Research Article - Neuropsychiatry (2017) Volume 7, Issue 6
Plasma Fibrinogen: An Independent Risk Factor for Post- Stroke Depression
- Corresponding Author:
- Jincai He
Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, 325000, Zhejiang Province, China
Tel: +86 0577 55579363
Fax: +86 0577 55579363
Abstract
Abstract
Background: Post-stroke depression (PSD) is a disease with high incidence in stroke patients. It is reported that about one third of stroke patients may develop depression after stroke. Elevated levels of plasma fibrinogen are found in stroke patients. Our study aims to find out whether there is a relationship between plasma fibrinogen level and PSD.
Methods and Findings: 406 acute ischemic stroke patients were admitted to our study. Plasma fibrinogen levels were assayed at hospital admission and measured by the method of Clauss and a Stago auto analyer with STA fibrinogen kit (Diagnostic Stago, Taverny, France). Clinical depression was diagnosed according to DSM-V criteria and a HAMD 17 score of ≥ 7 one month after the stroke.
Results: We found that 140 patients (34.5%) developed PSD, PSD group had a higher levels of plasma fibrinogen (3.73 g/l ±0.92), which was statistically different with non-PSD group (3.24 g/l ±0.73) (P<0.001). Meanwhile, elevated plasma fibrinogen levels (≥3.69 g/l) were independently associated with PSD, which developed at one month after acute ischemic stroke (OR 3.446, 95%CI 1.839-6.456, P<0.001).
Conclusions: Patients with a high level of plasma fibrinogen at admission are related to the development of PSD and may be an independent predictor for the generation of depression one month after stroke.
Keywords
Depression, Acute stroke, Fibrinogen, Plasma, Risk factor, Post stroke, Ischemic stroke
Introduction
Depression is a very common mental symptom in stroke patients. It is reported that about one third of the stroke patients will suffer from poststroke depression [1-3]. Post-stroke depression not only leads to poor functional outcome, but also increases mortality [4]. Furthermore, patients with post-stroke depression are more likely to reduce the quality of life and heavy the burden of a family [5-7]. With its high incidence and adverse event rates, it needs more attention. However, the mechanisms of the disease are still complicated and unknown.
Fibrinogen is an important blood coagulation factor and inflammatory factor. Previous studies have shown that fibrinogen levels are strongly associated with the risk of stroke [8]. Higher clot burden is related to more severe stroke symptoms and poor outcome [8,9]. High levels of fibrinogen may result in psychological distress. Marie et al conducted a study including 73367 individuals from the general population and found a positive association between fibrinogen and depression [10]. However, some studies found controversial conclusions [11].
High fibrinogen level is associated with increased risks of not accomplishing, of giving up, of self-reported antidepressant medication, of prescription antidepressant medication, and of hospitalization with depression [12]. However, whether increased level of fibrinogen is associated with post-stroke depression is still unknown. Accordingly, we focus on patients with poststroke depression to discover the relationship.
Methods
▪ Subjects
