Research Article - International Journal of Clinical Rheumatology (2016) Volume 11, Issue 6
Pigmented villonodular synovitis in type 1 diabetes mellitus: a cross-sectional study in Sardinian people
- Corresponding Author:
- Gianfilippo Caggiari
Orthopaedic Department, Medical School of Sassari, Italy
Tel: +393468464774
Fax: +393468464774
E-mail: gianfilippocaggiari@gmail.com
Abstract
Background: To evaluate the prevalence of Pigmented Villonodular Synovitis (PVNS) in Type 1 Diabetes Mellitus (T1DM) patients in Sardinian people. Materials and Methods: In this cross-sectional study, we evaluated 578 patients T1DM attending the division of Endocrinology and Metabolism in Sassari University Hospital. Age, gender, duration of diabetes, blood pressure levels and presence of neuropathy and retinopathy were noted. Metabolic control of diabetes was evaluated with glycosylated hemoglobin A1c (HbA1c) blood concentrations. 137 of these selected patients had articular pain at least one month, recurrent joint effusion, an inability to lie on the affected joints, and restricted active and passive articular movements. All patients denied a previous trauma. In 89 cases the most affected joints were the knees; in 13 cases the shoulders and in the remaining cases the hips. All patients were submitted to pharmacological therapy with NSAIDs obtaining a good resolution of clinical symptoms in 77 cases; the remaining 60 patients underwent arthroscopy and biopsy of the synovium. As control group 60 patients were selected and underwent knee arthroscopy with biopsy of synovium. Results: The prevalence of PVNS in the group of T1DM patients was in 17 patients; the mean age was 36.4 years with significant higher incidence on males than females. The age, sex and duration of diabetes mellitus were parameters statistically significant. The results achieved from the analysis of the control group data showed a prevalence of