Mini Review - Interventional Cardiology (2023)
Polyphenol-rich pequi extract protects human coronary artery endothelial cells against free radicals: Therapeutic potential of a Brazilian native plant
- Corresponding Author:
- M. Ruhul Abid
Division of Cardiothoracic Surgery, Cardiovascular Research Center, Rhode Island Hospital, Warren Alpert Medical School of Brown University, Providence, USA,
E-mail: Ruhul_Abid@brown.edu
Received date: 17-Nov-2022, Manuscript No. FMIC-22-80088; Editor assigned: 21-Nov-2022, PreQC No. FMIC-22-80088 (PQ); Reviewed date: 05-Dec-2022, QC No. FMIC-22-80088;Revised date: 12-Dec-2022, Manuscript No. FMIC-22-80088 (R);Published date: 22-Dec-2022, DOI: 10.37532/1755-5310.2023.15 (S15). 363
Abstract
The imbalance between production and elimination of free radicals such as Reactive Oxygen (ROS) and nitrogen species compromises endothelial cells and results in Cardiovascular Diseases (CVD). The antioxidant system plays an essential role in maintaining the homeostasis of organic functions, by promoting the balance between cellular oxidants and antioxidants. However, in pathophysiological conditions, the endogenous antioxidant defense mechanisms may be insufficient to control and eliminate free radicals accumulated within the cell. The supplement of external antioxidants is suggested to help fight and prevent oxidative lesions such as lipoperoxidation and oxidative DNA damage. Primary natural antioxidants polyphenols are found in a variety of vegetable sources and can assist cells in the neutralization of free radicals. Resveratrol, a non-flavonoid polyphenol abundant in red wine, is the most investigated compound of this class due to the healthy properties attributed to the Mediterranean Diet. Despite its proven beneficial roles in multiple in vitro and small animal research models, resveratrol supplementation has not yet to produce satisfactory results in human clinical trials. Phenolic compounds are secondary metabolites frequently formed in organisms as a defense mechanism against environmental adverse conditions, like drought and severe UV exposure, typical features of the Brazilian Cerrado biome. The pequi (Caryocar brasiliense, Cambess) is a typical Cerrado tree that produces fruits with polyphenol-rich barks. Recently, we have tested pequi bark’s ethanolic extract efficacy in protecting Human Coronary Artery Endothelial Cells (HCAEC) subject to oxidative stress or conditions that result in increased ROS production. Pequi bark extract increased antioxidative enzyme levels, decreased ROS, and favored Endothelial Cells (EC) proliferation, pointing to a protective effect of pequi extract. The therapeutical potential from native fruits worldwide may support treating and preventing cardiovascular disease and become a sustainable economic activity to low-income communities.
Keywords
Alternative medicine . Cardiovascular disease . Caryocar brasiliense . Reactive oxygen species . Pequi; Resveratrol
Introduction
The cellular antioxidant system serves as a crucial player in the maintenance of homeostasis of organic functions by balancing between the cellular oxidant and antioxidant levels. However, internal and external factors can tip the balance towards excessive synthesis of Reactive Oxygen and Nitrogen Species (RONS), generically known as free radicals [1].
RONS encompass two classes of chemically reactive molecules: Reactive Oxygen Species (ROS), which are generated as a byproduct of oxygen metabolism with significant effects on cell signaling and homeostasis, and Reactive Nitrogen Species (RNS), which are byproducts of nitrogen metabolism. The overproduction and accumulation of RONS may result in significant harm, such as oxidation of lipids and proteins, together with DNA damage, triggering chronic and degenerative diseases such as ischemic cardiovascular diseases, the leading cause of mortality in the world [2].
Oxidative stress arises from the imbalance between the formation of RONS and the (lack of) capacity of the antioxidant defense to neutralize and eliminate the harmful effects of excessive free radicals. Transformations in structure and modulation of function associated with the prominent oxidation of biomolecules such as proteins, lipids, carbohydrates, nucleic acids, and cell apoptosis are characteristic of oxidative stress [3]. Such biochemical reactions imply the reduction or loss of critical biological functions, with consequent discontinuity of cellular homeostasis, oxidative damage against cells and tissues, which under extreme conditions, can cause cell death [4,5]. Evidence suggests the significant contribution of oxidative stress in the etiology of numerous degenerative diseases, such as sclerosis, obesity, Parkinson’s, Alzheimer’s, diabetes, cancer, and cardiovascular diseases [6].
In response to free radical accumulation, evolution may have prompted the development of endogenous antioxidant defense mechanisms [7], which in turn can limit the intracellular levels of ROS by modulating cellular redox reactions [8].
The enzymes Superoxide Dismutase (SOD) 1 and 2, Catalase (CAT), and Glutathione Peroxidase (GPx) constitute the first-line enzymatic antioxidant defense system. These enzymes act very smoothly in neutralizing any molecule with the potential of developing into a free radical or any free radical with the ability to induce the production of other radicals, breaking down hydrogen peroxides and hydroperoxides to harmless molecules (H2O2/alcohol and O2) [9].
