Case Series - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 5
Pneumocystis jirovecii pneumonia in inflammatory rheumatic systemic diseases under immunosuppressive therapy: Two case reports, current overviews
- Corresponding Author:
- Gabriel Dischereit
Department of Rheumatology
Osteology, Physical Medicine
Justus-Liebig-University Giessen
Kerckhoff-Klinik, Bad Nauheim, Germany
E-mail: gabriel.dischereit@med.uni-giessen.de
Abstract
Infection with pneumocystis jirovecii is one of the most common AIDS (Acquired Immune Deficiency Syndromes) defining diseases. There is an increased risk of clinical apparent pneumocystis jirovecii infections due to cellular immunodeficiency. Other patients with a higher risk for an infection with pneumocystis jirovecii are those with malignant diseases, condition after stem cell transplantation or after transplantation of solid organs, and patients receiving immunosuppressive therapy due to autoimmune disorders. Therefore, patients with inflammatory rheumatic diseases are often at an increased risk for opportunistic infections such as pneumocystis jirovecii pneumonia (PcP). The PcP is transmitted aerogenically or it can be a result of reactivation of a latent infection after immunosuppression. Leading symptoms are dyspnea on effort, coughing and sub febrile temperature. For detection of the pathogen staining methods or molecular genetic methods are used. Furthermore, imaging, in particular highresolution computed tomography, plays an essential role. The most important medication for treatment is Trimethoprim and Sulfamethoxazole. Sometimes an adjuvant corticosteroid treatment is recommended, whereas evidence for benefits in patients with inflammatory rheumatic disease is lacking. A primary chemoprophylaxis is recommended for patients on high intensive or combined immunosuppression or under high-dose systemic corticosteroid treatment. Also in this case Trimethoprim/Sulfamethoxazole are treatment of choice. Here we describe two case reports on clinical pneumocystis jirovecii infection under immunosuppressive medication in patients with rheumatic diseases and we give an overview on current literature with regard to rheumatic therapy options.
Keywords
pneumocystis jirovecii pneumonia, inflammatory rheumatic diseases, immunosuppressive therapy, methotrexate, biological
Introduction
Infection with pneumocystis jirovecii is one of the most common AIDS (Acquired Immune Deficiency Syndromes) defining diseases. There is an increased risk of clinical apparent pneumocystis jirovecii infections due to cellular immunodeficiency. Other patients with a higher risk for an infection with pneumocystis jirovecii are those with malignant diseases, condition after stem cell transplantation or after transplantation of solid organs, and patients receiving immunosuppressive therapy due to autoimmune disorders. Therefore, patients with inflammatory rheumatic diseases are often at an increased risk for opportunistic infections such as pneumocystis jirovecii pneumonia (PcP). The PcP is transmitted aerogenically or it can be a result of reactivation of a latent infection after immunosuppression. Leading symptoms are dyspnea on effort, coughing and sub febrile temperature.
Pneumocystis jirovecii - the pathogen
The initial description of a Pneumocystis infection in human’s dates from to van der Meer and Brug in 1942 [1]. Initially, an infection with a protozoon was assumed, whereas more recent phylogenetic investigations based on rRNA sequences, despite the absence of ergosterol in the cell wall, suggest a closer relationship to Regnum Fungi [2]. Biomolecular and immunological studies suggest that the causes of Pneumocystis-associated diseases in humans and animals differ, leading to a reclassification of the organism isolated from human samples as Pneumocystis jirovecii, whereas P. carinii describes the pathogen in rodentlike animals [3]. The abbreviation PcP, which is commonly used in clinical terminology, continues to be used and now stands for Pneumocystis pneumonia. Besides geographical factors, the risk for the occurrence of PcP is largely dependent on the underlying disease and the pharmacotherapy used [4]. Various pathomechanisms play a role, which can favour the occurrence of infections, reinfections and reactivations of latent infections [5].
Pneumocystis infections
There is an increased risk of clinical apparent pneumocystis infections due to cellular immunodeficiency, which, also after introduction of antiretroviral therapy, is one of the most common AIDS (Acquired Immune Deficiency Syndromes) defining diseases [6]. Other patients at increased risk are those with malignant diseases, condition after stem cell transplantation or after transplantation of solid organs, and patients receiving immunosuppressive therapy due to systemic inflammatory rheumatic disease [4].
Diagnosis of PcP
Sensitivity and specificity data of diagnostic methods for detection and exclusion of PcP are derived from studies of Human Immunodeficiency Virus (HIV) infected patients in AIDS stage. The analysis of spontaneous sputum and the morning sputum showed only a low sensitivity, which could be increased to about 95% by induction of stimulus sputa by hypertonic NaCl solution [7]. To detect the pathogen, immunofluorescence methods are used. If, despite the induction of stimulus sputa, no sufficient amount of sputum can be obtained, or if, despite negative examination findings, there is still a clinical and laboratory suspicion of PcP, bronchoscopy with BAL should be attempted. By means of toluidine blue staining, Grocott silver staining, modified Giemsa staining and immunofluorescence, diagnosis was confirmed in over 80% of all patients and in over 95% of all patients with AIDS. Repetitive transbronchial biopsies considerably increased the diagnostic yield [8-10]. However, the detection of pathogens by PCR from a BAL is now regarded as gold standard [11].