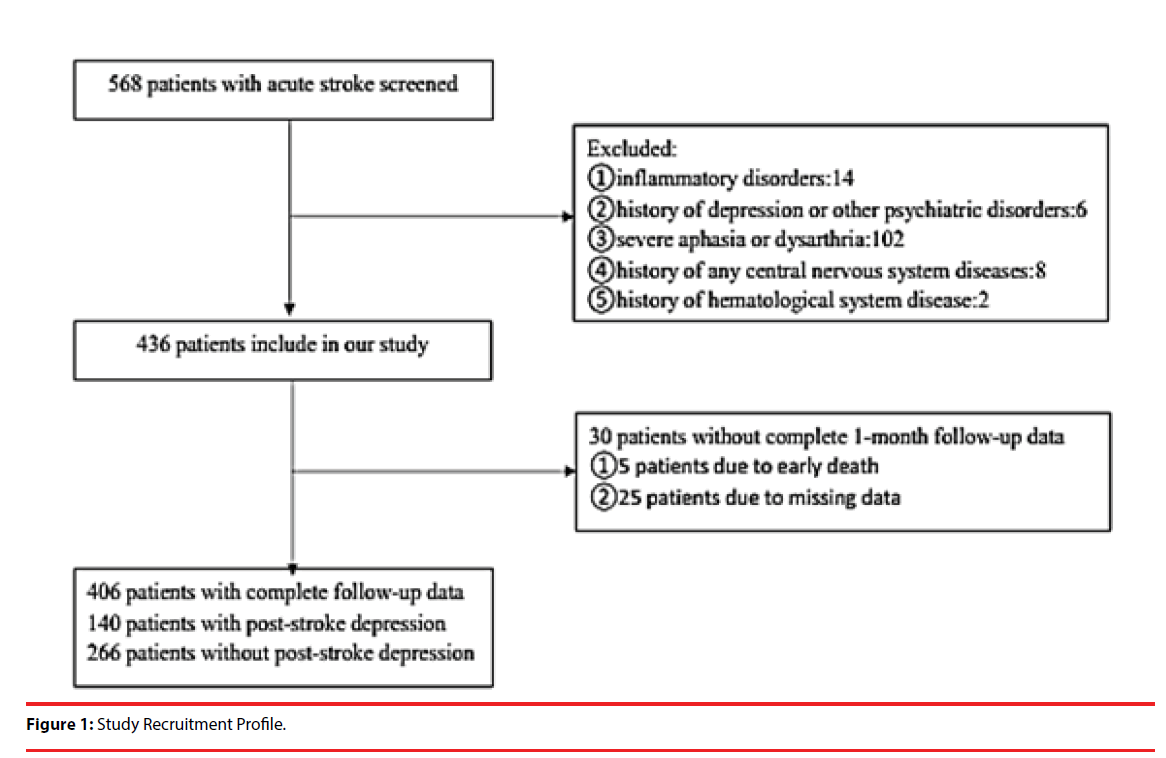
406 patients with acute ischemic stroke from the First Affiliated Hospital of Wenzhou Medical University’s Stroke Unit between January 2013 and June 2015 were admitted to our study. The study was approved by the hospital’s Medical Ethics Committee and patients or their relatives signed the informed consent. The inclusion criteria were as follows: (1) age 18– 80 years; (2) acute stroke occurring within 7 days before admission; (3) voluntary signature of informed consent. The exclusion were as follows: (1) people with inflammatory disorders, as Fibrinogen is one of the acute phase reactants; (2) patients with a history of depression (clinical diagnosis or previous treatment) or other psychiatric disorders; (3) patients with severe aphasia or dysarthria that can’t complete the evaluation; (4) patients with a history of any central nervous system diseases such as dementia, Parkinson’s disease or tumour; (5) patients with a history of hematological system disease, such as coagulation disorders. The flowchart of filtered patients was shown in Figure 1.
▪ Clinical Measures
Depressive symptoms were evaluated one month after stroke. All patients were screened using the 17-item Hamilton Depression Scale by doctors holding professional qualifications who were blind to Laboratory results. Patients with a Hamilton Depression Scale score of ≥7 were given the diagnosis of PSD according to DSM IV.
Stroke severity was assessed by experienced neurologists using the National Institutes of Health Stroke Scale (NIHSS) at admission. Functional outcome was assessed by the Barthel Index (BI) and the modified Rankin Scale (mRS) at one month follow-up.
▪ Fibrinogen Measurements
The blood samples were analysed as soon as possible in an independent laboratory blinded to clinical and nervous system data in the First Affiliated Hospital of Wenzhou Medical University. We obtained blood samples from antecubual vein and placed them in plastic tubes which contain 3.8% trisodium citrate or ethylenediaminetetraacetic acid (EDTA)·K2 anticoagulant. Blood was centrifuged at 4°C at 2,500 × g for 15 min. Our study complied by the principle from Declaration of Helsinki. FIB was measured by a STAGO Coagulation analyzer (Diagnostica Stago, Asnières, France). The STA®-fibrinogen kit (Diagnostica Stago) is intended for use with STA-R®analyzers for the quantitative determination of FIB levels in plasma using the clotting method of Clauss.
Statistical analysis
For categorical variables, the results were indicated as percentages. For continuous variables, it depends on their normal distribution by mean standard deviation (SD) or median (interquartile range, IQR). We used the Chisquared test for proportions and used the Student t test and analysis of variance (ANOVA) to analysis statistics. Plasma fibrinogen levels were divided into tertiles (≤ 2.97g/l, 2.99 – 3.68 g/l, ≥ 3.69 g/l). We recorded the number of patients in each tertiles. The influence of plasma fibrinogen level on PSD was estimated by binary logistic regression analysis. In the meanwhile, the results were indicated as adjusted odds ratios (ORs) (95% confidence intervals, CIs). All statistical analysis was used SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL). Statistical significance was identified as P < 0.05.
