Review Article - Interventional Cardiology (2013) Volume 5, Issue 6
Plaque characterization to identify patients at high risk of acute complications during PCI
- Corresponding Author:
- Ryan Madder
Frederik Meijer Heart & Vascular Institute
Spectrum Health,100 Michigan Street NE
Grand Rapids, MI 49503, USA
Tel: +1 616 391 9553
Fax: +1 616 391 0801
E-mail: ryan.madder@spectrumhealth.org
Abstract
Despite advancements in the equipment and technique used to perform percutaneous coronary intervention (PCI), acute coronary complications during PCI continue to occur with considerable frequency.
Keywords
intravascular ultrasound;lipid core plaque;near-infrared spectroscopy;no-reflow;optical coherence tomography;periprocedural myocardial infarction;plaque characterization;vulnerable plaque
Acute coronary complications during percutaneous coronary intervention
Despite advancements in the equipment and technique used to perform percutaneous coronary intervention (PCI), acute coronary complications during PCI continue to occur with considerable frequency [1–3]. These acute complications often occur unexpectedly during balloon inflation or stent deployment and include abrupt vessel closure, intimal dissection, vessel perforation, angiographic no-reflow and periprocedural myocardial infarction.
Often defined as reduced coronary f low (less than thrombolysis in myocardial infarction grade 3) in the absence of a mechanical obstruction in the epicardial coronary artery, angiographic no-ref low indicates reduced myocardial tissue perfusion and is caused by microvascular obstruction [3–6]. Depending on the clinical setting in which angiographic no-reflow occurs, the underlying microvascular obstruction may be attributable to multiple pathophysiologic mechanisms, including reperfusion injury, vasospasm, and embolization of thrombus and plaque components [3–6]. Supporting the concept that embolization of plaque components may precipitate no-reflow, plaque debris, such as necrotic core, inflammatory cells and cholesterol, have been retrieved from distal portions of infarct-related arteries after primary PCI [7,8]. Similar to angiographic no-reflow, periprocedural myocardial infarction may, in some cases, arise directly from embolization of debris from the target plaque during PCI [3–6,9]. Although the definition continues to evolve [10,11], periprocedural myocardial infarction remains commonplace, complicating 3–15% of PCI procedures [5,12]. Importantly, both periprocedural myocardial infarction and angiographic no-reflow have been associated with both short- and long-term mortality [6,13–15].
With embolization of plaque components probably playing a role in many cases of noreflow and periprocedural myocardial infarction, the susceptibility of patients to suffer from these acute complications during PCI may directly relate to morphologic characteristics of the target plaque. Accordingly, both no-reflow and periprocedural myocardial infarction have been shown to occur with greater frequency when PCI is performed on target lesions having certain morphologic features detectable by advanced imaging. As a result, pre-PCI imaging using advance techniques may play a role in identifying target lesions prone to triggering acute PCI-related complications. The focus of this review is to highlight the ability of advanced imaging techniques, including coronary computed tomographic angiography (CTA), intravascular ultrasound (IVUS), optical coherence tomography (OCT) and nearinfrared spectroscopy (NIRS), to assess the risk of acute procedural complications prior to performing PCI.
Coronary CTA predictors of angiographic no-reflow
Coronary CTA is a noninvasive imaging technique, capable of accurately detecting atherosclerosis, that provides considerable information regarding coronary stenosis severity and plaque morphology [16–18]. Given the advent of coronary CTA as a diagnostic tool to evaluate patients with acute chest pain [19,20], interventionalists may increasingly encounter patients in the catheterization laboratory who have undergone coronary CTA prior to PCI.
Preprocedural coronary CTA imaging may be useful to assess the risk of acute PCI-related complications [21–23]. Accordingly, Kodama et al. identified both low-attenuation plaque and circumferential plaque calcification detected by preprocedural CTA to be independently associated with the occurrence of angiographic noreflow during PCI [21]. Low-attenuation plaque is defined by attenuation <30 HU and plaque calcification is defined by a rim of calcification extending >180° around the periphery of a plaque. Whereas plaque calcification has been hypothesized to increase the risk of plaque rupture and distal embolization by creating a buttress against which angioplasty leads to excessive intraplaque pressure [21], low-attenuation plaque by CTA is thought to increase the risk of PCI-related complications owing to its presumably lipid-rich nature [18,21,24]. Two additional reports have similarly demonstrated low-attenuation plaque by CTA to be associated with angiographic no-reflow during PCI [22,23].
IVUS predictors of angiographic no-reflow & periprocedural myocardial infarction
Current clinical practice guidelines support the use of IVUS to assess lesions of intermediate stenosis severity, to guide coronary stent implantation and to determine the mechanism of stent restenosis or thrombosis [25]. Although not formally addressed by these guidelines, IVUS may be used to image target lesions prior to PCI in order to assess the risk of acute PCI-related complications. Several studies have found that when certain morphologic characteristics of the target lesion are identified by IVUS prior to PCI, there is an increased risk of angiographic no-reflow and periprocedural myocardial infarction during PCI. These morphologic characteristics include attenuated plaque, larger plaque burden, positive remodeling, a lipid pool-like image and intracoronary thrombus.