Imaging with PcP
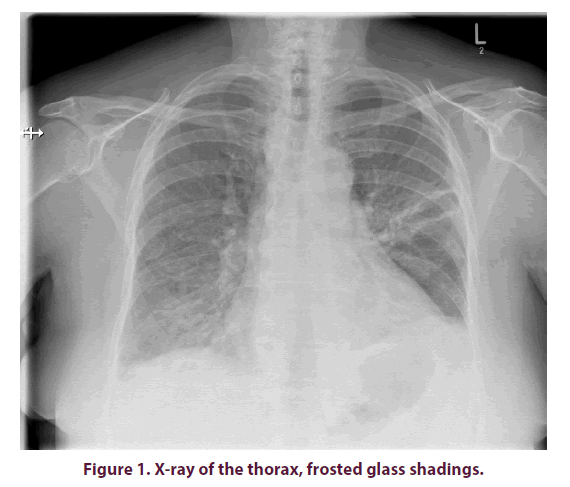
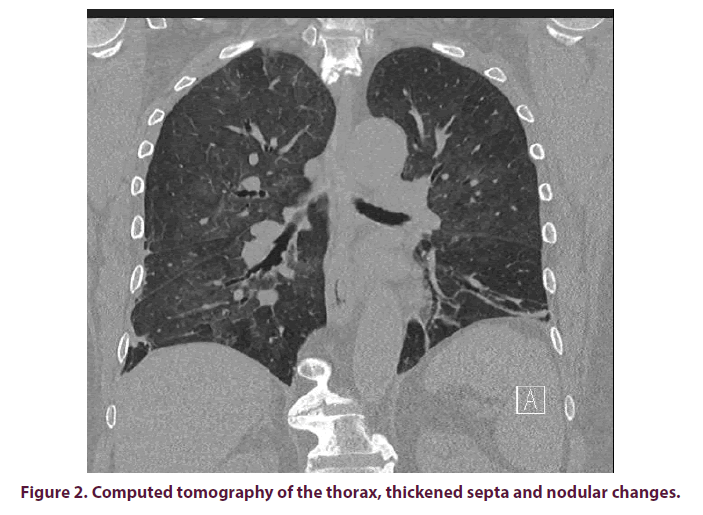
X-ray images of the thorax often show diffuse bilateral, frost-like shadows (Figure 1). As described in the above case report, the pulmonary X-ray findings can, at least initially, also impress inconspicuously. If there is a well-founded suspicion and a corresponding indication, a CT of the thorax should therefore be sought at an early stage. In presence of PcP, this usually shows hilarily emphasized frosted glass-like turbidity, thickened septa and nodular changes, as well as granulomas and cysts in some cases (Figure 2). Pleural effusions are rather atypical [12].
PcP in patients with inflammatory rheumatic diseases
The number of documented PcP cases in patients with inflammatory rheumatic diseases has increased since the 80ies. On the one hand, this may be due to the fact that immunosuppressive drugs are given more frequently, in higher doses and increasingly in combination [13], on the other hand, since the late 1990s, biologics have been at the disposal of practitioners an evergrowing armamentarium of immunomodulatory active substances which promote susceptibility to Opportunistic Infections (OI) [14]. Increased sensitivity and specificity of diagnostic procedures probably also contribute to an increased detection of PcP [13].
In case HIV-negative patients with confirmed evidence of PcP are examined, it becomes apparent that the proportion of inflammatory rheumatic diseases is not insignificant, with PcP occurring almost exclusively under immunosuppressive therapy. In a collection of 78 PcP cases between 1980 and 1993, the proportion of vasculitis and other autoimmune diseases was 22%, in patients diagnosed with granulomatosis with polyangiitis (GPA; Wegener granulomatosis) accounting for the largest proportion [15]. In other studies, an increased risk of PcP of up to 12% could be demonstrated in patients with GPA as well [4,16,17]. In other inflammatory rheumatic systemic diseases, the occurrence of PcP appears to be significantly less frequent. The incidence of PcP in one case collection was 0.13% in RA, 0.8% in Systemic Lupus Erythematosus (SLE), 1.2% in panarteritis nodosa and 2% in polymyositis/dermatomyositis [16,17]. Due to the very small number of cases, however, these figures are probably not representative. In addition, there are individual case reports of PcP in psoriatic arthritis (PsA), SHARP syndrome (mixed connective tissue disease - MCTD) and giant cell arteritis [18,19]. PcP without immunosuppressive therapy is reported in 2 cases of SLE [16].
The majority of cases of PcP occurred during the first 3 months after initiation of immunosuppressive therapy. The mortality rate is high and averages about 40%; if mechanical ventilation is required, it even rises to over 60% [20].
PcP for patients with inflammatory rheumatic diseases under therapy with conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs)
PcP data for inflammatory rheumatic diseases under csDMARDs can at best be described as poor and are almost exclusively based on individual case reports. Thus, there are 2 case reports of patients with RA under MTX monotherapy with a 7- or 8-month substitution period of 15 mg MTX 1x/week each before the occurrence of PcP. Both cases have in common that additionally a systemic steroid therapy of 10 mg or 12.5 mg prednisolone equivalent per day was available [21]. An analysis of 20 cases of PcP in MTX pretreated RA patients also showed that PcP occurred predominantly in the first months after MTX exposure, usually within one year, with lymphopenia and systemic steroid therapy reported in 2/3 of the cases [21].
A 70-year old patient suffering from longstanding RA received 7.5 mg MTX 1x/week in combination with 7.5 mg prednisolone per day due to vasculitic complications and developed a PcP after 2.5 months of therapy, so that even under low-dose MTX an increased susceptibility can be assumed [22]. This is supported by a further case study in which a 62-year-old patient with RA developed pancytopenia and PcP after 12 weeks of low-dose MTX therapy. The authors report on 13 documented cases of PcP under MTX (literature search) and postulate from the few available data an increased risk for the occurrence of pancytopenia under MTX in patients over 60 years, renal insufficiency and intake of NSAIDs [23].
Combination of MTX with trimethoprim/ sulfamethoxazole (TMP/SMX) appears to induce severe pancytopenia in individual cases. A 64-year-old RA patient is reported to have undergone low-dose MTX therapy to develop PcP, and to have presented severe pancytopenia after initiation of TMP/SMX, which persisted even after discontinuation of TMP/SMX. The pulmonary situation of the patient, who now requires ventilation, could only be improved and a recovery of the cell lines observed by switching to pentamidine [24]. On the other hand, the occurrence of PcP with low-dose MTX therapy in RA is considered by some authors to be the reason for a general recommendation for prophylaxis with TMP/SMX [23,25] This must certainly be judged rather cautiously on the basis of the limited data available.
PcP in patients with inflammatory rheumatic diseases under biological (bDMARD) and targeted synthetic DMARD (tsDMARD) therapy
The few available data on the risk of PcP in patients with inflammatory rheumatic systemic diseases under biologic and tsDMARD therapy originate from individual case reports [26], case series and register data. Since many patients receive MTX in combination with a biologic or a tsDMARD, an isolated risk assessment for monotherapy with a substance from one of the two substance classes is virtually impossible based on the currently available data. The situation is aggravated by the fact that the majority of patients have a permanent or temporary need for steroids, for example in the acute relapse of the underlying disease. The resulting immunosuppressive effects must also be taken into account when assessing the risk of PcP under biologic or tsDMARD therapy.