PVNS in 6 cases with higher mean age. Conclusions: PVNS is a no rare disorder in T1DM patients in Sardinian people respect remaining population without history of diabetes mellitus. It is associated with age, sex and duration of diabetes. Although no significant association was demonstrated between PVNS and the control of diabetes the most good therapy is prophylaxis with good control of glycemic values.
Keywords
type 1 diabetes mellitus, sardinian people, knee, villonodular synovitis
Introduction
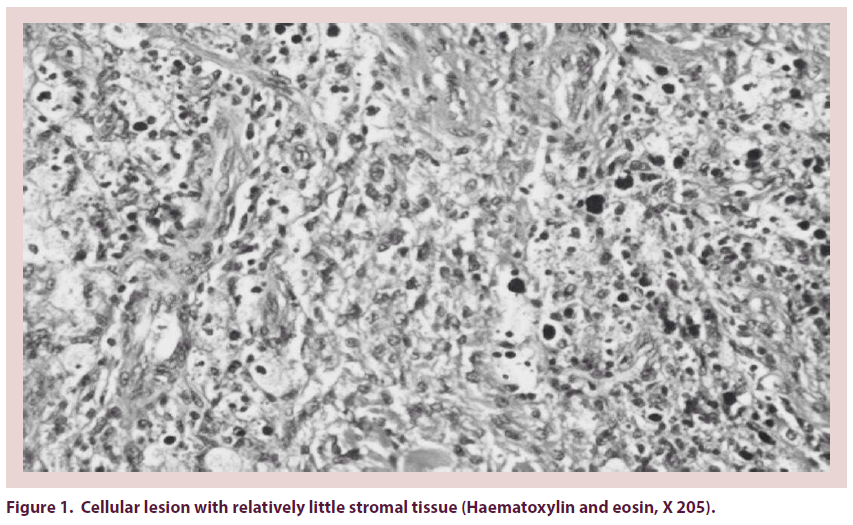
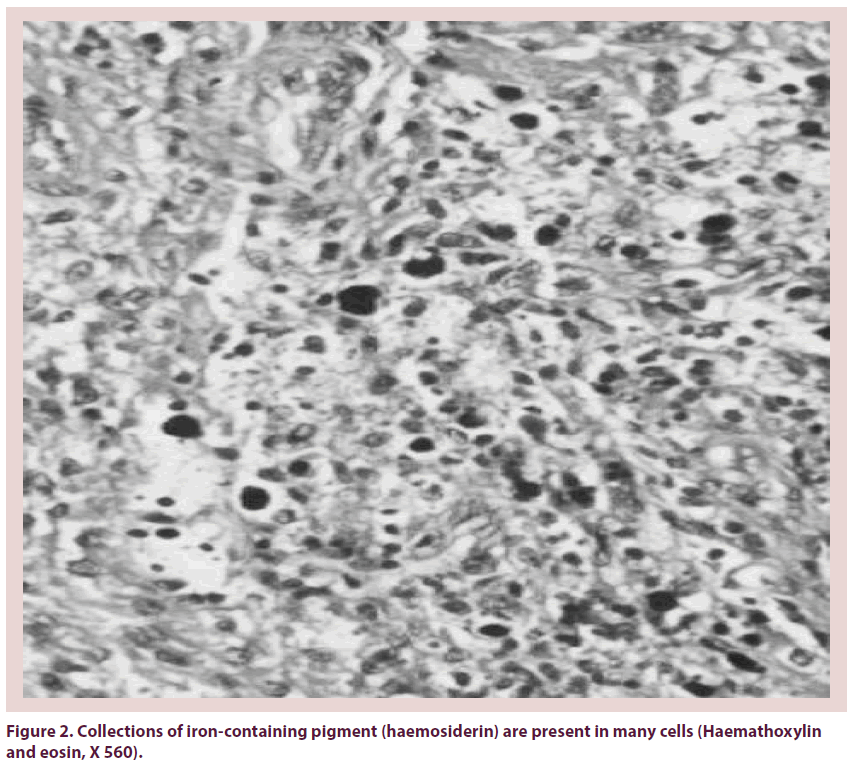
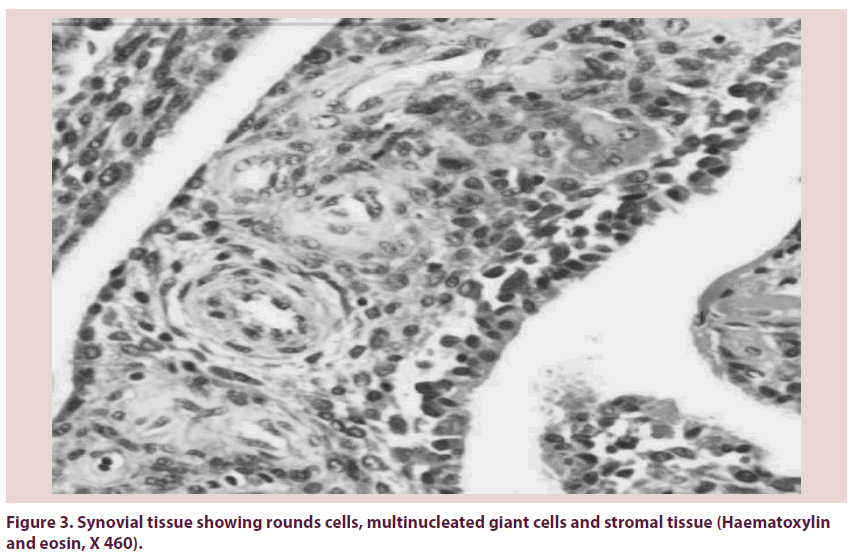
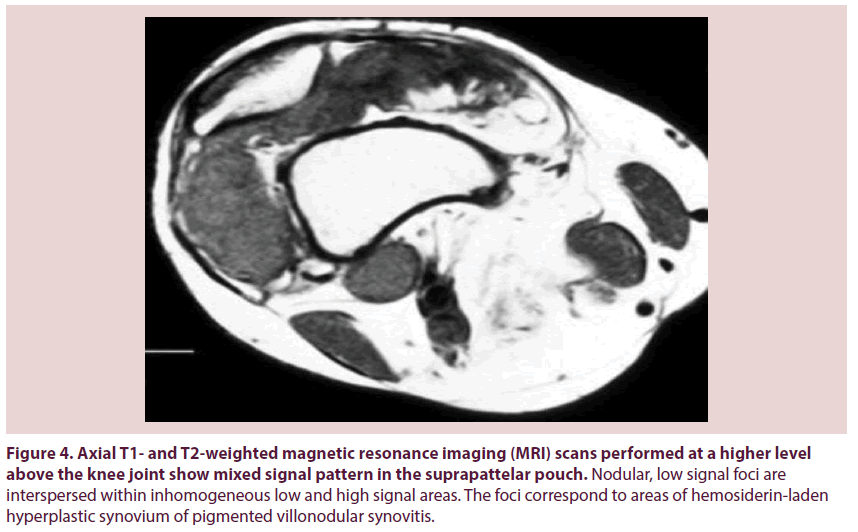
Pigmented Villonodular Synovitis (PVNS) is a painful condition of the joints that is associated with recurrent effusion and loss of range of motion. PVNS was first described by Jaffe et al. as a benign tumor of the synovial membrane of joints, tendon sheaths, and bursae [1]. It may present as a localized or a diffuse lesion, but the histology is identical [2,3]. It’s etiology is unknown, although a neoplastic or inflammatory origin has been suggested [4]. The pathogenesis has not been elucidated although recent studies have demonstrated clonal chromosomal abnormalities, findings that support a neoplastic mechanism [5]. It affects patients in the age group of 20–40 years. Prevalence by sex has been reported to range between a 2:1 male-tofemale ratio [6]. Knee involvement accounts for 80% of cases, followed by the hip, ankle, shoulder, and elbow [7]. Localized PVNS is a relatively rare form, representing 27% of PVNS cases [8]. In the hip, the disease is usually of the diffuse villonodular type [9]. The average time lapse between symptom onset and diagnosis is 2 years. However, there are outliers in whom the disease was diagnosed as soon as a few days after symptom onset to as long as 12 years afterward. Localized PVNS is commonly seen in the