debate of PFO closure after CS has been ongoing for over a decade. Several studies such as RESPECT, CLOSURE I and PC suggested there was no benefits [10-12]. However, multiple recent The debate of PFO closure after CS has been ongoing for over a decade. Several studies such as RESPECT, CLOSURE I and PC suggested there was no benefits [10-12]. However, multiple recent randomized control trials have demonstrated a significant reduction in stroke recurrence with PFO closure compared to antithrombotic therapy alone, perhaps owing to better patient selection [9,13-15]. DEFENSE-PFO and CLOSE trials in particular only included CS patients with high-risk PFO features, such as large inter-atrial shunts or the presence of ASA, thus reinforcing the need for better selection tools. Recently, the American Academy of Neurology updated their guidelines to recommend PFO closure in patients <60 with cryptogenic stroke where no other higher risk cause of stroke is elucidated [16].
There are several possible mechanisms certain PFOs may be pathogenic. Authors have proposed that PFOs with anatomical features such as a long-tunneled PFO, ASA, or chiari’s network may in fact themselves be thrombogenic [17,18]. These features may act as a nidus for in-situ clot formation due to slow or turbulent blood flow [17,18].
Classically it has been thought that PFOs may act as conduit for a pre-formed clot-in-transit: paradoxical embolization. This involves a dynamic right-to-left (R-L) cardiac shunt whenever the pressure in the right atrium exceeds that of the left atrium. This transient flow gradient occurs physiologically at rest during early diastole; it is inducible by the Valsalva maneuver or any straining- activity such as heavy lifting, moving bowels, sexual intercourse, coughing, sneezing, or vomiting, which elicit a disproportionate increase in intrathoracic pressure. In one case series of 148 patients with PFO, a R-L shunt was observable in 57% of patients at rest compared to 92% of patients while straining [3]. Several authors have demonstrated cryptogenic stroke is associated with preceding valsalva-like straining [19-21]. Regardless of whether a thrombus is in-transit via the PFO or formed in-situ, it is conceivable that a valsalva maneuver could play the terminal step in its forward propagation.
In this case series, we present four patients who presented to our facility with acute ischemic stroke that was preceded by a Vasalva- like-straining; intense push-ups or heavy weight-bearing exercise. All were found to have a PFO with a large R-L shunt, notably exacerbated by Valsalva maneuver with microbubbles in the left atrium that were too numerous to count within three cardiac cycles on bubble echocardiogram. For reference, the CLOSE trial considered a shunt to be large when there were greater than 30 microbubbles present in the left atrium. Valsalva-like straining may have led to the hemodynamic conditions for embolization to occur.
Case Presentation
Case 1
A previously healthy 20-year-old male presented after sudden onset expressive aphasia, fragmented speech, inability to write at school. The night prior he had been intensely weightlifting, “maxing out” his shoulder press, whereby he lifted more weight in one set than he ever had before. His National Institutes of Health Stroke Scale (NIHSS) was two on arrival at our center. He was out of the therapeutic window for thrombolytics so he was loaded with Dual Antiplatelet Therapy (DAPT) with aspirin and clopidogrel. Brain MRI demonstrated acute infarcts involving left frontal cortex and corona radiata. Transesophogeal Echocardiogram bubble study (bTEE) confirmed a PFO with a moderate R-L shunt at rest, and a large shunt with Valsalva. Additionally, he was found to have an ANA titer of 1:320, mixed homogeneous and speckled pattern.
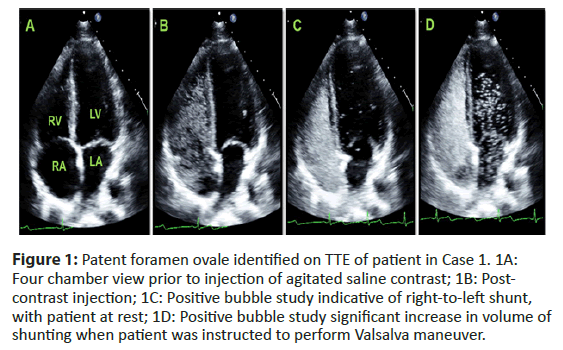
His expressive aphasia significantly improved over his 3-day hospitalization. He was discharged home on rivaroxaban 20 milligrams daily [4]. Months later, he had percutaneous endovascular closure of the PFO and was without further strokes at one year (Figure 1).
Figure 1: Patent foramen ovale identified on TTE of patient in Case 1. 1A: Four chamber view prior to injection of agitated saline contrast; 1B: Post- contrast injection; 1C: Positive bubble study indicative of right-to-left shunt, with patient at rest; 1D: Positive bubble study significant increase in volume of shunting when patient was instructed to perform Valsalva maneuver.