Nevertheless, this endogenous defense system may become insufficient in controlling and eliminating RONS in high concentrations in the body, generating a dangerous imbalance between oxidation and antioxidation [9,10]. Furthermore, external conditions also disrupt the cellular environment and overload the internal defenses. For example, usual modern life conditions like radiation exposure, environmental toxins, smoking, changes in dietary priorities, excessive intake of alcoholic beverages, and foods rich in carbohydrates and fats, combined with a sedentary lifestyle, increase the generation of free radicals and, consequently, the demand for antioxidant countermeasures [3].
Supplementation might be a reasonable strategy to increase the antioxidants available to fight and prevent oxidative damage [11]. In addition, several researchers and clinicians claim that the long-term combination of antioxidant-rich diets, such as the Mediterranean diet, protects RONS, resulting in a better quality of life by preventing or delaying the development of cardiovascular diseases [12-14].
In this context, exogenous antioxidants are essential molecules that help prevent the formation of RONS, carry out concomitant redox, and repair lesions within the cell. Therefore, supplemental antioxidants are frequently necessary to minimize the adverse reactions caused by oxidative stress. In addition, they inhibit the initiating or developing factors of oxidative chain reactions, which can cause cell death [15].
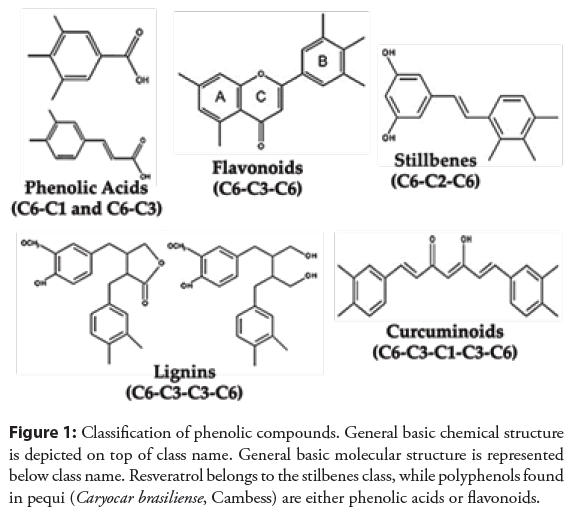
Important exogenous antioxidant sources are found in nature, mainly in flowers, fruits, cereals, spices, and medicinal plants [4]. In a wide variety of vegetables, the primary natural antioxidants recognized as polyphenols (phenolic acids, flavonoids, anthocyanins, lignins, and stilbenes) Shown in the Figure 1, carotenoids (carotenes and xanthophylls), and vitamins (vitamin C and E) are found. Polyphenols exert potential biological anti-inflammatory, anti-atherosclerosis, anti-aging, and anticancer effects [16].
Figure 1: Classification of phenolic compounds. General basic chemical structure is depicted on top of class name. General basic molecular structure is represented below class name. Resveratrol belongs to the stilbenes class, while polyphenols found in pequi (Caryocar brasiliense, Cambess) are either phenolic acids or flavonoids.
Literature Review
General basic molecular structure is represented below class name. Resveratrol belongs to the stilbenes class, while polyphenols found in pequi (Caryocar brasiliense, Cambess) are either phenolic acids or flavonoids.
Stilbenes belong to the class of non-flavonoid polyphenols, and resveratrol is the most studied compound of this class and is present in foods such as grapes, peanuts, berries, and red wine. Despite its low water solubility during digestion, low chemical stability, and thus relatively low bioavailability, resveratrol, particularly as a pivotal component of the Mediterranean Diet, is reported to play an essential role in human health in countries where this particular diet is standard [17].
Once ingested, resveratrol or its precursors move along the gastrointestinal tract, and around 70% of the resveratrol intake is absorbed [13]. In the intestine, resveratrol binds to several nutrients, such as proteins, and this solubility will influence its absorption or elimination in feces. Besides, the gut microbiota is highly responsible for metabolizing resveratrol by increasing its availability from resveratrol precursors and producing resveratrol derivatives [18].
Dr. Sellke’s research group has previously investigated the role of resveratrol in cardiovascular disease [19-28], and type II diabetes/metabolic syndrome [29-33], using pigs as experimental models. Pig models of cardiovascular disease have helped to bridge the gap between the results of in vitro and small animal models and clinical trials, particularly with resveratrol.
In most of the experiments, resveratrol was supplemented in a purified state, mimicking the supplements available in drugstores. Still, red wine was used as the source of resveratrol in two investigations, the first in the ischemic myocardium in a model of endothelial dysfunction [23], and the other in a model of metabolic syndrome to study insulin signaling pathways in the liver and skeletal muscle [31]. Our results suggest that resveratrol ameliorates chronic metabolic disease, improving myocardial perfusion and cardiovascular health. The results with red wine appeared more convincing than those with resveratrol, and we attributed that to the effects of alcohol.
The results of clinical trials to determine the precise mechanism of resveratrol action in cells have been inconclusive due to differences in supplementation and the treated individuals’ characteristics, such as age, sex, presence of single nucleotide polymorphisms, presence of metabolic disturbances, and gut microbiota composition [18]. Therefore, the effects of resveratrol on human health appear to be more beneficial in populations that ingest the polyphenol as formed in nature, as a part of a biological solution from a plant or modified after fermentation grape juice to become wine, and not as an isolated compound.