third decade of life [10], with mononuclear stromal cell hyperplasia, multinuclear giant cells, and macrophages with hemosiderin [11]. Hemosiderin can accumulate in both macrophages and extracellular compartments (Figure 1); its accumulation is responsible for the “pigmented” portion of the disease’s name (Figures 2 & 3). The key features of PVNS seen on plain radiographs include dense soft-tissue swelling, scalloped bony erosions with sclerotic margins, and joint-space preservation during the early stage of the disease. Magnetic resonance imaging is currently the best diagnostic investigation. It typically appears as an intraarticular effusion of low intensity on both T1 and T2-weighted images because of hemosiderin deposits, with thick fibrous tissue, synovial hyperplasia, bone erosion, and preserved bone density and joint-space width. PVNS can be demonstrated as a dark lesion on all pulse sequences of Magnetic Resonance Imaging (MRI) because of the ferromagnetic properties of hemosiderin [12] (Figure 4). Management of PVNS consists of synovectomy that is generally accepted as the appropriate surgical treatment [13-15], and it can be performed through an open approach or arthroscopically. In patients with irreversible damage of articular surface the indication is arthroplasty.
Figure 2: Collections of iron-containing pigment (haemosiderin) are present in many cells (Haemathoxylin and eosin, X 560).
Figure 3: Synovial tissue showing rounds cells, multinucleated giant cells and stromal tissue (Haematoxylin and eosin, X 460).
Figure 4: Axial T1- and T2-weighted magnetic resonance imaging (MRI) scans performed at a higher level above the knee joint show mixed signal pattern in the suprapattelar pouch. Nodular, low signal foci are interspersed within inhomogeneous low and high signal areas. The foci correspond to areas of hemosiderin-laden hyperplastic synovium of pigmented villonodular synovitis.