Case 2
A previously healthy 34-year-old US Army service-woman on hormonal contraceptives presented with sudden onset left-sided weakness and hemianopsia while doing push-ups. She was given intravenous tissue plasminogen activator (IV tPA) with an NIHSS of five.
Brain MRI demonstrated acute right thalamic infarct. bTEE revealed a PFO with a moderate R-L shunt at rest, and a large shunt with Valsalva. Additional work-up revealed mildly elevated Anticardiolipin IgM antibody of 21 antiphospholipid (APL) units, with normal IgG. On hospital-day five she was discharged home on daily ASA 81 mg, Plavix 75 mg, and atorvastatin 20 mg.
She presented four days later with acute worsening of residual leftsided numbness and weakness and new onset aphasia during mildmoderate aerobic exercise and MRI demonstrated new embolicappearing infarcts in the right thalamus and right inferior occipital lobe. She underwent PFO closure during that hospitalization and was ultimately discharged home on apixaban 5 mg twice daily and atorvastatin 20 mg.
At 6-month follow-up, she reported no new stroke-like episodes. Repeat echocardiogram confirmed PFO closure with no residual shunt. Her Anticardiolipin IgM decreased to 17 APL units.
Case 3
A previously healthy 49-year-old male presented with word-finding difficulty following a transient episode of right-sided weakness and right facial droop immediately after doing push-ups and bench-pressing 300 pounds in rapid succession. NIHSS was 1 on arrival. He did not receive IV tPA, as his symptoms had markedly improved; he was loaded with Asa 325 mg and clopidogrel 300 mg.
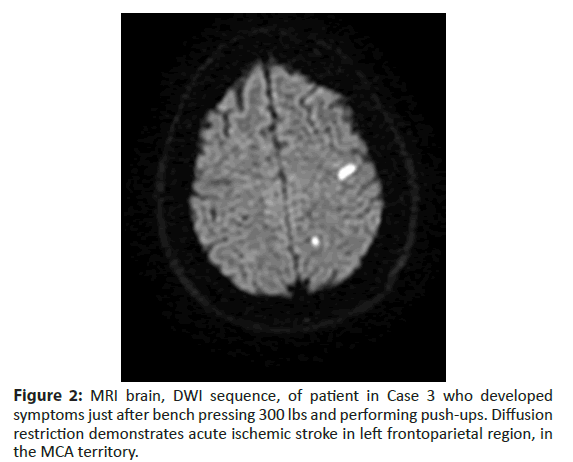
Brain MRI revealed scattered multifocal infarcts within left frontal and parietal lobes and chronic infarcts in the right cerebellum. bTEE demonstrated a PFO with a moderate R-L shunt at rest, and a large shunt with Valsalva. He was discharged home on apixaban 5 mg twice daily and atorvastatin 80 mg. He received an implantable loop monitoring (ILR) device to evaluate for occult atrial fibrillation, which was negative after four months and he subsequently underwent PFO closure. He continued anticoagulation for three months post-closure and was subsequently changed to Aspirin 81 mg. No atrial fibrillation was detected at one year (Figure 2).
Figure 2: MRI brain, DWI sequence, of patient in Case 3 who developed symptoms just after bench pressing 300 lbs and performing push-ups. Diffusion restriction demonstrates acute ischemic stroke in left frontoparietal region, in the MCA territory.
Case 4
A 60-year-old male smoker with a history of hypertension and asthma presented with sudden onset double vision, slurred speech and right-sided weakness immediately following intense lifting of heavy boxes for several hours. On arrival, he was severely dysarthric with left gaze preference and dense right hemiplegia with an NIHSS of 17. He received IV tPA.
Brain MRI demonstrated acute left thalamic infarct and evidence of chronic cerebral infarcts bilaterally in the hemispheres. bTEE revealed an atrial septal aneurysm and PFO with a large R-L shunt at rest, severely exacerbated with Valsalva. His dysarthria, diplopia and hemiparesis resolved over the 6-day hospitalization to NIHSS was zero. He was discharged home on apixaban 5 mg twice daily, aspirin 81 mg daily, and atorvastatin 80 mg daily.
Results and Discussion
We have presented four patients with cryptogenic strokes preceded by Valsalva-like-straining such as intense pushing or heavy lifting just prior to, or during symptom onset; each patient had a PFO with a large R-L shunt, exacerbated during Valsalva maneuver. The authors propose that such exercises may have increased intrathoracic pressure, worsening the R-L shunt, thus creating the terminal conditions for embolism to occur. Notably, three of the four patients are under the age of 50 and had a RoPE score of at least eight, suggesting an 84% chance that the stroke is related to the PFO.