Abatacept
Abatacept (ABC) treatment was associated with a slightly higher risk of severe infection in an integrated safety analysis of over 4000 patients with RA and Juvenile Idiopathic Arthritis (JIA) with over 10,000 Patient Years (PY). Several fungal infections were observed, including aspergillosis, blastomycosis and systemic candidosis, but no PcP [27]. In addition, there are a number of other ABC studies with several thousand PY in which no occurrence of PcP was observed [28-31]. A Colombian working group published the only case report of a PcP under ABC. Due to a worsening of general condition with dry cough, dyspnea and fever (38.5°C), a 64-year-old RA patient who had already been treated with ABC for 4 years was admitted to hospital via the emergency department. Besides appropriate imaging, BAL was used to detect P. jirovecii. It should be mentioned that the dose of ABC was 750 mg per month intravenously and at the same time there was a co-medication with methotrexate 15 mg per week. In addition, it is not clear whether the patient had additional systemic steroid therapy in the period prior to infection [32].
Apremilast
For apremilast, no data are available on the risk of PcP occurrence.
Interleukin(IL)-1 inhibitors
A study with almost 2800 RA patients showed an increased risk of severe infections in high dose Anakinra (≥ 100 mg/day). PcP was observed neither in the treatment arm nor in the placebo group [33,34].
Canakinumab
For Canakinumab, no data are available on the risk of PcP occurrence.
Interleukin(IL)-6 inhibitors
RA patients who were treated with Tocilizumab (TCZ, 8 mg/kg bw every 4 weeks i.v.) and MTX had almost twice the risk of developing severe infections in comparison to patients who were treated with MTX monotherapy [35]. In a combined analysis of over 4000 RA patients receiving TCZ with a mean duration of 2.4 years, 11 cases of invasive fungal infections occurred, including 6 cases of invasive candidosis and one case each of PcP and Cryptococcus pneumonia. All these infections occurred in patients receiving TCZ at a dose of 8 mg/kg bw every 4 weeks, with the exception of PcP, within the dose was only 4 mg/kg bw every 4 weeks. No OI were observed in 1555 patients in the placebo arms of the studies [36]. In another study of 600 RA patients treated with TCZ monotherapy, the risk of severe respiratory infection in the TCZ group was twice as high as in the age and gender comparable control group treated with other therapies including non-biological(nb) DMARDs. However, the respiratory tract infections in the studies cited were primarily bacterial pneumonia. However, there was no significant difference between the two groups with regard to the risk of invasive fungal infections [37].
Sarilumab
For Sarilumab, no data on the risk of PcP are available yet.
Interleukin(IL)-17A inhibitors
In two randomized phase III studies 2400 psoriasis patients with two different doses of Ixekizumab (IXZ) were compared to Etanercept (ETC) or placebo. 80 mg of IXZ every two weeks (12 infections/1.6 % of patients) showed about twice as many infections of the skin, oral cavity, esophagus and urogenital tract than under IXZ 80 mg every 4 weeks (4/0.5%), ETC (5/0.6%) or placebo (2/0.6%) [38]. Except for one single infection, all cases could be adequately treated with oral or topical standard therapy, IXZ was not discontinued in any of the cases. No other fungal infections, in particular the occurrence of PcP, were observed. In a 64-week open-label extension study in over 300 RA patients treated with IXZ, there was no evidence of invasive fungal infections, particularly no report of the occurrence of PcP [39].
In two Randomized Controlled Trials (RCTs) with Secukinumab (SEM) versus ETC and versus placebo in 1200 psoriasis patients, the risk of infection was higher in the SEM arms than in the ETC or placebo arms. Candida infections were more frequent in SEM than in ETC and placebo, with 2-5% of patients in the SEM groups developing Candida infections only temporarily during the observation period. None of the infections resulted in chronic mucocutaneous candidosis or discontinuation of SEM and all cases were either self-limited or well treatable with standard therapy [40]. Similar results with an increased risk for noninvasive Candida infection were obtained with another RCT with SEM in patients with PsA and Ankylosing Spondylitis (AS) [41,42]. So far, the occurrence of PcP under SEM has not been described. However, since the available RTCs were predominantly associated with relatively short follow-up periods, data on the long-term risk of infection under SEM are lacking.
Januskinase (JAK) inhibitors
In one RTC, in which Tofacitinib (TOF) was compared at doses of 10 mg/d and 5 mg/d respectively in combination with csDMARDs (predominantly MTX) with csDMARD monotherapy, the rates of severe infections in the group with the higher dose TOF were higher than in the groups with the lower dose TOF and also higher than in the placebo arm. One of 391 patients in the 10 mg TOF arm developed a Cryptococcus pneumonia after 6 months of treatment. None of the other study arms showed invasive fungal infections [43]. A combined safety analysis of TOF involving more than 5000 patients observed 0.21 OI per 100 PY not associated with tuberculosis. Patients with concomitant systemic steroid therapy were expected to have a higher risk of OIs than patients without steroids. Invasive fungal infections detected in this study included esophageal candidiasis (n=9), PcP (n=4) and cryptococcus infections (n=3) [44]. In a Swiss study, a 78-yearold male RA patient undergoing TOF, MTX and low dose steroid therapy was admitted to hospital due to severe arthralgia and nausea. Among other things, severe hypercalcemia prompted the colleagues to perform a CT of the thorax in which bilateral pneumonic infiltrates showed up. PCR from BAL was used to detect P. jirovecii. Hypercalcemia decreased under the PcP therapy, other possible causes were excluded. The authors postulate an increased suspicion of PcP with the simultaneous presence of hypercalcemia and pneumonic infiltrates even with otherwise rather atypical clinical symptoms [45].
Baricitinib
No data on the risk of occurrence of PcP are available for baricitinib.
Rituximab
The overall attrition risk of severe infections from rituximab (RTX) compared to placebo does not appear to be elevated in RA patients [33]. OIs have a low incidence of 0.05/100 PY. In a 10- year follow-up study in RA patients treated with RTX (almost 12,000 years of observation), 7 OIs were reported with three fungal infections: candidiasis, Scedosporium pneumonia and PcP [46].