▪ Attenuated plaque by IVUS
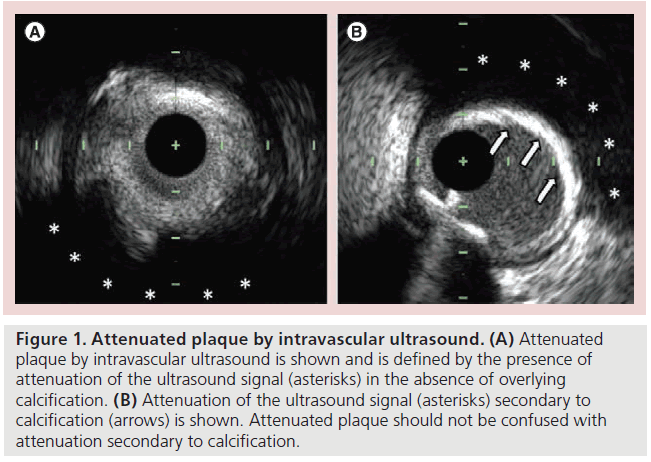
Defined by the presence of attenuation of the ultrasound signal in the absence of overlying calcification (Figure 1), attenuated plaque by IVUS has been associated with an increased risk of both angiographic no-reflow and periprocedural myocardial infarction during PCI [26–28]. In acute coronary syndromes (ACS), attenuated plaque at the target lesion is associated with worse thrombolysis in myocardial infarction flow after first balloon inflation [27]. In some cases, this deterioration in thrombolysis in myocardial infarction flow may not improve during the procedure as final coronary flow remains less favorable after PCI is performed on attenuated plaques compared with nonattenuated lesions [27]. In addition to impairing coronary blood flow, attenuated plaques may adversely impact myocardial perfusion and have been associated with worse myocardial blush grades after PCI compared with nonattenuated lesions [27].
Figure 1: Attenuated plaque by intravascular ultrasound. (A) Attenuated plaque by intravascular ultrasound is shown and is defined by the presence of attenuation of the ultrasound signal (asterisks) in the absence of overlying calcification. (B) Attenuation of the ultrasound signal (asterisks) secondary to calcification (arrows) is shown. Attenuated plaque should not be confused with attenuation secondary to calcification.
In further support of attenuated plaques being associated with an increased risk of PCI-related complications, Kimura et al. found attenuated plaque at the target lesion to be the strongest independent predictor of no-reflow [27]; furthermore, Lee et al. found attenuated plaque at the target site to be the only independent IVUS predictor of periprocedural myocardial infarction [28]. Although more commonly found in ACS, attenuated plaque has also been described at nonculprit locations in stable patients and at target lesions in stable angina [27–29]. Among patients with stable angina, attenuated plaque at the target lesion has been similarly associated with an increased risk of acute PCI-related complications [27,28].
The connection between attenuated plaque and the occurrence of no-reflow and periprocedural myocardial infarction may be attributable to embolic release of plaque material during PCI. Histologic analysis of atherectomy specimens shows that attenuated plaques are composed of greater amounts of lipid-rich atheromatous tissue and cholesterol clefts compared with nonattenuated plaques [27]. Similarly, attenuated plaques contain more fibrofatty content and necrotic core than nonattenuated plaque as demonstrated by post-mortem analysis of cadaveric hearts [30]. As further evidence of the lipid-rich nature of attenuated plaques, a recent comparison of IVUS and OCT findings demonstrated that nearly 90% of attenuated plaques by IVUS met OCT criteria for being lipid-rich [28].
Whereas the mere presence of attenuated plaque may be associated with acute PCI-related complications, the extent of attenuated plaque, including the circumferential degree and length of attenuation, may likewise be a marker of PCIrelated risk. In patients with ST-segment elevation myocardial infarction, the mean attenuation score, which reflects the circumferential degree of attenuation, was the only IVUS characteristic associated with angiographic no-reflow in an analysis of patients in the HORIZONS-AMI trial [26]. This study showed that an attenuation angle >90° predicted the occurrence of angiographic no-reflow with a sensitivity and specificity that exceeded 80% [26]. Similarly, Shiono et al. recently demonstrated that attenuated plaque having an attenuation angle >180° and a length of >5 mm was an independent predictor of microvascular obstruction after primary PCI for ST-segment elevation myocardial infarction, whereas the mere presence of attenuation without consideration for its extent was not associated with PCI-related complications [31].
▪ Other grayscale IVUS characteristics
Additional plaque characteristics by IVUS associated with angiographic no-reflow during PCI include larger plaque burden, positive remodeling, intracoronary thrombus and a lipid poollike image [8,32,33]. A lipid pool-like image is characterized as a localized hypoechoic plaque surrounded by hyperechoic material [8,32,33]. In patients with acute myocardial infarction, Tanaka et al. demonstrated that a lipid pool-like image at the culprit site was the strongest independent predictor of no-reflow [8]. However, the study by Tanaka et al. did not evaluate for attenuated plaque by IVUS. More recent studies that have assessed lesions for both a lipid pool-like image and attenuated plaque by IVUS have not shown a lipid pool-like image to be a significant independent predictor of periprocedural infarction or angiographic no-reflow by multivariate analysis [26–28].
▪ Virtual histology-IVUS predictors of acute PCI-related complications
By using radiofrequency analysis of backscattered IVUS signals, virtual histology (VH)-IVUS provides information regarding plaque composition. VH-IVUS classifies intracoronary plaque using a color-code scheme, wherein fibrotic plaque is displayed as green, fibrofatty plaque as light green, densely calcified plaque as white and necrotic core plaque as red [34].