There are some confounding limitations. In the second case, the woman was on hormonal contraceptives and was positive for anticardiolipin IgM antibodies with persistent elevation at three months. While this could have produced a pro-coagulable state that increased her risk of paradoxical embolism, it could have also increased her risk of primary thrombotic stroke. Interestingly, Liu et al. recently demonstrated that PFO closure was superior to medical therapy alone in patients with underlying thrombophilia’s [22], furthering the idea that a PFO may just be the terminal step of a complex pathogenesis (Table 1).
| Case | Patient Demographics | Straining Activity Associated with Symptoms | Stroke Location | PFO Characteristics (per TEE) | CTA Head and Neck | RoPE Score* | Stroke Risk Factors | Hypercoagulable, Rheumatological Labs |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 yo Caucasian Male | Weight-lifting: Shoulder press | Left frontal cortex, corona radiata | Moderate R-L shunt at rest Large shunt with Valsalva | Normal | 9(88%) | A1c: 5.8% LDL: 65 HDL: 44 TSH: 1.15 |
ANA titer 1:320 (mixed homogeneous and speckled pattern) |
| 2 | 34 yo Caucasian Female | Push-ups | Right thalamus, occipital cortex | Moderate R-L shunt at rest Large shunt with Valsalva | Normal | 8(84%) | A1c: 4.7% LDL: 102 HDL: 90 TSH: 0.759 |
Anticardiolipin IgM: 21 Anticardiolipin IgM: Normal |
| 3 | 49 yo African American Male | Weight-lifting: Bench press (300 lbs), push-ups | Left frontal cortex, corona radiata | Moderate R-L shunt at rest Large shunt with Valsalva | Normal | 8(84%) | A1c: 5.6% LDL: 158 HDL: 44 TSH: 2.57 |
Normal |
| 4 | 60 yo African American Male | Lifting Heavy Boxes | Left thalamus | Large R-L shunt at rest, worsened shunting with Valsalva Atrial septal aneurysm present | Normal | 3(0%) | Former Smoker HTN A1c: 6.2% LDL: 61 HDL: 42 TSH: 0.451 | Normal |
PFO: Patent Formen Ovale; TEE: Trans-esophageal echocardiogram; CTA: Computed Tomography Angiography; *RoPE Score relates to percent chance stroke is attributable to PFO; A1c: Hemoglobin A1c. LDL: Low-Density Lipoprotein; HDL: High-Density Lipoprotein; TSH: Thyroid Stimulating Hormone; HTN: Hypertension. Large shunt: >30 microbubbles observed in the left atrium with 3 cardiac cycles. Moderate shunt: >10 mm excursion of septum into the left atrium
Table 1: Summary of cases.
In Case 4, the 60-year-old male had multiple cardiovascular risk factors and his RoPE score was 3, which comports with a low probability that his CS was attributable to the PFO. The presence of severe resting inter-atrial shunt and an atrial septal aneurysm make PFO the more likely etiology. Additionally, there was a clear temporal relationship between the Valsalva-inducing-exercise with symptom onset. This patient further illuminates the shortcomings in the RoPE score.
Several prospective randomized trials demonstrated benefit with percutaneous PFO closure, particularly in younger patients without cardiovascular risk factors, and with the aforementioned high-risk PFO features [9,13-15]. In the CLOSE trial, which selected only patients with large inter-atrial shunts or atrial septal aneurysm: none of patients in the PFO closure group had stroke recurrence over the five year follow up period, versus 5.9% in the antiplatelet only group (P<0.001) [13]. Likewise in DEFENSE-PFO, which only selected patients with atrial septal aneurysm, large PFO or hypermobility of the septum: none had stroke recurrence in the intervention group vs. 10.5% in the medical arm in a 2 year follow up period (P=0.023) [9]. These studies demonstrated the most robust treatment response when compared to their predecessors, perhaps in part because of stricter inclusion criteria (Table 2).