Type 1 Diabetes Mellitus (T1DM) results from an autoimmune destruction of insulinproducing β cells [16]. Although T1DM accounts for 5–10% of the total burden of diabetes mellitus, it is the predominant endocrine disease in childhood and adolescence. There are approximately 500,000 children aged under 15 with T1DM in the world [17]. Worldwide, the incidence of T1DM increased, on average, 3% per year between 1960 and 1996 in children under age 15 [18]. This trend is especially troubling in the youngest children; for every hundred thousand children under age 5, 4% were diagnosed every year, on average, worldwide [19]. From an epidemiological point of view, Sardinia, the second largest Italian Mediterranean island, has the second highest incidence of T1DM in the world, after Finland [20,21]. Both areas have shown significant annual increases in incidence rates over the second half of the 20th century and the beginning of the new millennium. Both populations are genetically stable implying a significant role for environmental factors. Analysis of data from Sardinia provides valuable insights into the epidemiology and positive aetiological factors of T1DM. Sardinia has an incidence rate for T1DM over 300 times that of continental Italy. A recent report observed a rising trend of incidence from 37.7/100 000 in 1989 to 49.3/100 000 at the end of 1999 [22]. For the relation between T1DM and PVNS has been thought that chronic inflammation due to diabetes may cause a systemic inflammation, which also involves the joints, including the synovial membrane, in susceptible people. The purpose of this study was to investigate the prevalence of PVNS in T1DM Sardinian patients.
Materials and methods
In this cross-sectional study, we evaluated 578 patients (247 women, 331 men) with T1DM attending the division of Endocrinology and Metabolism in Sassari University Hospital. This study was conducted in accordance with Good Clinical Practice guidelines and principles of the Declaration of Helsinki. Their ages ranged from 29 to 71 (mean ± SD: 51.33 ± 8.45) years. The following variables were noted: age, gender, duration of diabetes, blood pressure levels. Metabolic control of diabetes was evaluated with Glycosylated Hemoglobin A1c (HbA1c) blood concentrations. The specific symptoms of somatic peripheral symmetrical neuropathy were pain, paraesthesiae, tickling, and muscle weakness. The patients were considered to have neuropathy if they had typical symptoms and impaired vibratory sensation or if ankle jerks were absent. Nerve conduction of these patients measured with electroneuromyography. The criteria for synovitis were articular pain at least one month, recurrent joint effusion, an inability to lie on the affected joints, restricted active and passive articular movements without a history of previous trauma. 137 of these selected patients had clinical signs and symptoms of synovitis. All patients denied a previous trauma. In 89 cases the most affected joint was the knee; in 13 cases the shoulder and in the remaining cases the hip. All patients were submitted to pharmacological therapy with NSAIDs obtaining a good resolution of clinical symptoms in 77 cases; the remaining 60 patients underwent arthroscopy and biopsy of the synovium. As control group 60 patients were selected and underwent knee arthroscopy with biopsy of synovium; all patients had uniform clinical characteristics with the patients enrolled in the cross-sectional study as age, sex, no trauma history, clinical signs of synovitis resistant to pharmacological therapy with NSAIDs without history of T1DM.
Statistical evaluation was made with Pearson’s χ2 test, Spearman’s correlation, Mann–Whitney U test, independent samples t test, and Wilcoxon signed-rank test. Statistical significance defined as p<0.05.
Results
The prevalence of PVNS in 578 patients with diabetes mellitus was 17 cases whereas in the control group the rate of PVNS is lower with a prevalence of 6 cases (p<0.01). When we evaluated articular involvement of PVNS, we found diffuse involvement in 13 cases and localized lesion in 4 cases. The mean age was 36.4 (SD 8.7) years in T1DM patients with PVNS and 45.9 (SD 7.6) years in patients with PVNS of the control group (p<0.01). PVNS was associated with patients gender with prevalence for male (11 cases) than female (6 cases) (p<0.01). T1DM patients with and without PVNS had had diabetes for 29.3 (7.1) and 16.1 (8.4) years, respectively (p<0.01). Twenty per cent of T1DM patients with hypertension and 6% of patients without hypertension had PVNS (p<0.01). Three per cent of T1DM patients without neuropathy and 20% with neuropathy had PVNS (p<0.01). There was significant association with proliferative retinopathy (p=0.010). There was no significant difference in mean levels of HbA1c in T1DM patients with PVNS. Patients with poor control of diabetes (HbA1c >8%) seemed to have more articular painful symptoms than patients with better control of diabetes, but this association was not significant.