The impact of the various VH-IVUS plaque types on periprocedural complications during PCI has been extensively studied [35–44]. Although two singular studies have reported no-reflow to occur more frequently in the setting of fibrofatty plaque [35,36], the majority of studies have implicated VH-IVUS necrotic core plaque in PCI-related complications, including angiographic no-reflow [37,38], periprocedural myocardial infarction [39,40] and distal embolization [41]. Importantly, VH-IVUS necrotic core has been linked to these complications in both ACS and in the setting of elective PCI for stable angina [42]. The association between necrotic core plaque by VH-IVUS and PCI-related complications has been further highlighted by a recent systematic review [43] and meta-analysis [44].
OCT predictors of periprocedural myocardial infarction & angiographic no-reflow
OCT works on the principle of back reflection of infrared light and produces high-quality images having an axial resolution of 10–20 μm [45–47]. Owing to this superb spatial resolution, OCT provides visualization of structures currently beyond the capabilities of traditional IVUS imaging. Hence, OCT remains the only intracoronary imaging technique with sufficient spatial resolution to visualize the fibrous cap of coronary atheroma. This unique aspect of OCT imaging may be beneficial in evaluating the risk of acute coronary complications during PCI, since OCT-derived thin-capped fibroatheroma (TCFA) requires visualization of the fibrous cap and has been associated with adverse outcomes during stent placement [9,48].
▪ OCT-derived TCFA
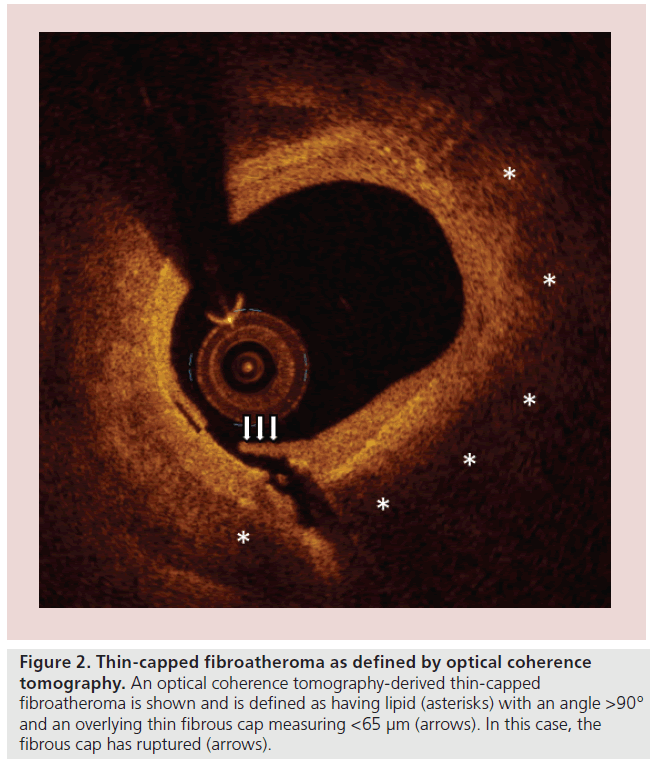
An OCT-derived TCFA is typically defined as a plaque that is both lipid-rich and has an overlying thin-fibrous cap (Figure 2). For a plaque to be considered ‘lipid-rich’ by OCT, most studies have required the presence of a lipid arc involving at least two quadrants in a cross-sectional image, or covering an angle >90° [9,48,49]. These same studies have defined a thinfibrous cap as one having a thickness of <65 or 70 μm [9,48].
Figure 2: Thin-capped fibroatheroma as defined by optical coherence tomography. An optical coherence tomography-derived thin-capped fibroatheroma is shown and is defined as having lipid (asterisks) with an angle >90° and an overlying thin fibrous cap measuring <65 μm (arrows). In this case, the fibrous cap has ruptured (arrows).
Using time-domain OCT technology, which requires vessel occlusion for image acquisition, Lee et al. imaged target lesions prior to PCI to delineate plaque characteristics associated with periprocedural myocardial infarction [48]. Multivariable analysis showed that the presence of OCT-derived TCFA at the target lesion was the strongest independent predictor of periprocedural myocardial infarction [48]. Using frequency-domain OCT technology, which does not require vessel occlusion for imaging and is currently available for clinical use in the USA, Porto et al. similarly studied plaque characteristics associated with periprocedural myocardial infarction [9]. Consistent with the findings of Lee et al. [48], Porto et al. found OCT-derived TCFA to be the only morphologic feature significantly associated with periprocedural myocardial infarction [9]. Taken together, these two studies demonstrate that, when identified at the target lesion prior to PCI, OCT-derived TCFA is associated with periprocedural myocardial infarction in 50–76% of cases [9,48]. Furthermore, these studies provide evidence that OCT-derived TCFA may be associated with an increased risk of periprocedural myocardial infarction in the setting of both ACS and stable angina [9,48].
▪ Lipid-rich plaque by OCT
Although the presence of a lipid-rich plaque as a standalone variable was not found to be associated with periprocedural infarction in the above studies [9,48], it may be that the quantity of lipid, rather than its mere presence, is a marker of acute complications during PCI. In a study by Tanaka et al., target lesions characterized by OCT as having a lipid arc of 1–90, 91–180 and >180° were associated with angiographic no-reflow in 4.7, 35.0 and 75.0% of cases, respectively [49]. Interestingly, angiographic no-reflow did not occur in any patient in the absence of lipid-rich plaque [49]. By multivariable analysis, the degree of lipid arc was determined to be the only significant predictor of angiographic no-reflow after PCI [49]. Yonetsu et al. further highlighted the importance of the lipid arc in a study where increasing arc was associated with a greater frequency of periprocedural myocardial infarction [50]. These observations are congruent with those of IVUS studies that have demonstrated the circumferential extent of attenuated plaque, a potential surrogate marker of the extent of lipid core, to be similarly associated with acute complications during PCI [26,31].
