Research Article - Pharmaceutical Bioprocessing (2018) Volume 6, Issue 3
Phytochemical analysis, antibacterial, antifungal and insecticidal activity of Berberis royleana roots
- *Corresponding Author:
- Zeb MA
Department of Chemistry
Mohi Ud Din Islamic University
AJ & K, Pakistan
E-mail: muhammad_aurangzeb@hotmail.com
Abstract
Background: Berberis royleana is rare species belongs to genus Berberis. In the current research work the plant roots were investigated for their phytochemical analysis, antifungal, antibacterial and insecticidal activities.
Materials: B. royleana roots were collected from Trarkhal, Azad Kashmir, Pakistan in July 2017. Extraction, fraction preparation and phytochemical analysis were done using standard procedures. Antibacterial and antifungal activities of the plant roots were checked against the bacterial strains E. coli, C. freundii, K. pneumoniae, V. pestis, S. aureus, P. aeruginosa and S. typhi and fungal strains A. flavus, A. niger, Penicillium and White rot fungi. Also, the insecticidal activities of fractions were tested by direct contact application process of the fractions against insects Kelosobrocus meleticulatis at three different time intervals.
Results: Phytochemical analysis of the fractions identified the presence of alkaloids, tannins, anthraquinones, glycosides, reducing sugars, saponins, flavonoids, phlobatanins, steroids, terpenoid in the roots of B. royleana. All of the fractions showed inhibition against the growth of tested bacterial strains. The chloroform fraction showed maximum inhibition 39 mm against E. coli while the n-hexane fraction exhibits minimum inhibition 10 mm against S. aureus. The crude fractions n-hexane, chloroform, ethyl acetate and water showed significant antifungal activity against tested fungal strains. The crude fractions were tested for insecticidal activity at different time intervals 24, 48 and 72 hours. The maximum mortality rate was shown by water fraction which was recorded to be 60% at 72 hours intervals.
Conclusion: This study showed that the roots of plant B. royleana are very important from medicinal point of view and should be further explore for phytochemicals and their biological potentials.
Keywords
Berberis royleana, phytochemicals, antibacterial, antifungal, insecticidal
Introduction
The genus Berberis belongs to the family Berberidaceae is comprised of approximately 500 species distributed in Siberia, Nepal, Afghanistan, China, India, Europe, North and South America [1]. The genus Berberis species being comprised of alkaloids are very important from pharmacological point of view. Some species of this genus are comprised of important constituent and are used in herbal medication systems such as in Unani, Ayurvedic, Eastern and modern system of medicines [2,3]. It has been reported that these species phytochemical constituents act against jaundice, infectious diseases, ocular trachoma, AIDS, diarrhea, enlargement of spleen, eye troubles, dysentery, diabetes, osteoporosis, cancer, leprosy, bone fractures, cholera, cardiovascular ailments and hypertension etc. [4]. Various disease such as fever, stones of gall bladder and kidney, rheumatism, eye disease etc. are cured by other species of genus Berberis since ancient times due to the presence of berberine alkaloids which are biologically active. The different parts of all species of genus Berberis have been found to possess various biological activities like anti-inflammatory, anti-emetic, anti-oxidant, anti-pyretic, anti-microbial, sedative, anti-arrhythmic, anti-cholinergic, anti-leishmaniasis, cholagogic and anti-malaria. The major phytochemicals detected in various species of Berberis are berbamine and berberine [5].
B. royleana is rare species amongst the members of Berberis (Berberidaceae). This specie is still imperfectly known and the flower is not specified. It differs from other species of the genus by its smaller leaves, narrow fruits, inflorescence and pedicels [6]. B. royleana is a deciduous plant with height up to 3 m. The roots are thick and broaden easily. The stem has red-brown color and has spines. Leaves are usually 7-15 mm long, 6-12 mm broad. The ripe fruit is ovoid with red color and about 1 cm in length. The fruits developed in clusters form are bitter to taste. Berries are somewhat black, pruinose grey, oblong, 8 mm long and 3.5 mm broad (immature) [6]. Keeping in view the above mentioned pharmacological importance of genus berberis, its species B. royleana was selected for phytochemical and biological investigation to further explore its hidden medicinal potential.
Material and methods
Plant material
B. royleana roots were collected from Trarkhal, Azad Jammu and Kashmir, Pakistan in July 2017. A voucher specimen number BRZ-31 of the plant sample was kept as a record in the Department of Botany, Abdul Wali Khan University, Pakistan.