Results
▪ Demographics and symptoms
Among the 406 patients with acute ischemic stroke, 140 patients develop depression symptoms, at the percentage of 34.5%. In the depression group, about 58.27% were male patients, while in the non-depression group, male patients accounting for 68.54% (P=0.037). The patients didn’t have significant differences in BMI, educational years and income. There is no significant difference of their vascular risk factors and lesion location in two groups. Furthermore, NIHSS score, BI score, mRS score are statistically significant between depression group and nondepression group (Table 1), the same as the past studies [13,14].
| Clinical characteristics | Depression (140) | Non-Depression (266) | p |
|---|---|---|---|
| Demographics | |||
| Age (years), Mean ± SD | 62.43 ± 11.36 | 62.52 ±10.02 | 0.846 |
| Gender (male/female) | 81/58 | 183/84 | 0.037* |
| BMI (kg/m2) | 24.33 ± 4.28 | 24.57 ±7.81 | 0.646 |
| Educational years, Mean ± SD | 3.99 ± 3.55 | 4.74± 5.70 | 0.169 |
| Income, Mean ± SD | 2.38 ± 1.15 | 2.45± 1.16 | 0.581 |
| Vascular risk factors | |||
| History of hypertension | 93 | 203 | 0.925 |
| History of diabetes | 33 | 61 | 0.460 |
| History of hyperlipidaemia | 16 | 22 | 0.164 |
| CAD | 8 | 22 | 0.506 |
| Current smoking | 36 | 89 | 0.695 |
| Current drinking | 34 | 116 | 0.071 |
| History of stroke | 16 | 25 | 0.314 |
| Lesion location | |||
| Frontal lobe | 40(18.1) | 13(16.7) | |
| Parietal lobe | 25(11.3) | 10(12.8) | |
| Temporal lobe | 18(8.1) | 8(10.3) | |
| Occipital lobe | 21(9.5) | 7(9.0) | |
| Basal ganglia | 68 (30.8) | 22(28.2) | |
| Brainstem | 22(10.0) | 9(11.5) | |
| Cerebellum | 19(8.6) | 6(7.7) | |
| Other | 8(3.6) | 3(3.8) | |
| Neuropsychological function | |||
| NIHSS score, median (IQR) | 2 (1-3) | 1 (1-2) | <0.001* |
| BI score, median (IQR) | 95(80-100) | 100(95-100) | <0.001* |
| mRS score, median (IQR) | 2(1-3) | 1(1-2) | <0.001* |
| WBC | 7.11 ±1.89 | 6.70±1.92 | 0.033* |
| HSCRP | 9.15 ±17.76 | 5.25 ±12.74 | 0.024* |
| FIB (nmol/l), mean SD | 3.73±0.92 | 3.24±0.73 | <0.001* |
The P values reflect comparisons between PSD group and non-PSD group.
Table 1: Clinical and Demographic Characteristics of the Samples under Study.
▪ Laboratory index of depression and non-depression patients
Of the 140 post-stroke depression patients, the mean of WBC level was 7.11*109/L ± 1.89, while the non-depression group was 6.70*109/L ± 1.92, the difference was statistically significant (P=0.033). Furthermore, the result of hsCRP level was higher in depression patients than non-depression patients (9.15mg/l ± 17.76 vs. 5.25mg/l ± 12.74). Plasm fibrinogen level was significantly higher in PSD patients compared to non-PSD patients (3.73 g/l ± 0.92 vs. 3.24 g/l ± 0.73).