NIRS predictors of periprocedural myocardial infarction
Intracoronary NIRS has been validated to detect the presence of lipid core plaque within the coronary arteries [51,52]. NIRS is performed in a manner analogous to IVUS, in which the NIRS catheter is positioned distal to a region of interest within an artery and an automated pullback is performed, thereby generating a NIRS chemogram (Figure 3) [53]. The NIRS technique is relatively simple to perform and NIRS chemograms are easy to interpret; a yellow color indicates the presence of lipid and red indicates the absence of lipid at any given location [53].
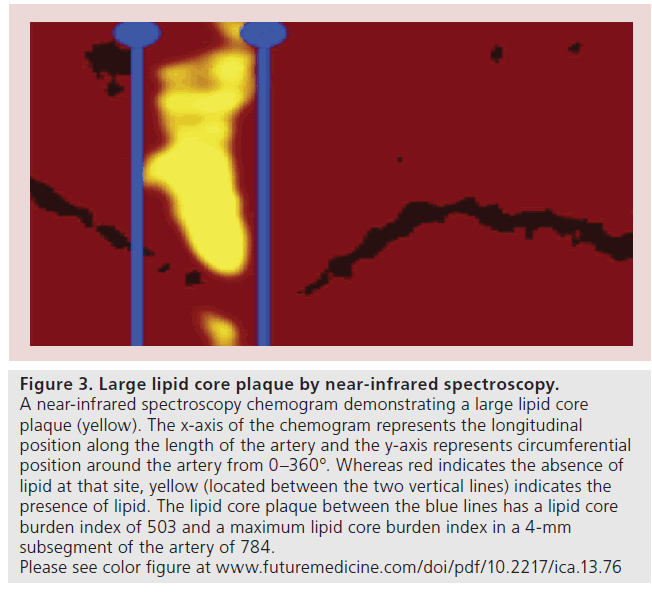
Figure 3: Large lipid core plaque by near-infrared spectroscopy. A near-infrared spectroscopy chemogram demonstrating a large lipid core plaque (yellow). The x-axis of the chemogram represents the longitudinal position along the length of the artery and the y-axis represents circumferential position around the artery from 0–360°. Whereas red indicates the absence of lipid at that site, yellow (located between the two vertical lines) indicates the presence of lipid. The lipid core plaque between the blue lines has a lipid core burden index of 503 and a maximum lipid core burden index in a 4-mm subsegment of the artery of 784. Please see color figure at www.futuremedicine.com/doi/pdf/10.2217/ica.13.76
▪ Lipid core burden index
Not only can NIRS detect the presence of lipid within the arterial wall but, unlike IVUS and OCT, it is also capable of providing a rapid automated assessment of lipid quantity, reported as the lipid core burden index (LCBI). The LCBI is a metric defined as the fraction of pixels within a region of interest indicating lipid multiplied by 1000 [53–56]. The maximum LCBI in a 4-mm subsegment of the artery (maxLCBI4 mm) has been used to characterize the lipid burden of individual lesions [55,56]. An example of a large lipid core plaque detected by NIRS and its associated LCBI and maxLCBI4 mmis provided in Figure 3.
Using data from a large multicenter registry, Goldstein et al. studied patients with ACS and stable angina undergoing NIRS imaging of the target lesion prior to PCI and found the quantity of lipid within the target lesion to be associated with periprocedural myocardial infarction risk [55]. Consequently, the median maxLCBI4 mm of target lesions in those suffering from a periprocedural infarction was 592 compared with a median maxLCBI4 mm of only 219 in those without a periprocedural infarct. In further support of the concept that larger lipid cores are associated with a greater risk of periprocedural infarction, 50% of patients with a maxLCBI4 mm ≥500 suffered a periprocedural myocardial infarction compared with only 4% of those with a maxLCBI4 mm<500 [55].
▪ Combined NIRS–IVUS imaging
The only intracoronary NIRS imaging technology currently available for clinical use is a combined NIRS and IVUS imaging system [57,58]. Using a single catheter and requiring only one pullback within the artery, this combined system provides coregistered NIRS images with traditional grayscale IVUS images [57,58]. Although NIRS alone is capable of identifying lesions at risk of periprocedural myocardial infarction [55], the combined NIRS–IVUS system, by providing information on lipid quantity and the presence of attenuated plaque, may be particularly wellsuited to assess the risk of acute coronary complications during PCI. However, future studies are needed to determine the utility of combined NIRS–IVUS in this regard.
Limitations of pre-PCI plaque characterization
There are several potential limitations to consider with respect to performing intracoronary imaging prior to PCI. First, although the risk of serious complications, such as coronary dissection or inducing plaque rupture with the imaging catheter,is low, coronary spasm has been reported to occur in as many as 1–2% of IVUS cases [59,60]. Owing to this risk of catheter-induced coronary spasm, it is commonplace to administer intracoronary nitroglycerin prior to intracoronary imaging. Second, in the case of OCT, additional intravenous contrast is required for imaging. As a result, OCT carries a risk of contrast-induced acute kidney injury in those with underlying kidney disease; OCT should be used judiciously in such patients. Third, the use of intravascular imaging with OCT, NIRS or IVUS requires the operator to conceptually place the intracoronary imaging findings in the appropriate location within the artery. This process can theoretically lead to geographic miss and may be overcome in the future by the development of imaging systems that automatically coregister the angiographic and intravascular images [61].