| Trail (Year Published) | N | Follow-Up | Patient and PFO Baseline Characteristics | lntervention Arm: PFO Closure | Control Arm(s): Medical Therapy | Primary Outcome Measures | Results | ||
|---|---|---|---|---|---|---|---|---|---|
| Event Rate: PFO Closure Arm vs. Medical Therapy Arm | NNT to Prevent One Stroke | Conclusions | |||||||
| RESPECT: Long Term (2017) | 980 | Median: 5.9 years | Patients 18-60 years with CS and PFO* | Amplatzer PFO Occluder | Aspirin, Clopidogrel, Warfarin, or Aspirin with extended-release Dipyridamole | Composite of recurrent non-fatal ischemic stroke, fatal ischemic stroke, or early death after randomization | Recurrent stroke: 3.6% vs.5.8%(P=0.46) | 42 in 5 years | Closure is superior to antiplatelets therapy alone on extended follow up on intention-to-treat analysis |
| REDUCE: (2017) | 664 | Median: 3.2 years | Patients 18-59 years with CS and PFO** | Helex Septal Occluder and Cardiolorm Septal Occluder | Aspirin, Clopidogrel, Warfarin, or Aspirin with Dipyridamole | 1. Recurrent stroke 2. New brain infarct (clinical ischemic stroke or silent infarct) |
Recurrent stroke: 1.4% vs.5.4% (P=0.002) New brain infarct: 5.7% vs.11.3% (P=0.04) | 25 in 3.2 years | Closure is superior to antiplatelets therapy alone |
| CLOSE (2017) | 663 | Mean: 5.3 years | Patients 16-60 years with CS and a PFO associated with ≥ 10 mm atrial septal aneurysm or large interatrial shunt, as defined by >30 microbubbles in the left atrium in <3 cardiac cycles | Any CE marked PFO device | 1. Antiplatelet: Aspirin, Clopidogrel, or Aspirin with extended-release Dipyridamole 2. Oral Anticoagulant: Vitamin K antagonists or NOACs | Recurrent stroke (fatal or non-fatal) | (Closure vs. Antiplatelet) Recurrent stroke: 0% vs.5.9% (P=<0.001) (Anticoagulant vs. Antiplatelet) Recurrent stroke: 1.5% vs. 3.8% (P=0.17) | 16.9 in 5.3 years | Closure is superior to antiplatelet therapy alone in patients with PFO and associated atrial septal aneurysm or large interatrial shunt. Anticoagulant is equivalent to antiplatelet |
| DEFENSE-PFO (2018) | 120 | Median: 2.8 years | Patients with CS and PFO with high-risk features: Atrial septal aneurysm(15 mm protrusion), hypermobility of the septum (10 mm excursion), or PFO size (>2 mm) | Amplatzer PFO Occluder | Aspirin, Aspirin and Clopidogrel, Aspirin and Cilostazol, or Warfarin | Composite of stroke, vascular death, or thrombolysis in myocardial infarction defined major bleeding | Recurrent ischemic stroke: 0% vs. 10.5% (P=0.023) | 10 in 2 years | Closure in patients with high-risk PFO features resulted in lower rate of ischemic stroke vs. medical therapy |
ESPECT: Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. REIDUCE: Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. CLOSE: Patent Foramen Ovale Closure or Anticoagulation Versus Antiplatelet after Stroke. DEFENSE-PFO: Cryptogenic Stroke and High-Risk Patent Foramen Ovale; PFO: Patent Foramen Ovale; CS: Cryptogenic Stroke; CE: Conforme Europeenne. NOAC: Non-vitamin K antagonist oral anticoagulant NNT: Number Needed to Treat.
*Note: In "Final Results," 48.8% of patients had PFO with substantial shunt (i.e. shunt size grade 3); 35.7% with atrial septal aneurysm.
**Note: In "Final Results," 81% of patients had PFO with moderate or large interatrial shunt (as defined by 6-25 or 25+ microbubbles, respectively); 20% with atrial septal aneurysm
Table 2: PFO clinical trials.
Patient selection for PFO closure will be important for future management of these patients. One proposed screening tool is the RoPE score but it has not yet been validated for this purpose. In at least one meta-analysis it has failed to correlate with eligibility criteria outlined in CLOSE, REDUCE or RESPECT trials [23]. One major limitation of the score is that it does not take into account various features associated with PFO, such as shunt size, presence of an atrial septal aneurysm, or endothelial function. While some of these features including large shunt or atrial septal aneurysm have been associated with increased risk of stroke, the role of PFO promoting endothelial dysfunction has been controversial. One study suggested a PFO facilitates impairment of endothelial function acutely by the transfer of microbubbles into the arterial circulation [24] and other suggested improved endothelial function in PFO patients compared to patients without PFO [25]. Scicchitano et al. showed no change in endothelial function after PFO closure compared to ASD closure showing improved function [26]. Nevertheless most relevant to this series, the ROPE score also does not take into account clinical provoking factors such as Valsalva-straining prior to the patient’s stroke.