Extraction and fractionation
The plant B. royleana roots (4 kg) were shade dried for two months. The dried roots were chopped, crushed and powdered. The powdered material (4 kg) was soaked in methanol, with occasional stirring at room temperature for one week. The filtrate was concentrated through the use of rotary evaporator at 45°C. The crude extract obtained (1 kg) of B. royleana roots was suspended in minimum amount of water and fractionated with n-Hexane thrice which afforded n-hexane fraction weighed (180 g). The remaining water-soluble part was further fractionated with adequate amount of chloroform which afforded chloroform fraction weighed (200 g). The remaining aqueous soluble part was partitioned with ethyl acetate three time which resulted into ethyl acetate fraction weighed (350 g) and aqueous fraction (190 g). These crude fractions were further tested for phytochemical and biological potency.
Phytochemical analysis
Qualitative phytochemical analysis of the crude fractions of B. royleana roots were carried out in order to detect the constituents as described by [7-9].
Alkaloids
In this test 0.2 g of each crude fraction was added with 2% H2SO4 and heated for two minutes, filtered followed by pouring of few drops of Dragendorffs reagent. Orange red precipitates specify the occurrence of alkaloids.
Tannins
In order to detect the presence of tannins, small quantity of each crude fraction was mixed with water and heated on water bath and filtered. Few drops of ferric chloride were added to the filtrate. The appearance of dark green solution indicates the presence of tannins.
Anthraquinones
To determine the presence of anthraquinones, about 0.5 g of each crude fraction was boiled with 10% HCl for few minutes on water bath. The sample was then filtered and cooled. To the filtrate equal volume of CHCl3 was mixed besides adding few drops of 10% ammonia. Appearance of rose-pink color authenticates the occurrence of anthraquinones.
Glycosides
In this phytochemical test, the crude fractions were initially hydrolyzed with HCl followed by neutralization with NaOH solution. To the mixture few drops of Fehling solution A and B were mixed. Formation of red precipitates showed the existence of glycosides.
Reducing sugars
In this test each crude fraction was stirred with distilled water, filtered and boiled after the mixing of few drops of Fehling solution A and B. Formation of orange red precipitates confirms the presence of reducing sugars.
Saponins
For the recognition of saponins, approximately 0.2 g of each crude fraction was mixed with 5 ml of distilled water followed by heating to boil. Frothing (emergence of creamy small bubbles) exposed the occurrence of saponin.
Flavonoids
Each crude fraction of 0.2 g was dissolved in dilute NaOH and then to it HCL was added.
The appearance of a yellow solution that turns to colorless authenticates the existence of flavonoids.
Phlobatanins
Each crude fraction (0.5 g) was dissolved in specified amount of distilled water and filtered. The filtrate was subjected to boil with 2% HCl solution. The appearance of red precipitate articulated the existence of phlobatanins.
Steroids
In this test acetic anhydride (2 ml) was mixed to 0.5 g of each crude fraction of B. royleana roots followed by adding of 2 ml of H2SO4. The color changed from violet to green or blue in some samples authenticate the presence of steroids.
Terpenoid
To check the presence of terpenoids 0.2 g of each crude fraction was mixed one by one with a mixture of 2 ml of chloroform and conc. H2SO4 (3 ml) to form a layer. The appearance of reddish brown coloration at the interface was developed which specify positive results for the existence of terpenoids.
Antibacterial assay
In this biological evaluation, a total of seven bacterial strain were chosen to be used. The bacterial strain used were classified as Escherichia coli, Citobactor freundii, klebsiella pneumoniae, yersinia pestis, Staphylococcus aureus, Pseudomonas aeruginosa and Salmonella typhi. Through the method of agar well diffusion, in the presence of a cell suspension of roughly 1.5 × 106 CFU/ mL, arranged from Macfarland turbidity standard No. 0.5 [10], the anti-bacterial activity was tested. The shimadzu, UV-VIS spectrophotometer [11] was used to regulate the optical density to 0.1 at 600 nm, in order to standardize the suspension. On (8 mm thick) the Mueller Hinton agar (MHA) plate, holes with diameter of 6 mm were bored and filled with different concentration of the sample 50, 100, 150 and 200 μg/ml obtained by dissolution in DMSO with concentration 8 mg/1 ml and standard drug(s) were tested against different bacterial strains. For 24 hours, the plates after inoculation were incubated at temperature of 37°C. In order to determine the antibacterial activity, the zone of inhibition was calculated. The average diameter was measured by repeating the assay thrice. The standard antibiotic used in this bioassay for comparison was imipenem.