Finally, although considerable evidence that intracoronary imaging can identify target lesions at increased risk of inducing no-reflow and periprocedural myocardial infarction, it is unclear whether identification of such ‘high-risk’ lesions prior to PCI should alter clinical management. The absence of studies demonstrating a clinical benefit to morphologic characterization of the target lesion prior to PCI represents a barrier to widespread adoption of pre-PCI intracoronary imaging. Whereas routine use of distal embolic protection devices in PCI without consideration of underlying plaque morphology is not beneficial [62], a study evaluating the selective use of an embolic protection device based upon plaque morphology is underway. The CANARY study, which requires performance of NIRS to evaluate the target lesion prior to PCI, is randomizing patients with large lipid cores by NIRS to a strategy of distal embolic protection or routine care during PCI [101]. Although it is not yet known whether such an approach will reduce the incidence of periprocedural myocardial infarction, similarly designed studies using IVUS and OCT are needed.
Conclusion & future perspective
In summary, IVUS, OCT and NIRS are all capable of identifying target lesions at heightened risk of inducing acute PCI-related complications. The morphologic characteristics most associated with these complications include attenuated plaque by IVUS, necrotic core by VH-IVUS, TCFA by OCT and lipid core plaque by NIRS (Table 1), all of which seemingly indicate the presence of lipid within the target lesion. As underscored by studies with each imaging modality, it is not the mere presence of lipid per se, but rather the extent of lipid within the target lesion that may be best associated with the risk of acute PCI-related complications. Interestingly, these same imaging markers have all been identified at culprit sites in ACS [54,56,63–71], which may, in a broader sense, identify plaques that are likely to rupture, whether induced by PCI or occurring spontaneously and thereby triggering acute coronary events or plaque progression [72–76]. The value of advanced coronary imaging to characterize vulnerable plaques with the goal of predicting future acute coronary events has been recently published [77].
| Modality | Plaque | Definition | Threshold associated with | Associated | Ref. |
|---|---|---|---|---|---|
| characteristic | adverse events | outcomes | |||
| Coronary | Low-attenuation | <30 HU | Unknown | No-reflow | [21–23] |
| CTA | plaque | ||||
| Circumferential | Rim of calcium extending | Unknown | No-reflow | [21] | |
| plaque calcification | >180° around the plaque | ||||
| IVUS | Attenuated plaque | Attenuation of the ultrasound | Attenuation angle >180° and | No-reflow and PPMI | [26–28,31] |
| signal in the absence of | length >5 mm | ||||
| overlying calcification | |||||
| Lipid pool-like image | Localized hypoechoic plaque | Unknown | No-reflow | [8,32,33] | |
| surrounded by hyperechoic | |||||
| material | |||||
| VH-IVUS | Necrotic core | Plaque that is displayed as red | Unknown | No-reflow and PPMI | [35–44] |
| by VH–IVUS | |||||
| OCT | TCFA | Lipid present over an angle | Unknown | PPMI | [9,48] |
| >90° and fibrous cap <65 µm | |||||
| Lipid-rich plaque | Lipid present over an | Circumferential extent of lipid | No-reflow and PPMI | [49] | |
| angle >90° | arc is associated with adverse | ||||
| events | |||||
| NIRS | MaxLCBI4 mm | Maximum LCBI in a | MaxLCBI4 mm ≥500 | PPMI | [55] |
| 4-mm coronary segment |
Table 1. Morphologic characteristics of target lesions associated with an increased risk of acute complications during percutaneous coronary intervention.
Although advanced intracoronary imaging with IVUS, OCT and NIRS are capable of predicting acute PCI-related complications, periprocedural myocardial infarction and angiographic no-reflow continue to occur with considerable frequency in clinical practice. Techniques to prevent these complications at the present time are lacking. Clearly, additional studies are needed to determine whether routine plaque characterization using IVUS, OCT or NIRS prior to PCI can improve the safety of PCI performance. This will probably be achieved through prospective clinical trials in which patients are randomized to protective therapies based on the presence of high-risk intracoronary imaging findings.
Financial & competing interests disclosure
R Madder has received research support and speaker honoraria from Infraredx (MA, USA) and has served as a consultant for St Jude Medical (MN, USA). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Acute coronary complications during percutaneous coronary intervention
▪ Acute percutaneous coronary intervention (PCI)-related complications, including angiographic no-reflow and periprocedural myocardial infarction, are often due to embolization of plaque components and are associated with increased short- and long-term mortality.
▪ The susceptibility of patients to suffer no-reflow and periprocedural myocardial infarction relates to the morphologic characteristics of the target plaque that are detectable by advanced coronary imaging.
Coronary computed tomographic angiography predictors of angiographic no-reflow
▪ Low-attenuation plaque and circumferential plaque calcification detected by coronary computed tomographic angiography are independently associated with angiographic no-reflow during PCI.
Intravascular ultrasound predictors of angiographic no-reflow & periprocedural myocardial infarction
▪ Attenuated plaque by intravascular ultrasound (IVUS), which is probably a marker of lipid-rich plaques, is independently associated with an increased risk of both angiographic no-reflow and periprocedural myocardial infarction during PCI.
▪ Additional IVUS characteristics associated with angiographic no-reflow include a large plaque burden, positive remodeling, intracoronary thrombus and a lipid pool-like image.