▪ Predictors of the development of depression
We observed significant differences between the depression patients and non-depression patients in the plasm fibrinogen levels tertiles of patients (P<0.001) (Table 2 and 3). The distribution of patients in the highest tertile (≥3.69 g/l) was significantly higher in the depression patients (p<0.001). With all patients taken as a whole, PSD occurrence taken as a dependent variable and tertile 1 and tertile 3 taken as the reference used for FIB levels in the logistic analysis, FIB levels (≥3.69g/l) were independently associated with the development of PSD (OR 3.446, 95%CI 1.839-6.456, P<0.001). However, other factors have no significantly independent association with depression.
| PSD patients (n=78) | Non-PSD patient (n=221) | P value | |
|---|---|---|---|
| FIB, n(% of total population) | <0.001 | ||
| Quartile 1 | 63(45.0%) | 65(24.4%) | <0.001 |
| Quartile 2 | 46(32.9%) | 92(34.6%) | 0.727 |
| Quartile 3 | 31(22.1%) | 109(41.0%) | <0.001 |
Table 2: Plasma Fibrinogen levels tertiles of patients.
| Variables | OR(95%CI) | P value |
|---|---|---|
| FIB | 0.001 | |
| Tertile 1 | 0.068 | |
| Tertile 3 | 3.446(1.839-6.456) | <0.001 |
| SEX | 0.103 | |
| NIHSS | 0.471 | |
| BI score | 0.119 | |
| mRS score | 0.116 | |
| WBC | 0.276 | |
| HSCRP | 0.855 |
Table 3: Multivariate logistic model of the clinical determinants of post-stroke depression.
Discussion
Several researches have examined the relationship between fibrinogen and stroke, on the fields of whether high levels of fibrinogen are related to the prevalence and mortality of stroke [9,15,16]. Our research is the first research which observes the levels of fibrinogen and its relationship with post-stroke depression.
Fibrinogen is an acute phase protein, which is produced by Hepatocytes. Human fibrinogen is an independent predictor of coronary heart disease and stroke [17]. Elevated fibrinogen levels are associated with a blood coagulation condition [18]. A lot of evidence suggests that fibrinogen is the mediator in the development of coronary artery thrombi and future cardiac events [19]. High levels of fibrinogen are related to stroke severity and increased mortality in one year after stroke and may result in poor functional outcome [15,16]. Patients with lower initial fibrinogen levels had better functional outcomes when adjusting age factors and initial stroke severity [8].
Fibrinogen levels seem to be higher in stroke patients compared with the non-stroke patients. Many inflammatory markers will increase after stroke, such as C-reactive protein and D-Dimer levels [16,20]. Inflammatory markers like C-reactive protein, and fibrinogen, are associated with the acute-phase response so as to be associated with risk of recurrent stroke [21]. Vascular endothelial injury can activates up regulation of hepatic fibrinogen and initiates coagulation cascade [22]. On the other hand, fibrinogen levels can be raised by many physiological variables and inflammatory conditions, such as be raised by the cytokine interleukin-6 because of inflammatory stimulus [10,15]. Cytokine interleukin-6 is increased in plasma and cerebrospinal fluid when ischemic stroke occurs [23]. Elevated cytokine interleukin-6 may result in the elevated of fibrinogen. Studies also have showed that interleukin-6 is strongly related to mental disorder both in men and women. In addition, animal studies have showed that fibrinogen has an intrinsically central nervous system toxicity, which may increase the severity of stroke [24].
Depression is becoming more and more common in the modern society. Elevated plasma fibrinogen level is connected with major depression disorder [25]. Depression may be bidirectionally linked with an unhealthy lifestyle such as lack of activity and smoking and finally turns out that pro-inflammatory markers such as CRP or fibrinogen may increase [26]. Depression was believed as a chronic inflammation process by a frequently cited hypothesis. Inflammation biomarkers, such as CRP and fibrinogen were related to major depression disorder [11]. Studies have found that depressive patients have higher levels of Cytokine interleukin-6,[27] suggesting an active inflammatory state. The endothelium can produce anticoagulant factors like thrombomodulin. Thrombomodulin is an anticoagulant by formatting a thrombin– thrombomodulin complex, which can inhibit fibrin formation [28]. Endothelial dysfunction, such as change of thrombomodulin levels, has some connection with depression [29].
Fibrinogen can induce rapid and sustained microglial responses; fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation [30]. Stroke is a serious injury to brain neurons, axonal remodeling is common after stroke, [31] High levels of fibrinogen may accelerate axonal injury and increase stroke severity. Fibrinogen might reflect clot perturbations on a finer scale [9,32]. High fibrinogen levels can help to form denser and less permeable clots, more resistant to lysis, which may lead to thrombosis and stroke. However, elevated PAI-1 can impair fibrinolysis. Components of the hemostasis system, such as PAI-1, could also be related to depression pathogenesis [28]. Animal experiment has shown that inject fibrinogen into mice cortices induced microglial responses and the development of axonal damage [32]. These changes can influence white matter fibres may have something to do with the depression after stroke [33].