Antifungal assay
The strains used for antifungal activity includes; Aspergillus flavus, Aspergillus niger, Penicillium and White rot fungi. For the determination of anti-fungal activity, the laminar flow cabinet was used. The extract fractions (10 mg/mL) each were dissolved in DMSO and were diluted with sterile water in micro plates. Then the cultures of actively growing test fungi were poured into various wells and grow whole night in hundred percent humid atmospheres at 37°C. At early morning, the fungal development was observed through the use of a violet coloration of the culture following mixing of tetrazolium violet to all wells. The test solutions in low conc. were used as the Minimum Inhibitory Concentration (MIC). In this bioassay studies, the miconazole and amphotericin B were used as positive controls.
Insecticidal assay
The valuation of insecticidal potential was conceded by direct contact application of the fractions by means of filter paper [12]. In this assay, all crude fractions 3 mL (1 mg/ mL) each were located on the filter papers (90 mm diameter). The filter papers were allowed to dried and placed in a different Petri dishes accompanied by 10 adults of Kelosobrocus meleticulatis. Afterward, ten insects were sited in every plate and (27°C) as incubation temperature was maintain for twenty-four, forty-eight and seventy-two hours in growth chamber with 50% relative humidity. To check the mortality number, the insects were kept on standing in the dearth of food for 24, 48 and 72 hours respectively. The results were deliberate as percent death by comparing with reference to both the negative and positive controls. In this experimentation, permethrin (235.71 μ/cm3) was used which act as a reference insecticide and acetone with test insects was used as negative controls.
Results and Discussions
Phytochemical analysis
The results obtained from phytochemical analysis of B. royleana revealed the presence of all compounds including alkaloids, tannins, anthraquinones, glycosides, reducing sugars, saponins, flavonoids, phlobatanins, steroids, terpenoid in chloroform fraction while flavonoids were detected only in n-hexane fraction. The ethyl acetate fraction showed the presence of all compounds except saponins while the water fraction only contains steroids, tannins and glycosides (Table 1).
| Phytochemicals | n-hexane | Chloroform | Ethyl acetate | Water |
|---|---|---|---|---|
| Alkaloids | - | + | + | - |
| Tannins | - | + | + | + |
| Anthraquinones | - | + | + | - |
| Glycosides | - | + | + | + |
| Reducing sugars | - | + | + | - |
| Saponins | - | + | - | - |
| Flavonoids | + | + | + | - |
| Phlobatannins | - | + | + | - |
| Steroids | - | + | + | + |
| Terpenoids | - | + | + | - |
Table 1. Phytochemical screenings of the crude fractions of B. royleana roots
Anti-bacterial activity
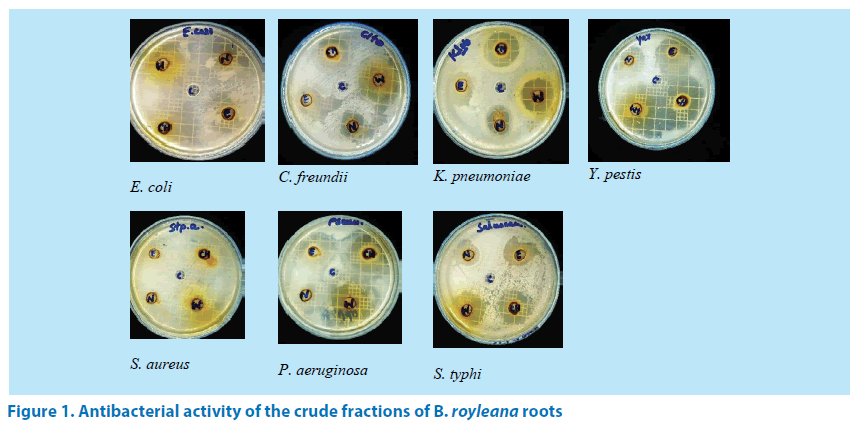
In order to check the antibacterial activity, the fractions of B. royleana roots were checked in resistance to seven strains of bacteria which included E. coli, C. freundii, K. pneumoniae, Y. pestis, S. aureus, P. aeruginosa and S. typhi. The results obtained were expressed as inhibition zones (mm) in (Table 2) and (Figure 1).