.▪ Necrotic core plaque by virtual histology-IVUS is independently associated with angiographic no-reflow and periprocedural myocardial infarction.
Optical coherence tomography predictors of periprocedural myocardial infarction & angiographic no-reflow
▪ Optical coherence tomography-derived thin-capped fibroatheroma is independently associated with the occurrence of periprocedural myocardial infarction.
▪ It is not the mere presence of lipid-rich plaque per se, but rather the degree of the lipid arc by optical coherence tomography that is associated with periprocedural myocardial infarction.
Near-infrared spectroscopy predictors of periprocedural myocardial infarction
▪ Unlike IVUS and optical coherence tomography, near-infrared spectroscopy is capable of providing a rapid automated assessment of lipid quantity reported as the lipid core burden index, which is defined as the fraction of pixels within a region of interest indicating lipid multiplied by 1000.
▪ A maximum lipid core burden index in a 4-mm subsegment of the artery ≥500 is associated with a 50% rate of periprocedural myocardial infarction during PCI.
▪ Although near-infrared spectroscopy alone is capable of identifying lesions at risk of periprocedural myocardial infarction, the combined near-infrared spectroscopy–IVUS system, by providing information on lipid quantity and the presence of attenuated plaque, may be particularly well-suited to assess the risk of acute coronary complications during PCI.
Limitations of pre-PCI plaque characterization
▪ The risk of serious complications imposed by pre-PCI intracoronary imaging is low.
▪ Although intracoronary imaging can identify target lesions at increased risk of inducing no-reflow and periprocedural myocardial infarction, it is currently unclear how identifying high-risk plaque morphology prior to PCI could alter clinical management.
References
- Park DW, Kim YH, Yun SC et al. Frequency, causes predictors, and clinical significance of peri-procedural myocardial infarction following percutaneous coronary intervention. Eur. Heart J. 34, 1662–1669 (2013).
- Wang TY, Peterson ED, Dai D et al. Patterns of cardiac marker surveillance after elective percutaneous coronary intervention and implications for the use of periprocedural myocardial infarction as a quality metric: a report from the national cardiovascular data registry. J. Am. Coll. Cardiol. 51, 2068–2074 (2008).
- Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117, 3152–3156 (2008).
- Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstruction-related myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. Am. Coll. Cardiol. Interv. 3, 695–704(2010).
- Prasad A, Herrmann J. Myocardial infarction due to percutaneous coronary intervention. Engl. J. Med. 364, 453–464 (2011).
- Morishima I, Sone T, Okumura K et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J. Am. Coll. Cardiol. 36, 1202–1209 (2000).
- Sutsch G, Kiowiski W, Bossard A et al. Use of an emboli containment and retrieval system during percutaneous coronary angioplasty in native coronary arteries. Schweiz. Med. Wochenschr. 130, 1135–1145 (2000).
- Tanaka A, Kawarabayashi T, Nishibori Y et al. No-reflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation 105, 2148–2152 (2002).
- Porto I, Di Vito L, Burzotta F et al. Predictors of periprocedural (type IVa) myocardial infarction, as assessed by frequency-domain optical coherence tomography. Circ. Cardiovasc. Interv. 5, 89–96 (2012).
- Thygesen K, Alpert JS, White HD et al. Joint ESC/ACCF/AHA/WHF task force for the redefinition of myocardial infarction. Universal definition of myocardial infarction. Circulation 116, 2634–2653 (2007).
- Thygesen K, Alpert JS, Jaffe AS et al. The writing group on behalf of the Joint ESC/ACCF/AHA/WHF task force for the universal definition of myocardial infarction. Third universal definition of myocardial infarction. Circulation 126, 2020–2035 (2012).
- Goldstein JA, Grines C, Fischell T et al. Coronary embolization following balloon dilation of lipid-core plaques. JACC Cardiovasc. Imaging 2, 1420–1424 (2009).
- Lansky AJ, Stone GW. Periprocedural myocardial infarction: prevalence, prognosis, and prevention. Circ. Cardiovasc. Interv. 3, 602–610 (2010).
- Park DW, Kim YH, Yun SC et al. Frequency, causes, predictors, and clinical significance of peri-procedural myocardial infarction following percutaneous coronary intervention. Eur. Heart J. 34, 1662–1669 (2013).
- Ndrepepa G, Tiroch K, Fusaro M et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 55, 2383–2389 (2010).
- Achenbach S, Moselewski F, Ropers D et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment based comparison with intravascular ultrasound. Circulation 109, 14–17 (2004).
- Leber AW, Becker A, Knez A et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J. Am. Coll. Cardiol. 47, 672–677 (2006).
- Madder RD, Chinnaiyan KM, Marandici AM, Goldstein JA. Features of disrupted plaque by coronary computed tomographic angiography: correlates with invasively proven complex lesions. Circ. Cardiovasc. Imaging4, 105–113 (2011).
- Hoffmann U, Bamberg F, Chae CU et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT trial. J. Am. Coll. Cardiol. 53, 1642 (2009).
- Goldstein JA, Chinnaiyan KM, Abidov A et al. The CT-STAT trial. J. Am. Coll. Cardiol. 58, 1414–1422 (2011).
- Kodama T, Kondo T, Oida A, Fujimoto S, Narula J. Computed tomographic angiography-verified plaque characteristics and slow-flow phenomenon during percutaneous coronary intervention. J. Am. Coll. Cardiol. Interv. 5, 636–643 (2012).