Limitations
Our study still has some limitations. Firstly, the sample size is not really large, lead to a decrease in statistical credibility of the results. Secondly, we removed severe stroke patients and patient’s comorbid with other nervous disease, which may result in an error in assessing the incidence of the disease. Fourthly, only the baseline period fibrinogen was measured and lack of dynamic observations, it has caused the collection data and analysis results not adequate enough. Lastly, we did not collect the information of D-Dimer. But in the future, we will include more detailed information of lab investigations in this study and we will try to make this research even deeper.
Conclusion
In summary, our study found a strong relationship between fibrinogen and post-stroke depression. Patients with a high level of FIB are more likely to develop PSD one month after stroke; fibrinogen is an independent risk factor of post-stroke depression.
Acknowledgements
Authors Jincai He, Xiaoqian Luan, Huiping Shen designed the study and wrote the protocol. Authors Huijun Chen, Kai Zhao, Huihua Qiu conducted literature searches and provided summaries of previous research studies. Author XiaoqianLuan conducted the statistical analysis. Author Xiaoqian Luan wrote the first draft of the manuscript and all authors contributed to and have approved the final manuscript.
Funding
This research was supported by a grant from Wenzhou Municipal Sci-Tech Bureau Program (Y20160002) and National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant number: 2015BAI13B01). We are greatly indebted to the staff and to the patients with stroke for their contributions during this study.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Abbreviations
PSD: Post-Stroke Depression; BMI: Body Mass Index; CAD: Coronary Artery Disease; NIHSS: National Institutes Of Health Stroke Scale; BI: Barthel Index; Mrs: Modified Rankin Scale; FIB: Fibrinogen; WBC: White Blood Cell; Hscrp: High Sensitivity C Reactive Protein; HAMD: Hamilton Depression Scale; NIHSS: National Institutes of Health Stroke Scale; SD: Standard Deviation; IQR: Interquartile Range; OR: Odds Ratios; CI: Confidence Intervals.
References
- Towfighi A, Ovbiagele B, El Husseini N, et al. Poststroke Depression: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 48(7), e30-e43 (2017).
- Babkair LA. Risk Factors for Poststroke Depression. J. Neurosci. Nurs 49(2), 73-84.
- Rogers SC. Poststroke Depression Screening: An Executive Summary.. J. Neurosci. Nurs 49(2), 66-68 (2017).
- Yue Y, Liu R, Cao Y, et al. New opinion on the subtypes of poststroke depression in Chinese stroke survivors. Neuropsychiatr. Dis. Treat 13(1), 707-713 (2017).
- Kapoor A, Lanctot KL, Bayley M, et al. "Good Outcome" Isn't Good Enough: Cognitive Impairment, Depressive Symptoms, and Social Restrictions in Physically Recovered Stroke Patients. Stroke 48(6), 1688-1690 (2017).
- Lu CY, Huang HC, Chang HH, et al. Acupuncture Therapy and Incidence of Depression After Stroke. Stroke 48(7), 1-7 (2017).
- Lee S-J, Hong JM, Lee SE, et al. Association of fibrinogen level with early neurological deterioration among acute ischemic stroke patients with diabetes. BMC. Neurol 7(1), 101 (2017).
- del Zoppo GJ, Levy DE, Wasiewski WW, et al. Hyperfibrinogenemia and functional outcome from acute ischemic stroke. Stroke 40(5), 1687-1691 (2009).
- Pikija S, Trkulja V, Mutzenbach JS, et al. Fibrinogen consumption is related to intracranial clot burden in acute ischemic stroke: a retrospective hyperdense artery study. J. Transl. Med 14(1), 250 (2016).
- Wium-Andersen MK, Ørsted DD, Nordestgaard BG. Elevated plasma fibrinogen, psychological distress, antidepressant use, and hospitalization with depression: Two large population-based studies. Psychoneuroendocrinology 38(5), 638-647 (2013).
- Baune BT, Neuhauser H, Ellert U, et al. The role of the inflammatory markers ferritin, transferrin and fibrinogen in the relationship between major depression and cardiovascular disorders - The German Health Interview and Examination Survey. Acta. Psychiatr. Scand 121(2), 135-142 (2010).