Tumour necrosis factor inhibitors (TNFi)
A retrospective analysis of British registry data revealed 14 confirmed PcP cases under TNFi therapy in 13,905 RA patients. Of these, 9 patients had a combination with MTX, 8 patients were under systemic steroid therapy at the time of infection. In summary, the authors postulate a rather low additional risk for the occurrence of PcP under TNFi therapy, but final conclusions are not drawn due to the small number of cases. The infections occurred at an early stage in treatment with a TNFi (on average 5.8 months after the start of therapy), which was also observed for tuberculosis (TB) and could argue for the reactivation of a latent infection [47].
Retrospectively, a Japanese working group examined 702 RA patients for whom biologic therapy was initiated during the course of the disease. 281 patients received Infliximab (IFX), 197 ETC, 117 Adalimumab (ADM) and 107 TCZ. 78.5 % of the patients received MTX therapy at the same time in a mean dose of 8.96 mg/week. Originally, 141 patients received primary prophylaxis with TMP/SMX due to an increased risk of PcP (long-term steroid therapy, age ≥ 65 years, pulmonary involvement). The group of patients with primary prophylaxis did not develop PcP during an observation period of more than 16 months. The 561 patients without primary prophylaxis showed an outbreak of PcP in 9 cases in average about 7 months after initiation of the biologic therapy. In comparison to the 552 patients with no primary prophylaxis, 9 patients with PcP were significantly older, had higher steroid doses and a higher proportion of pulmonary comorbidities [48].
Occurrence of PcP in TNFi therapy ranges widely from <0.01 per 1000 patient years in North America and Europe to 8.8 per 1000 patient years in Japan [49]. The different incidence is due to the application of different test methods and to differences in the actual geographical distribution of the disease. Research has revealed that up to a quarter of RA patients are asymptomatically populated with P. jirovecii. Treatment with MTX and glucocorticoids as well as the use of IFX for more than 3 years are regarded as risk factors for colonisation. In summary, the risk of a PcP occurring under ADM and IFX is estimated to be somewhat higher than under ETC [50]. For Certolizumab and Golimumab there are only a few case reports suggesting a slightly increased risk of infection compared to placebo. The data were obtained from patients with RA, AS, PsA, M. Crohn (MC) and ulcerative colitis [51-55].
Ustekinumab
Psoriasis patients under treatment with Ustekinumab (USM) had a lower risk of developing a severe infection compared to IFX and other biologics [56]. In a pooled safety analysis of USM in 3000 patients with psoriasis no invasive fungal infections were observed in the follow-up of 1.7 years [57,58]. RCT in 312 adults with PsA also showed no cases of invasive fungal infections in the USM arm, especially no PcP [59]. MC and AS patients treated with USM did not show invasive fungal infections and especially no PcP in the investigations and observation periods performed [60,61].
Prophylaxis and therapy of PcP for patients with inflammatory rheumatic diseases
The usefulness of primary prophylaxis should be evaluated on the basis of the individual risk for the development of PcP, the adverse drug reactions and the expected morbidity and mortality. In principle, primary prophylaxis should be considered in case of long-term steroid therapy (≥ 20mg/day prednisolone equivalent for at least 4 weeks) with simultaneous immunodeficiency or chronic obstructive pulmonary disease [62]. Systemic vasculitis plays a special role in primary prevention of autoimmune diseases. Primary prophylaxis should be indicated in vasculitis patients under immunosuppressive combination therapy (e.g. MTX+prednisolone>15mg/day) as well as in ANCA-associated vasculitis (ANCA: anti-neutrophilic cytoplasmic antibodies) under treatment with cyclophosphamide, especially if systemic steroid therapy is also in place. Patients with GPA or Microscopic Polyangiitis (MPA) are recommended for prophylaxis, if necessary, during or after RTX treatment, according to the specialist information [63]. For RA patients under TNFi therapy, primary prophylaxis is recommended when 2 of the following factors apply: Long-term steroid therapy, age ≥ 65 years or pulmonary involvement or concomitant disease [48].
Secondary prophylaxis is indicated as long as the immunosuppressive therapy of the underlying disease has to be continued. TMP/SMX is used for prophylactic treatment in a dose of 160/800 mg 3 times per week, which is comparable to the daily dose of 80/400 mg in terms of effectiveness. Much better tolerated and potentially similarly effective seems to be the daily administration of 40/200 mg TMP/SMX [64]. Alternatively, inhaled pentamidine, dapsone and atovaquone can be administered, whereby the use of the latter two drugs is an off-label use.
The treatment of choice for PcP in patients with and without HIV is TMP/SMX. Due to frequently occurring gastrointestinal side effects with oral administration, intravenous therapy is preferred, at least initially. For trimethoprim the standard is 15-20 mg/kg bw per day. Side effects of toxicity are common and can lead to gastrointestinal, cutaneous, renal, hepatic and hematological complications, limiting therapeutic adherence. There are approaches for lower dosages (e.g. TMP 10 mg/kg bw per day), which are also considered effective by some authors [65,66]. The initial therapy with a mean TMP dose (10-15 mg/kg bw per day) and partial further dose reduction during the course (4-6 mg/kg bw per day) in selected patients seemed to be safe in a retrospective analysis (104 patients) and did not affect the treatment outcome [67]. Under TMP/SMX therapy, blood count, electrolytes, liver and kidney values must be checked at least 3 times a week.
Different therapy alternatives are available, but they are either less well tolerated or less effective. Intravenous pentamidine is best studied and similarly effective as TMP/SMX. Side effects such as glucose metabolism disorders and nephrotoxicity, however, limit its regular use. Atovaquon is better tolerated but less effective than TMP/SMX. Further alternatives with less evidence are Dapson plus Trimethoprim and Clindamycin plus Primaquin. Adjuvant corticosteroid administration reduces mortality in HIV patients with hypoxemic respiratory insufficiency by approximately 40% [68]. This has not yet been proven for patients without HIV. However, case series usually also show a benefit of systemic steroid therapy.