- Kinohira Y, Akutsu Y, Li HL et al. Coronary arterial plaque characterized by multislice computed tomography predicts complications following coronary intervention. Int. Heart J. 48, 25–33 (2007).
- Nakazawa G, Tanabe K, Onuma Y et al. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am. Heart J. 155, 1150–1157 (2008).
- Motoyama S, Kondo T, Anno H et al. Atherosclerotic plaque characterization by 0.5‑mm-slice multislice computed tomographic imaging. Circ. J. 71, 363–366 (2007).
- Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology foundation/American Heart Association Task Force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124, 574–651 (2011).
- Wu X, Mintz GS, Xu K et al. The relationship between attenuated plaque identified by intravascular ultrasound and no-reflow after stenting in acute myocardial infarction. J. Am. Coll. Cardiol. Interv. 4, 495–502(2011).
- Kimura S, Kakuta T, Yonetsu T et al. Clinical significance of echo signal attenuation on intravascular ultrasound in patients with coronary artery disease. Circ. Cardiovasc. Interv. 2, 444–454 (2009).
- Lee T, Kakuta T, Yonetsu T et al. Assessment of echo-attenuated plaque by optical coherence tomography and its impact on post-procedural creatinine kinase-myocardial band elevation in elective stent implantation. Am. Coll. Cardiol. Interv. 4, 483–491(2011).
- Bayturan O, Tuzcu EM, Nicholls SJ et al. Attenuated plaque at nonculprit lesions in patients enrolled in intravascular ultrasound atherosclerosis progression trials. J. Am. Coll. Cardiol. Cardiovasc. Interv. 2, 672–678(2009).
- Yamada R, Okura H, Kume T et al. Histologic characteristics of plaque with ultrasonic attenuation: a comparison between intravascular ultrasound and histology. Cardiol. 50, 223–228 (2007).
- Shiono Y, Kubo T, Tanaka A et al. Impact of attenuated plaque as detected by intravascular ultrasound on the occurrence of microvascular obstruction after percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am. Coll. Cardiol. Interv. 6, 847–853(2013).
- Iijima R, Shinji H, Ikeda N et al. Comparison of coronary arterial finding by intravascular ultrasound in patients with transient no-reflow versus reflow during percutaneous coronary intervention in acute coronary syndrome. Am. J. Cardiol. 97, 29– 33 (2006).
- Fukada D, Tanaka A, Shimada K et al. Predicting angiographic distal embolization following percutaneous coronary intervention in patients with acute myocardial infarction. Am. J. Cardiol. 91, 403–407 (2003).
- Garcia-Garcia HM, Mintz GS, Lerman A et al. Tissue characterization usingintravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention 5, 177–189 (2009).
- Bae JH, Kwon TG, Hyun DW, Rihal CS, Lerman A. Predictors of slow flow during primary percutaneous coronary intervention: an intravascular ultrasound-virtual histology study. Heart 94, 1559–1564 (2008).
- Nakamura T, Kubo N, Ako J, Momomura S. Angiographic no-reflow phenomenon and plaque characteristics by virtual histology intravascular ultrasound in patients with acute myocardial infarction. J. Interv. Cardiol. 20, 335–339 (2007).
- Hong YJ, Jeong MH, Choi YH et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis. Eur. Heart J. 32, 2059–2066 (2011).
- Higashikuni Y, Tanabe K, Tanimoto S et al. Impact of culprit plaque composition on the no-reflow phenomenon in patients with acute coronary syndrome: an intravascular ultrasound radiofrequency analysis. Circ. J. 72, 1235–1241 (2008).
- Hong YJ, Mintz GS, Kim SW et al. Impact of plaque composition on cardiac troponin elevation after percutaneous coronary intervention: an ultrasound analysis. J. Am. Coll. Cardiol. Imaging 2, 458–468 (2009).
- Bose D, von Birgelen C, Zhou XY et al. Impact of atherosclerotic plaque composition on coronary microembolization during percutaneous coronary interventions. Basic Res. Cardiol. 103, 587–597 (2008).
- Kawamoto T, Okura H, Koyama Y et al. The relationship between coronary plaque characteristics and small embolic particles during coronary stent implantation. J. Am. Coll. Cardiol. 50, 1635–1640 (2007).
- Yamada R, Okura H, Kume T et al. Target lesion thin-cap fibroatheroma defined by virtual histology intravascular ultrasound affects microvascular injury during percutaneous coronary intervention in patients with angina pectoris. Circ. J. 74, 1658–1662 (2010).
- Claessen BE, Maehara A, Fahy M, Xu K, Stone GW, Mintz GS. Plaque composition by intravascular ultrasound and distal embolization after percutaneous coronary intervention. J. Am. Coll. Cardiol. Imaging 5, S111–S118 (2012).
- Jang JS, Jin HY, Seo JS et al. Meta-analysis of plaque composition by intravascular ultrasound and its relation to distal embolization after percutaneous coronary intervention. Am. J. Cardiol. 111, 968–972 (2013).
- Tearney GJ, Regar E, Akasaka T et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies. J. Am. Coll. Cardiol. 59, 1058–1072 (2012).
- Lowe HC, Narula J, Fujimoto JG, Jang IK. Intracoronary optical diagnostics: current status, limitations, and potential. J. Am. Coll. Cardiol. Interv. 4, 1257–1270 (2011).
- Ferrante G, Presbitero P, Whitbourn R, Barlis P. Current applications of optical coherence tomography for coronary intervention. Int. J. Cardiol. 165, 7–16 (2013).