| Bacterial strains | Zone of Inhibition (mm) | ||||
|---|---|---|---|---|---|
| Imipenem (drug) | A | B | C | D | |
| Escherichia coli | 35 | 20 | 39 | 25 | 35 |
| Citobactorfreundii | 25 | 19 | 27 | 20 | 27 |
| Klebsiella pneumoniae | 0.39 | 16 | 25 | 23 | 24 |
| Yersiniapestis | 32 | 24 | 36 | 31 | 31 |
| Staphylococcus aureus | 43 | 10 | 24 | 15 | 22 |
| Pseudomonas aeruginosa | 32 | 13 | 30 | 25 | 28 |
| Salmonella typhi | 40 | 18 | 25 | 21 | 27 |
Keywords: A = n-hexane, B = Chloroform, C = Ethyl acetate, D = Water
Table 2. Antibacterial activity of the crude fractions of B. royleana roots
From the data given in the table, it is clear that all of the fractions displayed inhibition against the tested microorganisms. The chloroform fraction B displays maximum inhibition 39 mm against E. coli while the n-hexane fraction A exhibits minimum inhibition 10 mm against S. aureus.
Antifungal activity
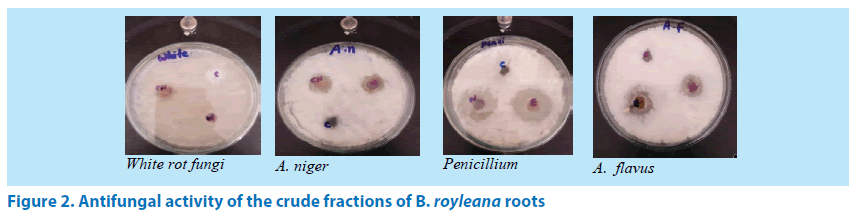
Four fungal strains were used to determined antifungal activity of B. royleana. The fungal strains used in this study were namely A. flavus, A. niger, Penicillium and white rot fungi. The results of the antifungal activity were articulated as Minimum Inhibitory Conc. (MIC) and are being depicted in (Table 3) and (Figure 2). From the data set in the table it is clear that the crude fractions n-hexane, ethyl acetate and water showed resistance against white rot fungi while the chloroform fraction displayed 17 mm minimum inhibitory activity against white rot fungi. All the extract fractions exhibit antifungal activity against A. niger among them the chloroform fraction showed maximum inhibition 20 mm. Similarly, all the tested fractions exhibit antifungal activity against Penicilium in which ethyl acetate fraction revealed maximum inhibition 30 mm. In case of antifungal activity of fractions against A. flavus the water fraction showed no activity while the rest of fractions demonstrate antifungal activity. Among these the ethyl acetate fraction showed maximum inhibition 20 mm against A. flavus.
| Fungal strains | Minimum Inhibitory Concentration (µg/ml) | ||||
|---|---|---|---|---|---|
| Miconazole | A | C | B | D | |
| White rot fungi | 25 | Resistance | 17 | Resistance | Resistance |
| Aspergillus niger | 10 | 15 | 20 | 15 | 16 |
| Penicillium | 40 | 27 | 20 | 30 | 16 |
| Amphotericin B | |||||
| Aspergillus flavus | 20 | 16 | 14 | 20 | Resistance |
Keywords: A = n-hexane, B = Chloroform, C = Ethyl acetate, D = Water
Table 3. Antifungal activity of the crude fractions of B. royleana roots
Insecticidal activity
The insecticidal action of investigation fractions were tested by direct contact application process of the fractions against insects Kelosobrocus meleticulatis at three different time intervals. The consequences obtained are explained in (Table 4). From the data given below it is clear that after 24 hours intervals the water fraction showed maximum mortality rate 20% and the n-hexane, chloroform and ethyl acetate fractions displayed minimum mortality 10%. Subsequently after the time interval of 48 hours the water fraction revealed maximum mortality 40% while n- hexane fraction expressed minimum mortality 20%. Afterward with the passage of 72 hours the maximum mortality% was observed for the water fraction which was 60% while minimum results were obtained for the chloroform and ethyl acetate fractions which were 30%.