After a possible initial deterioration at the beginning of therapy, an improvement of the clinical condition should occur after about 7 days. A change in therapy should therefore not be made hastily. For all patients, the recommended therapy duration is 21 days, although a therapy duration of 14 days is also discussed for patients without HIV due to the lower pathogen load [69,70]. Corticosteroids should be administered for 5 to 10 days, but not more than 14 days [71]. Hospital mortality is still high in severe PcP (> 60%), with delayed intubation, prolonged ventilation and the occurrence of pneumothorax worsening the prognosis [72].
Case reports
Case 1
A 74-year-old female patient with a long-term course of HLA-B 27 positive spondyloarthritis (SpA) with leading peripheral joint involvement had been undergoing a combination of antirheumatic basic therapy for the past 16 months, consisting of methotrexate (MTX 7.5 mg s.c. 1x per week) and leflunomide (LEF 20 mg p.o. daily), which had been well tolerated by the patient so far under mandatory controls. There was no systemic steroid therapy.
As a result of deterioration of general condition with fatigue, weight loss, back pain, dry cough and stress dyspnea, the patient was admitted to hospital in December 2016. The patient neither reported any arthralgia, nor was there any morning stiffness in the joints. Thoracic pain could not be determined either.
In physical examination the patient showed a reduced general condition. Blood pressure was 100/70 mmHg, the pulse rate was 90/min and respiratory rate was 18/min. Auscultatory findings of the lung revealed a ubiquitous rattling noise on both sides. There were no abnormalities in heart auscultation. No joint swellings could be clinically delimited. In addition, there were no signs of congestion or oedema.
The laboratory examination showed an increased humoral inflammatory activity (blood sedimentation rate [BSR] 81 mm/h, C-reactive protein [CRP] 7.4 mg/dl) and discrete leukopenia (6.7/nl) were observed. The blood gas analysis (capillary blood) revealed a reduced oxygen partial pressure (pH 7.51, BE 1.9 mmol/l, pO2 57 mmHg, pCO2 30 mmHg). A lung function test was not possible due to the reduced general condition of the patient.
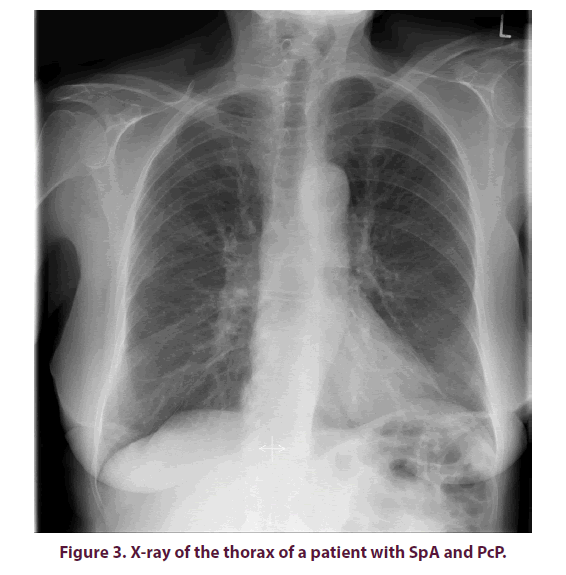
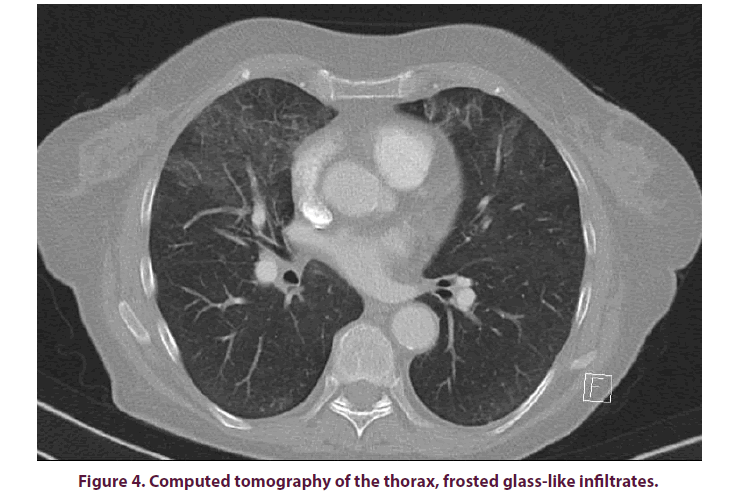
A nativradiological thoracic x-ray was taken to further clarify the cough and stress dyspnea, but no abnormalities were found (Figure 3). With progressive deterioration of condition with a CRP increase of up to 19.4 mg/dl and a procalcitonin (PCT) level 10 times higher than normal (1.02 ng/ml), a Computed Tomography (CT) of the thorax was performed, showing extensive flat, frost-like impressive infiltrates in all lung lobes (Figure 4). A bronchoscopy with Broncho Alveolar Lavage (BAL) was performed as blood cultures collected so far did not prove any germs. Pneumocystis jirovecii was detected from the lavage by Polymerase Chain Reaction (PCR).
A pronounced oxygenation disorder made it necessary to transfer the patient to intensive care and to use temporary, non-invasive ventilation. The application of an i.v. antibiotic therapy with high-dose trimethoprim/sulfamethoxazole (cotrimoxazole) and piperacillin/tacobactam as well as a systemic steroid therapy resulted in a rapid improvement of the clinical condition and the humoral inflammatory activity. After transfer to the peripheral ward, the i.e. antibiotic therapy was continued for a total of 14 days with further improvement of the pulmonary situation and finally oralization of the medication. In case of cotrimoxazole-associated bizytopenia, which occurred in the course of the disease and decreased with a treatment interruption, the therapy was resumed in prophylactic dosage after normalization of the blood count and continued for a total of 6 months on 3 days per week.
Case 2
A 76-year-old female patient with seronegative, Cyclic Citrullinated Peptide (CCP) autoantibodies of negative Rheumatoid Arthritis (RA) was diagnosed with MTX 15 mg/week in August 2016 and treated with MTX 15 mg/ week. (s.c.) and a low-dose steroid therapy with 5 mg prednisolone equivalent per day.
Due to acute resting and stress dyspnea in October 2018 the patient was admitted to hospital on suspicion of MTX-associated pneumonitis. Other clinical symptoms, such as recurrent subfebrile temperatures and an unproductive cough, were present for about 2 weeks at the time of admission. There was no clinical activity of the inflammatory rheumatic disease.