- Lee T, Tonetsu T, Koura K et al. Impact of plaque morphology assessed by optical coherence tomography on cardiac troponin elevation in patients with elective stent implantation. Circ. Cardiovasc. Interv. 4, 378–386 (2011).
- Tanaka A, Imanishi T, Kitabata H et al. Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. Eur. Heart J. 30, 1348–1355 (2009).
- Yonetsu T, Kakuta T, Lee T et al. Impact of plaque morphology on creatinine kinase-MB elevation in patients with elective stent implantation. Int. J. Cardiol. 146, 80–85 (2011).
- Gardner CM, Tan H, Hull EL et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. J. Am. Coll. Cardiol. Imaging 1, 638–648(2008).
- Waxman S, Dixon SR, L’Allier P et al. In vivo validation of a catheter- based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. J. Am. Coll. Cardiol. Imaging 2, 858–868 (2009).
- Goldstein JA, Madden SP, Sum ST et al. Assessment of plaque composition with near-infrared spectroscopy. Curr. Cardiovasc. Imaging Rep. 4, 298–308 (2011).
- Madder RD, Smith JL, Dixon SR, Goldstein JA. Composition of target lesions by near-infrared spectroscopy in patients with acute coronary syndrome versus stable angina. Circ. Cardiovasc. Interv. 5, 55–61 (2012).
- Goldstein JA, Maini B, Dixon SR et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of peri-procedural myocardial infarction. Circ. Cardiovasc. Interv. 4, 429–437 (2011).
- Madder RD, Goldstein JA, Madden SP et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. Interv. 6(8), 838–846 (2013).
- Madder RD, Steinberg DH, Anderson RD. Multimodality direct coronary imaging with combined near-infrared spectroscopy and intravascular ultrasound: initial US experience. Catheter. Cardiovasc. Interv. 81, 551–557 (2013).
- Shydo B, Hendricks M, Frazier G. Imaging of plaque composition and structure with the TVC imaging system and TVC insight catheter. J. Invasive Cardiol. 25(Suppl. A), 5A–8A (2013).
- Guédès A, Keller PF, L’Allier PL, Lesperance J, Gregoire J, Tardif JC. Long-term safety of intravascular ultrasound in nontransplant, nonintervened, atherosclerotic coronary arteries. J. Am. Coll. Cardiol. 45(4), 559–564 (2005).
- Mudra H, di Mario C, de Jaegere P et al. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study). Circulation 104(12), 1343–1349 (2001).
- Reiber JHC, Tu S, Tuinenburg JC, Koning G, Janssen JP, Dijkstra J. QCA, IVUS, and OCT in interventional cardiology in 2011.Cardiovasc. Diagn. Ther. 1, 57–70 (2011).
- Stone GW, Webb J, Cox DA et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction. JAMA 293, 1063–1072 (2005).
- Hong M, Mintz GS, Lee CW et al. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation 110, 928–933 (2004).
- Fujii K, Kobayashi Y, Mintz GS et al. Intravascular ultrasound assessment of ulcerated ruptured plaques. A comparison of culprit and non-culprit lesions of patients with acute coronary syndromes and lesions in patients without acute coronary syndromes. Circulation 108, 2473–2478 (2003).
- Lee SY, Mintz GS, Kim SY et al. Attenuated plaque detected by intravascular ultrasound: clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. J. Am. Coll. Cardiol. Interv. 2, 65–72 (2009).
- Sanidas EA, Maehara A, Mintz GS et al. Angioscopic and virtual histology intravascular ultrasound characteristics of culprit lesion morphology underlying coronary artery thrombosis. Am. J. Cardiol. 107, 1285–1290 (2011).
- Hong MK, Mintz GS, Lee CW et al. Comparison of virtual histology to intravascular ultrasound of culprit coronary lesions in acute coronary syndrome and target coronary lesions in stable angina pectoris. Am. J. Cardiol. 100, 953–959 (2007).
- Hong YJ, Jeong MH, Choi YH et al. Plaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patients. J. Am. Coll. Cardiol. Imaging 2, 339–349 (2009).
- Kubo T, Imanishi T, Takarada S et al. Assessment of culprit lesion morphology in acute myocardial infarction. J. Am. Coll. Cardiol. 50, 933–939 (2007).
- Ino Y, Kubo T, Tanaka A et al. Difference of culprit lesion morphologies between ST-segment elevation myocardial infarction and non-ST-segment elevation acute coronary syndrome. J. Am. Coll. Cardiol. Interv. 4, 76–82 (2011).
- Toutouzas K, Karanasos A, Riga M et al. Optical coherence tomography assessment of the spatial distribution of culprit ruptured plaques and thin-cap fibroatheromas in acute coronary syndrome. EuroIntervention8, 477–485 (2012).
- Yamagishi M, Terashima M, Awano K et al. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J. Am. Coll. Cardiol. 35, 106–111 (2000).
- Stone GW, Maehara A, Lansky A et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 364, 226–235 (2011).
- Calvert PA, Obaid DR, O’Sullivan M et al. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) study. J. Am. Coll. Cardiol. Imaging 4, 894–901 (2011).
- Stone PH, Saito S, Takahashi S et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION study. Circulation 126, 172–181 (2012).
- Uemura S, Ishigami K, Soeda T et al. Thin-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur. Heart J. 33, 78–85 (2012).
- Madder RD, Stone GW, Erlinge D, Muller JE. The search for vulnerable plaque – the pace quickens. J. Invasive Cardiol. 25(Suppl. A), 29A–33A (2013).