- Wium-Andersen MK, rsted DDO, Nordestgaard BG. Association between elevated plasma fibrinogen and psychological distress, and depression in 73,367 individuals from the general population. Mol. Psychiatr 18(1), 854-855 (2012).
- Wu C, Ren W, Cheng J, et al. Association Between Serum Levels of Vitamin D and the Risk of Post-Stroke Anxiety. Medicine (Baltimore) 95(18), e3566 (2016).
- Zhu L, Han B, Wang L, et al. The association between serum ferritin levels and post-stroke depression. J. Affect. Disord 190(1), 98-102 (2016).
- Di Napoli M, Papa F. Should neurologists measure fibrinogen concentrations? J. Neurol. Sci 46(1-2), 5-9 (2006).
- Swarowska M, Janowska A, Polczak A, et al. The Sustained Increase of Plasma Fibrinogen During Ischemic Stroke Predicts Worse Outcome Independently of Baseline Fibrinogen Level. Inflammation 37(4), 1142-1147 (2014).
- Wang L, Li L, Wang H, Liu J. Study on the influence of oxidative stress on the fibrillization of fibrinogen. Biochem. J 473(23), 4373-4384 (2016).
- Bielak LF, Klee GG, Sheedy PF 2nd, et al. Association of fibrinogen with quantity of coronary artery calcification measured by electron beam computed tomography. Arterioscler. Thromb. Vasc. Biol 20(9), 2167-2171 (2000).
- Koenig W. Fibrin(ogen) in cardiovascular disease: an update. Thromb. Haemost 89(4), 601-609 (2003).
- Zang R, Zhang H, Xu Y, et al. Serum C-reactive protein, fibrinogen and D-dimer in patients with progressive cerebral infarction. Transl. Neurosci 7(1), 84-88 (2016).
- Welsh P, Lowe GDO, Chalmers J, et al. Associations of Proinflammatory Cytokines With the Risk of Recurrent Stroke. Stroke 39(8), 2226-2230 (2008).
- Di Napoli M, Singh P. Is Plasma Fibrinogen Useful in Evaluating Ischemic Stroke Patients?: Why, How, and When. Stroke 40(5), 1549-1552 (2009).
- Gredal H, Thomsen BB, Boza-Serrano A, et al. Interleukin-6 is increased in plasma and cerebrospinal fluid of community-dwelling domestic dogs with acute ischaemic stroke. Neuroreport 28(3), 134-140 (2017).
- Ryu JK, McLarnon JG. VEGF receptor antagonist Cyclo-VEGI reduces inflammatory reactivity and vascular leakiness and is neuroprotective against acute excitotoxic striatal insult. J. Neuroinflammation 5(1), 18 (2008).
- Martins-de-Souza D, Maccarrone G, Ising M, et al. Plasma fibrinogen: now also an antidepressant response Transl. Psychiatry 4(1), e352-4 (2014).
- Hamer M, Molloy GJ, de Oliveira C, et al. Persistent depressive symptomatology and inflammation: to what extent do health behaviours and weight control mediate this relationship? Brain. Behav. Immun 23(4), 413-418 (2009).
- Eyre HA, Air T, Pradhan A, et al. A meta-analysis of chemokines in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 68(1), 1-8 (2016).
- Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, et al. Plasma levels of thrombomodulin, plasminogen activator inhibitor-1 and fibrinogen in elderly, diabetic patients with depressive symptoms. Aging. Clin. Exp. Res 28(5), 843-851 (2015).
- van Sloten TT, Schram MT, Adriaanse MC, et al. Endothelial dysfunction is associated with a greater depressive symptom score in a general elderly population: the Hoorn Study. Psychol. Med 44(7), 1403-1416 (2013).
- Davalos D, Ryu JK, Merlini M, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun 3(1), 1227 (2012).
- Okabe N, Narita K, Miyamoto O. Axonal remodeling in the corticospinal tract after stroke: how does rehabilitative training modulate it? Neural. Regen. Res 12(2), 185-192 (2017).
- Okafor ON, Gorog DA. Endogenous Fibrinolysis: An Important Mediator of Thrombus Formation and Cardiovascular Risk. J. Am. Coll. Cardiol 65(16), 1683-1699 (2015).
- Hattori K, Ota M, Sasayama D, et al. Increased cerebrospinal fluid fibrinogen in major depressive disorder. Sci. Rep 5(1), 11412 (2015).