During physical examination a speech dyspnea, a tachypnea and a reduced general condition were noticed. Fever could not be measured. Pulse and blood pressure values were documented as standard. Basal crackles could be auscultated bilaterally.
Laboratory chemistry showed a significantly elevated CRP of 11.4 mg/dl with discrete leukocytosis of 11.3/ml. The PCT level was 3-4 times elevated (0.35 ng/ml). In addition, renal dysfunction was found (eGFR of 34 ml/min and creatinine 1.7 mg/dl). The blood gas analysis (capillary blood) showed a reduced oxygen partial pressure (pO2 43 mmHg, pH 7.56, BE 4.4 mmol/l, pCO2 29 mmHg). A lung function test was not possible due to reduced general condition of the patient.
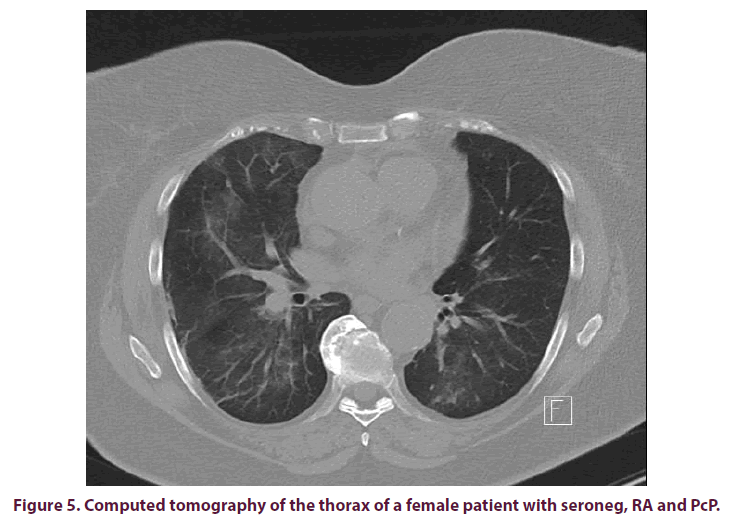
A CT of the lung was performed for further clarification and this revealed bilateral crosssegment pneumonitis (Figure 5). Intermittent, Non-Invasive Ventilation (NIV) had to be performed. The patient initially received an empirical antibiotic therapy with piperacillin/ tacobactam under suspicion of atypical pneumonia, while MTX was stopped immediately.
Despite increasing CRP values up to 13.4 mg/ dl and conventional radiological suspicions of increasing pulmonary infiltrates, the overall situation stabilised and a bronchoscopy could be performed. P. jirovecii was detected from the bronchial secretion both culturally and by PCR. The diagnosis of a Pneumocystis pneumonia was made. The antibiotic therapy was switched to cotrimoxazole. This resulted in a significant improvement of the clinical symptoms and a normalization of the laboratory parameters. The lung function test revealed a restrictive ventilation disorder during the final examination.
Discussion
PcP is a common, life-threatening complication in patients with inflammatory rheumatic systemic diseases requiring rapid, targeted diagnosis and effective therapy. Type and intensity of immunosuppression as well as gaps in the prophylaxis concept and special exposures play a crucial role in the risk of infection. Planning the diagnostic procedure often requires high-resolution CT. The standard method for pathogen extraction is flexible bronchoscopy with BAL. Close collaboration with the microbiologist should be sought to ensure optimal yield. Non-invasive antigen determinations and PCR tests complement the diagnostic spectrum. A standardized, guidelineoriented procedure in the initial therapy should be aimed for.
Summary and conclusion for use in practice
• Patients with inflammatory and rheumatic systemic diseases treated with immunosuppressive therapy have an increased risk of developing PcP.
• Among the factors that influence the risk of PcP are underlying disease, age, pulmonary disease and immunosuppressive therapy.
• Pathogen detection is usually achieved by smearing and special staining or by PCR from bronchoalveolar lavage. A cultivation is not possible in clinical everyday life.
• The therapy of PcP is primarily performed with trimethoprim/sulfamethoxazole. As long as the immunosuppressive therapy of the underlying disease has to be continued, secondary prophylaxis is indicated after having undergone PcP.
• For patients receiving high-dose or combined immunosuppressive therapy due to the underlying inflammatory rheumatic disease, primary prophylaxis with trimethoprim/ sulfamethoxazole should be considered.
Conflict of interest
None.
References
- van der Meer G, Brug SL. Infection à pneumocystis chez l'homme et chez les animaux. Ann. Soc. Belge Med. Trop. 22, 301–309 (1942).
- Ena J, Amador C, Pasquau F et al. Once-a-month administration of intravenous pentamidine to patients infected with human immunodeficiency virus as prophylaxis for Pneumocystis carinii pneumonia. Clin. Infect. Dis. 18(6), 901–904 (1994).
- Stringer JR, Beard CB, Miller RF et al. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerging. Infect. Dis. 8(9), 891–896 (2002).
- Rodriguez M, Fishman JA. Prevention of infection due to Pneumocystis spp. in human immunodeficiency virus-negative immunocompromised patients. Clin. Microbiol. Rev. 17(4), 770–782 (2004).
- Bertisch B, Ruef C. Pneumocystis-jiroveci-Pneumonie (PcP) bei Patienten mit rheumatologischen Erkrankungen. Fallbeschreibung und Review (Pneumocystis jiroveci pneumonia (PcP) in patients with rheumatic diseases: case report and review). Z. Rheumatol. 65(1), 18–20, 22–23 (2006).
- Kaplan JE, Hanson D, Dworkin MS et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 30(Suppl 1), 14 (2000)
- Leigh TR, Parsons P, Hume C et al. Sputum induction for diagnosis of Pneumocystis carinii pneumonia. Lancet. 2(8656), 205–206 (1989).
- Broaddus C, Dake MD, Stulbarg MS et al. Bronchoalveolar lavage and transbronchial biopsy for the diagnosis of pulmonary infections in the acquired immunodeficiency syndrome. Ann. Intern. Med. 102(6), 747–752 (1985).
- Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin. Infect. Dis. 33(8), 1397–1405 (2001).