| S.No | Crude fractions volume 100 µl | Total insects of k. meleticulatis | Live insects | Kill insects | Mortality (%) |
|---|---|---|---|---|---|
| Day 1: After 24 hours the following results were shown against kelosobrocus meleticulatis (Beatles) | |||||
| 1 | n- hexane | 10 | 9 | 1 | 10 |
| 2 | Chloroform | 10 | 9 | 1 | 10 |
| 3 | Ethyl acetate | 10 | 9 | 1 | 10 |
| 4 | Water | 10 | 8 | 2 | 20 |
| Day 2: After 48 hours the following results were shown against kelosobrocus meleticulatis (Beatles) | |||||
| 1 | n- hexane | 10 | 8 | 2 | 20 |
| 2 | Chloroform | 10 | 7 | 3 | 30 |
| 3 | Ethyl acetate | 10 | 7 | 3 | 30 |
| 4 | Water | 10 | 6 | 4 | 40 |
| Day 3: After 72 hours the following results were shown against kelosobrocus meleticulatis (Beatles) | |||||
| 1 | n- hexane | 10 | 6 | 4 | 40 |
| 2 | Chloroform | 10 | 7 | 3 | 30 |
| 3 | Ethyl acetate | 10 | 7 | 3 | 30 |
| 4 | Water | 10 | 4 | 6 | 60 |
Table 4. Insecticidal activity of the crude fractions of B. royleana roots
Conclusion
In the current study the medicinal plant botanically classified as B. royleana roots were selected for phytochemical analysis and in-vitro biological investigation. The preliminary qualitative analysis of the crude fraction showed the presence of phytochemicals including alkaloids, tannins, anthraquinones, glycosides, reducing sugars, saponins, flavonoids, phlobatanins, steroids, terpenoid in the crude fractions of B. royleana roots while the biological investigation of the crude fractions of roots of Berberis royleana showed significant biological activities. In case of antibacterial activity, the maximum activity was shown by the chloroform fraction B which displays maximum inhibition 39 mm against Escherichia coli. In case of antifungal activity, the ethyl acetate fraction C exhibited maximum inhibition 30 mm against Penicillium. The crude fractions of B. royleana roots were also tested for their insecticidal activity at different time intervals 24, 48 and 72 hours. The maximum mortality rate was shown by water fraction which was 60% at 72 hours intervals. The results obtained exhibit that this plant is very important from medicinal point of view, and it needs further phytochemical exploitation to isolate phytochemical constituents having antibacterial, antifungal and insecticidal activities.
References
- Ahrendt LWA. Berberis and Mahonia, taxonomic revision. Bot. J. Linn. Soc. 57(3), 1-410 (1961).
- Chopra M, Chatterji A, Pakrashi SC. The treatise on Indian medicinal plants. CSIR, New Delhi (1981).
- Chandra P, Purohit AN. Berberine contents and alkaloid profile of Berberis species from different altitudes. Biochem. Sys. Ecol. 8(4), 379-380 (1980).
- Ivanoska N, Philipov S. Study on the anti-inflammatory action of Berberis vulgaris root extracts, alkaloid fractions and pure alkaloid. Int. J. Immuno. Pharmacol. 18(10), 553-561 (1996).
- Rounsaville TJ, Ranney TG. Ploidy levels and genome sizes of Berberis L. and Mahonia nutt. Species, hybrids and cultivars. Hortscience. 45(7), 33-1029 (2010).
- Jafri SMH. Flora of West Pakistan. Nasir E, Ali SI, Eds. Ferozsons: Karachi, Pakistan, 4-31 (1975).
- Sofora A. Medicinal plants and traditional medicine in Africa. John Wiley and son Ltd. (1993).
- Evans WC. Pharmacology 11th Edtn. Brailliar Tiridel and Macmillian Publishers, London (2009).
- Harborne J. Phytochemical methods, a guide to modern techniques of plant analysis. JB Harborne. Chapman. London (1973).
- Jan AK, Shah MR, Anis I et al. In vitro antifungal and antibacterial activities of extracts of Galium tricornutum subsp. longipedunculatum. J. Enzyme. Inhibition. Med. Chem. 24(1), 192-196 (2009).
- Nisar M, Tariq SA, Marwat IK et al. Antibacterial, antifungal, insecticidal, cytotoxicity and phytotoxicity studies on Indigofera gerardiana. J. Enzyme. Inhibition. Med. Chem. 24(1), 224-229 (2009).
- Ahn YJ, Kim GH, Cho KY. Bioassay system for insecticidal compounds. In Proceedings of the third symposium on the biochemical methodology for the research and development of the bioactive substances. 495-506 (1995).