Conclusion
PFO closure is likely beneficial in younger patients with cryptogenic stroke who have a PFO with high-risk features. These four patients had high-risk PFO features and cryptogenic stroke that was preceded by exercise induced Valsalva just prior or during symptom onset. Future screening tools selecting CS patients for PFO closure, may warrant the addition of high-risk PFO characteristics in order to comport with the most current clinical trial data. It may also be worth considering a clinical question as well: “Did Valsalva-provocation occur prior to symptom onset?”
Acknowledgement
Written informed consent was obtained from the individual subjects of any potentially identifiable images or data included in this article.
References
- Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clinic Proceedings. 59(1): 17–20 (1984).
- Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: The SPARC Study. Mayo Clinic Proceedings. 74(9): 862–869 (1999).
- Lechat P, Mas J, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 318(18): 1148–1152 (1988).
- Saver JL, Mattle MP, Thaler D. Patent foramen ovale closure versus medical therapy for cryptogenic ischemic stroke. Stroke. 49(6): 1541–1548 (2018).
- Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 81(7): 619–625 (2013).
- Wessler BS, Kent DM, Thaler DE, et al. The RoPE score and right-to-left shunt severity by transcranial doppler in the CODICIA Study. Cerebrovasc Dis. 40(1-2): 52–58 (2015).
- Thaler DE, Ruthazer R, Weimar C, et al. Recurrent stroke predictors differ in medically treated patients with pathogenic vs other PFOs. Neurology. 83(3): 221–226 (2014).
- Morais LA, Sousa LD, Fiarresga A, et al. RoPE score as a predictor of recurrent ischemic events after percutaneous patent foramen ovale closure. Int Medical J. 59(6): 1327–1332 (2018).
- Lee PH, Song J-K, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: The DEFENSE-PFO trial. J Am Coll Cardiol. 71(20): 2335–2342 (2018).
- Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 368(12): 1092–1100 (2013).
- Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 368(12): 1083-91 (2013).
- Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 366: 991-999 (2012)
- Mas JL, Derumeaux G, Mattle HP, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 377: 1011-1021 (2017).
- Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 377(11): 1022–1032 (2017).
- Sondergaard L, Kasner S, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 377: 1033-1042 (2017).
- Messé SR, Gronseth G, Kent DM, et al. Practice advisory: Recurrent stroke with patent foramen ovale (update of practice parameter): Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 87(8): 815-821 (2016).
- Ioannidis SG, Mitsias PD. Patent foramen ovale in cryptogenic ischemic stroke: Direct cause, risk factor, or incidental finding? Front Neurol. 11: 567 (2020).
- Aggeli C, Verveniotis A, Andrikopoulou E, et al. Echocardiographic features of pfos and paradoxical embolism: A complicated puzzle. Int J Cardiovas Imag. 34: 1849-61 (2018).
- Ozdemir AO, Tamayo A, Munoz C, et al. Cryptogenic stroke and patent foramen ovale: Clinical clues to paradoxical embolism. J Neurol Sci. 275(1-2): 121-127 (2008).
- Ferguson T, Sansing LH, Herrmann H, et al. To close or not to close: PFO, sex and cerebrovascular events. J Invasive Cardiol. 18(12): E292-E293 (2006).
- Diot E, Turot V, Lesire V, et al. Paradoxical embolism: A cause of cerebral stroke in the young subject J Mal Vasc. 23(3): 199-200 (1998).
- Liu K, Song B, Palacios IF, et al. Patent foramen ovale attributable cryptogenic embolism with thrombophilia has higher risk for recurrence and responds to closure. JACC Cardiovasc Interv. 13(23): 2745-2752 (2020).
- Salari A, Al Banna M, Lakshminarayan K, et al. Does risk of paradoxical embolism (ROPE) score correlate with PFO closure criteria? (P4.3-065). Neurology. 92(S15): 4.3–065 (2019).
- Fok H, Jiang B, Chowienczyk P, et al. Microbubbles shunting via a patent foramen ovale impair endothelial function. JRSM Cardiovasc Dis. 4(1-6): 2015.
- Rodés-Cabau J, Noël M, Marrero A, et al. Atherosclerotic burden findings in young cryptogenic stroke patients with and without a patent foramen ovale. Stroke. 40(2): 419-25 (2009).
- Scicchitano P, Gesualdo M, Cortese F, et al. Atrial septal defect and patent foramen ovale: Early and long-term effects on the endothelial function after percutaneous occlusion procedure. Heart and Vessel. 34: 1499-1508 (2019).