- Stover DE, Zaman MB, Hajdu SI et al. Bronchoalveolar lavage in the diagnosis of diffuse pulmonary infiltrates in the immunosuppressed host. Ann. Intern. Med. 101(1), 1–7 (1984).
- Saito K, Nakayamada S, Nakano K et al. Detection of Pneumocystis carinii by DNA amplification in patients with connective tissue diseases: re-evaluation of clinical features of P. carinii pneumonia in rheumatic diseases. Rheumatology (Oxford). 43(4), 479–485 (2004).
- Kanne JP, Yandow DR, Meyer CA. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR Am J Roentgenol 198(6), W555–561 (2012).
- Ward MM, Donald F. Pneumocystis carinii pneumonia in patients with connective tissue diseases: the role of hospital experience in diagnosis and mortality. Arthritis. Rheum. 42(4), 780–789 (1999)
- Tai TL, O'Rourke KP, McWeeney M et al. Pneumocystis carinii pneumonia following a second infusion of infliximab. Rheumatology (Oxford). 41(8), 951–952 (2002).
- Arend SM, Kroon FP, van't Wout JW. Pneumocystis carinii pneumonia in patients without AIDS, 1980 through 1993. An analysis of 78 cases. Arch. Intern. Med. 155(22), 2436–2441 (1995).
- Godeau B, Coutant-Perronne V, Le Thi Huong D et al. Pneumocystis carinii pneumonia in the course of connective tissue disease: Report of 34 cases. J. Rheumatol. 21(2), 246–251 (1994).
- Ognibene FP, Shelhamer JH, Hoffman GS et al. Pneumocystis carinii pneumonia: a major complication of immunosuppressive therapy in patients with Wegener's granulomatosis. Am. J. Respir. Crit. Care. Med. 151(3), 795–799 (1995).
- Krebs S, Gibbons RB. Low-dose methotrexate as a risk factor for Pneumocystis carinii pneumonia. Mil. Med. 161(1), 58–60 (1996).
- Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo. Clin. Proc. 71(1), 5–13 (1996).
- Khellaf M, Godeau B. Pneumocystis pneumonia among patients with systemic diseases. Presse. Med. 38(2), 251–259 (2009).
- Roux N, Flipo RM, Cortet B et al. Pneumocystis carinii pneumonia in rheumatoid arthritis patients treated with methotrexate. A report of two cases. Rev. Rheum. Engl. Ed. 63(6), 453–456 (1996).
- Okuda Y, Oyama T, Oyama H et al. Pneumocystis carinii pneumonia associated with low dose methotrexate treatment for malignant rheumatoid arthritis. Ryumachi. 35(4), 699–704 (1995).
- Kitsuwa S, Matsunaga K, Kawai M et al. Pancytopenia and Pneumocystis carinii pneumonia associated with low dose methotrexate pulse therapy for rheumatoid arthritis- case report and review of literature. Ryumachi. 36(3), 551–558 (1996).
- Shimada T, Nishimura Y, Funada Y et al. A case of pneumocystis carinii pneumonia associated with low dose methotrexate treatment for rheumatoid arthritis and trimethoprim-sulphamethoxazole induced pancytopenia. Arerugi. 53(6), 575–581 (2004).
- Kaneko Y, Suwa A, Ikeda Y et al. Pneumocystis jiroveci pneumonia associated with low-dose methotrexate treatment for rheumatoid arthritis: report of two cases and review of the literature. Mod. Rheumatol. 16(1), 36–38 (2006).
- Kuroda T, Takeuchi H, Nozawa Y et al. Acute exacerbation of interstitial pneumonia associated with rheumatoid arthritis during the course of treatment for Pneumocystis jirovecii pneumonia: a case report. BMC. Res. Notes. 9, 240 (2016).
- Schiff M. Abatacept treatment for rheumatoid arthritis. Rheumatology (Oxford). 50(3), 437–449 (2011).
- Lovell DJ, Ruperto N, Mouy R et al. Long-term safety, efficacy, and quality of life in patients with juvenile idiopathic arthritis treated with intravenous abatacept for up to seven years. Arthritis. Rheum. 67(10), 2759–2770 (2015).
- Kremer JM, Peterfy C, Russell AS et al. Longterm safety, efficacy, and inhibition of structural damage progression over 5 years of treatment with abatacept in patients with rheumatoid arthritis in the abatacept in inadequate responders to methotrexate trial. J. Rheumatol. 41(6), 1077–1087 (2014).
- Alten R, Kaine J, Keystone E et al. Long-term safety of subcutaneous abatacept in rheumatoid arthritis: integrated analysis of clinical trial data representing more than four years of treatment. Arthritis. Rheum. 66(8), 1987–1997 (2014).
- Weinblatt M, Combe B, Covucci A et al. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: A one-year randomized, placebo-controlled study. Arthritis. Rheum. 54(9), 2807–2816 (2006).
- Ospina FE, Agualimpia A, Bonilla-Abadía F et al. Pneumocystis jirovecii Pneumonia in a Patient with Rheumatoid Arthritis Treated with Abatacept. Case. Rep. Rheumatol. 2014, 835050 (2014).
- Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann. Rheum. Dis. 68(1), 25–32 (2009).
- Mertens M, Singh JA. Anakinra for rheumatoid arthritis. Cochrane. Database. Syst. Rev. (1), CD005121 (2009).
- Campbell L, Chen C, Bhagat SS et al. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford). 50(3), 552–562 (2011).
- Schiff MH, Kremer JM, Jahreis A et al. Integrated safety in tocilizumab clinical trials. Arthritis. Res. Ther. 13(5), R141 (2011).
- Hoshi D, Nakajima A, Inoue E et al. Incidence of serious respiratory infections in patients with rheumatoid arthritis treated with tocilizumab. Mod. Rheumatol. 22(1), 122–127 (2012).
- Griffiths CEM, Reich K, Lebwohl M et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 386(9993), 541–551 (2015).
- Genovese MC, Braun DK, Erickson JS et al. Safety and Efficacy of Open-label Subcutaneous Ixekizumab Treatment for 48 Weeks in a Phase II Study in Biologic-naive and TNF-IR Patients with Rheumatoid Arthritis. J. Rheumatol. 43(2), 289–297 (2016).