Discussion
This paper shows that PVNS is a no rare disorder in T1DM patients in Sardinian people. The etiology of PVNS is not known, but patients with this disease reportedly live mainly in rural areas and it is thus speculated that environmental triggers could play an important role in its development in genetically susceptible individuals. While the mechanism is unknown, some cy-tokines such as interleukin 6, VEGF or TNF-α are reportedly increased. T1DM is well known to be associated with other autoimmune diseases such as autoimmune thyroiditis and Sjogren syndrome. However, cases with T1DM complicated by PVNS have been reported [26-28]. Furthermore there is one report suggesting an association with Polymyalgia Rheumatica (PMR), a disease considered to be similar to PVNS [23]. HLA analysis of our patient revealed DR9, DRB1- 0901 and DQB1-0303, HLA types, which are all known to be associated with T1DM. Morphological examination of pathological capsular tissue samples taken intra-operatively has shown the presence of newly vascularized synovial tissue, while immunohistochemical examination has revealed the presence of vascular endothelial growth factor, which has the capacity to induce intense synovial neovascularization. Furthermore, a high presence of intercellular adhesion molecule-1 has been found in articular capsule tissue of diabetic patients, correlating with inflammation and fibrosis [24]. It’s important to note that the patients examined in our study were enrolled at division of Endocrinology and Metabolism in Sassari University Hospital and came from the north-west of Sardinia, where the incidence of T1DM is significantly reduced compared to other areas of island and therefore the data obtained by us may be underestimated than the actual incidence of diabetes and its complications throughout our region; infact the geographical distribution of T1DM risk within the four provinces of the Sardinia was mapped, showing that the highest incidence (“hot spot”) was in the middle-western part of the island (Oristano Province, 45/100,000) followed by Cagliari (38/100,000) and Nuoro (35/100,000). The province with the lowest incidence (“cold spot”) was in the north-western part (Sassari Province, 30/100,000) of the island [25]. Our data report that the presence of PVNS was highly dependent on the age and the duration of diabetes and the prevalence of PVNS increased after the age of 40. Although no significant association between PVNS and the control of diabetes was found, the best therapy is the prophylaxis with good glycemic control. In conclusion, this study shows that PVNS is a no rare disorder in T1DM Sardinian people. It is associated with age, sex and duration of diabetes. All these factors, correlated with inflammation, may result in susceptible people, as it emerged could be the Sardinian population, a state of chronic inflammation in the synovial level, which would result in the emergence of PVNS.
Author contribution
All authors have made substantial contributions to the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content.
References
- Jaffe HL, Lichtenstein L, Sutro CJ. Pigmented villonodular synovitis, bursitis and tenosynovitis. Arch. Pathol. 31, 731–765 (1941).
- Granowitz SP, D’Antonio J, Mankin L. The pathogenesis and long-term end results of pigmented villonodular synovitis. Clin. Orthop. 114, 335–351(1976).
- Gumpel JM, Shawe DJ. Diffuse pigmented villonodular synovitis. Ann. Rheum. Diseases. 50(8), 531–533 (1991).
- Sciot R, Rosai J, Dal Cin P, et al. Analysis of 35 cases of localized and diffuse tenosynovial giant cell tumor: a report from the chromosomes and morphology (CHAMP) study group. Mod. Pathol. 12(6), 576-579 (1999).
- Choong PF, Willen H, Nilbert M, et al. Pigmented villonodular synovitis. Monoclonality and metastasis—a case for neoplastic origin? Acta. Orthop. Scand. 66(1), 64-68 (1995).