- Langley RG, Elewski BE, Lebwohl M et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 371(4): 326–338 (2014).
- Baeten D, Sieper J, Braun J et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N. Engl. J. Med. 373(26), 2534–2548 (2015).
- Mease PJ, McInnes IB, Kirkham B et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 373(14), 1329–1339 (2015).
- Kremer J, Li Z-G, Hall S et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 159(4), 253–261 (2013).
- Winthrop KL, Park S-H, Gul A et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann. Rheum. Dis. 75(6), 1133–1138 (2016).
- Pirker IFJ, Krane-Nuber J, Albrich WC et al. Atypical presentation of Pneumocystis jirovecii pneumonia in a patient with rheumatoid arthritis treated with tofacitinib: a case presentation. BMC. Rheumatol. 2, 34 (2018).
- van Vollenhoven RF, Fleischmann RM, Furst DE et al. Longterm Safety of Rituximab: Final Report of the Rheumatoid Arthritis Global Clinical Trial Program over 11 Years. J. Rheumatol. 42(10), 1761–1766 (2015).
- Bruce ES, Kearsley-Fleet L, Watson KD et al. Risk of Pneumocystis jirovecii pneumonia in patients with rheumatoid arthritis treated with inhibitors of tumour necrosis factor α: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford). 55(7), 1336–1337 (2016).
- Katsuyama T, Saito K, Kubo S et al. Prophylaxis for Pneumocystis pneumonia in patients with rheumatoid arthritis treated with biologics, based on risk factors found in a retrospective study. Arthritis. Res. Ther. 16(1), R43 (2014).
- Grubbs JA, Baddley JW. Pneumocystis jirovecii pneumonia in patients receiving tumor-necrosis-factor-inhibitor therapy: Implications for chemoprophylaxis. Curr. Rheumatol. Rep. 16(10), 445 (2014).
- Vallabhaneni S, Chiller TM. Fungal Infections and New Biologic Therapies. Curr. Rheumatol. Rep. 18(5), 29 (2016).
- Bykerk VP, Cush J, Winthrop K et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann. Rheum. Dis. 74(1), 96–103 (2015).
- Mease PJ, Fleischmann R, Deodhar AA et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann. Rheum. Dis. 73(1), 48–55 (2014).
- Sandborn WJ, Lee SD, Randall C et al. Long-term safety and efficacy of certolizumab pegol in the treatment of Crohn's disease: 7-year results from the PRECiSE 3 study Aliment. Pharmacol. Ther. 40(8), 903–916 (2014).
- Sandborn WJ, Feagan BG, Marano C et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 146(1), 85-95 (2014).
- Kay J, Fleischmann R, Keystone E et al. Golimumab 3-year safety update: an analysis of pooled data from the long-term extensions of randomised, double-blind, placebo-controlled trials conducted in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Ann. Rheum. Dis. 74(3), 538–546 (2015).
- Papp K, Gottlieb AB, Naldi L et al. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J. Drugs. Dermatol. 14(7), 706–714 (2015).
- Papp KA, Griffiths CEM, Gordon K et al. Long-term safety of ustekinumab in patients with moderate-to-severe psoriasis: final results from 5 years of follow-up. Br. J. Dermatol. 168(4), 844–854 (2013).
- Gordon KB, Papp KA, Langley RG et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J. Am. Acad. Dermatol. 66(5), 742–751 (2012).
- Ritchlin C, Rahman P, Kavanaugh A et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 73(6), 990–999 (2014).
- Sandborn WJ, Gasink C, Gao LL et al. Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N. Engl. J. Med. 367(16), 1519–1528 (2012).
- Poddubnyy D, Hermann KGA, Callhoff J et al. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 73(5), 817–823 (2014).
- Carmona EM, Limper AH. Update on the diagnosis and treatment of Pneumocystis pneumonia. Ther. Adv. Respir. Dis. 5(1), 41–59 (2011).
- Moosig F, Holle JU, Gross WL. Value of anti-infective chemoprophylaxis in primary systemic vasculitis: what is the evidence? Arthritis. Res. Ther. 11(5), 253 (2009).
- Utsunomiya M, Dobashi H, Odani T et al. Optimal regimens of sulfamethoxazole-trimethoprim for chemoprophylaxis of Pneumocystis pneumonia in patients with systemic rheumatic diseases: results from a non-blinded, randomized controlled trial. Arthritis. Res. Ther. 19(1), 7 (2017).
- Shibata T, Tonooka K, Tsuchida K et al. Retrospective investigation of side effects and prognoses of moderate-dose trimethoprim-sulfamethoxazole treatment for pneumocystis pneumonia that developed in patients with autoimmune diseases. Nihon. Rinsho. Meneki. Gakkai. Kaishi. 39(3), 213–218 (2016).
- Thomas M, Rupali P, Woodhouse A et al. Good outcome with trimethoprim 10 mg/kg/day-sulfamethoxazole 50 mg/kg/day for Pneumocystis jirovecii pneumonia in HIV infected patients. Scand. J. Infect. Dis. 41(11-12), 862–868 (2009).
- Creemers-Schild D, Kroon FP, Kuijper EJ et al. Treatment of Pneumocystis pneumonia with intermediate-dose and step-down to low-dose trimethoprim-sulfamethoxazole: lessons from an observational cohort study. Infection. 44(3), 291–299 (2016).
- Ewald H, Raatz H, Boscacci R et al. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV infection. Cochrane. Database. Syst. Rev. (4), CD006150 (2015).
- Cooley L, Dendle C, Wolf J et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern. Med. J. 44(12b), 1350–1363 (2014).
- Tasaka S, Tokuda H. Pneumocystis jirovecii pneumonia in non-HIV-infected patients in the era of novel immunosuppressive therapies. J. Infect. Chemother. 18(6), 793–806 (2012).
- Shibata S, Nishijima T, Aoki T et al. A 21-Day of Adjunctive Corticosteroid Use May Not Be Necessary for HIV-1-Infected Pneumocystis Pneumonia with Moderate and Severe Disease. PLoS ONE. 10(9), e0138926 (2015).
- Festic E, Gajic O, Limper AH et al. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest. 128(2), 573–579 (2005).