- Kallas KM, Vaughan L, Haghighi P, et al. Pigmented villonodular synovitis of the hip presenting as a retroperitonealmass. Skeletal. Radiol. 30(8), 469–474 (2001).
- Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin. Orthop. Rel. Res. 239, 154–160 (1989).
- deVisser E, Veth RPH, Pruszczynski M, et al. Diffuse and localized pigmented villonodular synovitis: evaluation of treatment of 38 patients. Arch. Orthop. Trauma. Sur. 119(7-8), 401–404 (1999).
- Aglietti P, di Muria GV, Salvate EA, et al. Pigmented villonodular synovitis of the hip joint (review of the literature and report of personal case material). Italian. J. Orthop. Traumatology. 9(4), 487–496 (1983).
- Schwartz HS, Unni KK, Pritchard DJ. Pigmented villonodular synovitis. A retrospective review of affected large joints. Clin. Orthop. Rel. Res. 247, 243–255 (1989).
- Szendroi M, Deodhar A. Synovial neoformations and tumours. Bailliere’s Best. Pract. Res. Clin. Rheumatol. 14(2), 363–383 (2000).
- Hughes TH, Sartoris DJ, Schweitzer ME, et al. Pigmented villonodular synovitis: MRI characteristics. Skeletal. Radiol. 24, 7-12 (1995).
- Gitelis S, Heligman D, Morton T. The treatment of pigmented villonodular synovitis of the hip. A case report and literature review. Clin. Orthop. 239, 154-160 (1989).
- Sim FH. Synovial proliferative disorders: role of synovectomy. Arthroscopy. 1, 198-204 (1985).
- Tartaglia L, Chiroff RT. Diffuse pigmented villonodular synovitis. An indication for total hip replacement in the young patient. Clin. Orthop. 115, 172-176 (1976).
- Borg WP, Sherwin RS. Classification of diabetes mellitus. Adv. Intern Med. 45, 279–295 (2000).
- Patterson C, Guariguata L, Dahlquist G, et al. Diabetes in the young - a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes. Res. Clin. Pract. 103, 161-175 (2014).
- Onkamo P, Väänänen S, Karvonen M, et al. Worldwide increase in incidence of Type I diabetes--the analysis of the data on published incidence trends. Diabetologia. 42, 1395–1403 (1999).
- DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet. Med. 23, 857–866 (2006).
- Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989-1998. Diabetologia. 44(S3), 3–8 (2001).
- Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet. 364, 1699–1700 (2004).
- Casu A, Pascutto C, Bernardinelli L, et al. Type 1 diabetes among sardinian children is increasing: the Sardinian diabetes register for children aged 0-14 years (1989-1999). Diabetes. Care. 27, 1623–1629 (2004).
- Nishikawa M, Shouzu A, Imai Y, et al. Histocompatibility antigens and polymyalgia rheumatica in a Japanese patient with insulin-dependent diabetes mellitus. Intern. Med. 36, 935–937 (1997).
- Kim YS, Kim JM, Lee YG, et al. Intercellular adhesion molecule-1 (iCAM-1, CD54) is increased in adhesive capsulitis. Joint. Surg. Am. 95, 181–188 (2013).
- Songini M, Lombardo C. The Sardinian way to type 1 diabetes. J. Diabetes. Sci. Tech. 4, 1248–1255 (2010).
- Sakoda H, Ito S, Kanda H, et al. Association between type 1 diabetes mellitus and remitting seronegative symmetrical synovitis with pitting edema: a case report. Diabetes. Res. Clin. Pract. 9(2), 43-44 (2011).
- Del Rosso A, Cerinic MM, De Giorgio F, et al. Rheumatological manifestations in diabetes mellitus. Curr. Diabetes. Rev. 2(4), 455-66 (2006).
- Lebiedz-Odrobina D, Kay J. Rheumatic manifestations of diabetes mellitus. Rheum. Dis. Clin. North. Am. 36(4), 681-99 (2010).