Phenolic compounds are considered secondary metabolites of vegetables. The secondary metabolites have low molecular weight, present a complex structure, and play a relevant role in the interaction of plants with the environment, especially in phytotherapy, through the production of micronutrients [34]. They are usually generated during the development and reproduction phases of the plants. However, they can be formed under adverse circumstances, as a defense against pathogen and pest infestations, or as an adaptive response to environmental challenges, such as water stress, resulting from climatic adversities with long drought periods and UV exposure radiation [35]. A similar vulnerability has been recently reported to increase polyphenol content in plants, namely the Tartary Buckwheat (Fagopyrum tataricum) [36], the Green Barley (Hordeum vulgare L.) [37], and the Amaranthus leafy vegetable [38].
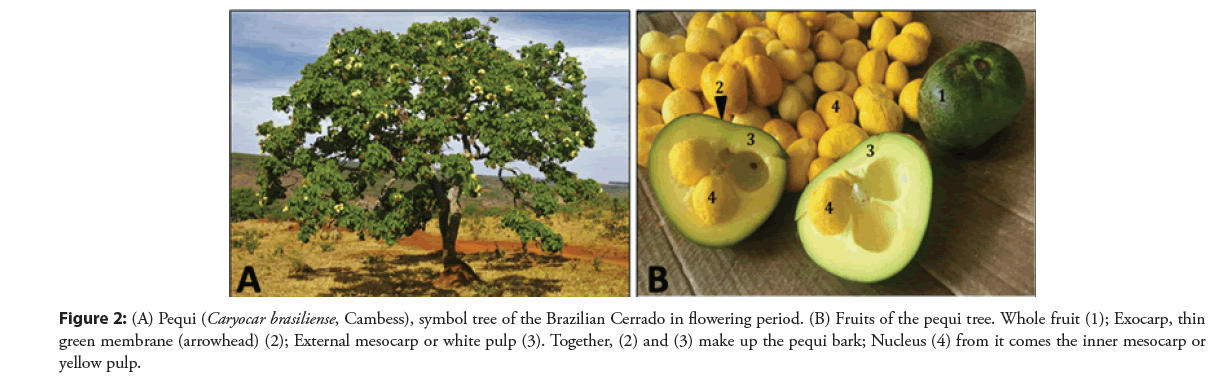
These elements are found in several biomes, especially under extreme conditions like the Brazilian Cerrado, the biologically richest tropical savanna in the world [39]. The pequi is a typical Cerrado tree that bears a remarkable fruit as shown in the Figure 2, that does not go unnoticed because of its pronounced and instigating sensory characteristics of aroma, color, and flavor, which are very particular [40]. The bark of the Caryocar brasiliense fruit amasses significant levels of polyphenols, dietary fiber, lipids, carbohydrates, minerals, proteins, and carotenoids [15].
Figure 2: (A) Pequi (Caryocar brasiliense, Cambess), symbol tree of the Brazilian Cerrado in flowering period. (B) Fruits of the pequi tree. Whole fruit (1); Exocarp, thin green membrane (arrowhead) (2); External mesocarp or white pulp (3). Together, (2) and (3) make up the pequi bark; Nucleus (4) from it comes the inner mesocarp or yellow pulp.
The mechanism of action of the phytochemical constituents of pequi lies in the antioxidant and protective activity against the harmful reactions of RONS in the complex cellular arrangement. Accordingly, expressive results have demonstrated the antioxidant potential of pequi byproducts from the experimental supplementation with the pulp oil in animals, with effective improvement in cardiac function [41].
Discussion
Our research team has recently tested the antioxidant properties of the ethanolic extract obtained from pequi fruit bark in Human Coronary Artery Endothelial Cells (HCAEC) subjected to stress by experimental in vitro hypoxia for 48 hours or 100 μM H2O2 for six hours. Remarkably, in cells supplemented with the extract, the levels of the endogenous first-line defense antioxidative enzymes superoxide dismutase 1 and 2, catalase, and glutathione peroxidase increased. In the meantime, cytosolic and mitochondrial ROS levels decreased, and HCAEC proliferation was boosted [42].
The pequi extract we prepared contained gallic, protocatechuic, gentisic, caffeic, p-coumaric, vanillic, and ellagic acids, as well as catechin, quercetin, epicatechin, rutin, naringenin, luteolin, and kaempferol [42]. Although all these phenolic compounds have been reported to have antioxidant activity per se, it is reasonable to assume that the decrease in ROS levels we observed and its subsidiary effects are consequences of a synergic effect rather than a single molecule action [24].
Conclusion
The pharmaceutical industry has been searching, in the last decades, for drugs that have specific effects on human and animal cells. In the case of antioxidants, however, clinical trials using single molecules, such as the best-known resveratrol, have not been as successful as the in vitro investigations. Unlike single molecules, plant extracts containing different polyphenols affect several important cell signaling pathways, favoring their use in metabolically complex diseases. Thus, natural products and their derivatives, such as plant extracts or juices rich in various polyphenolic compounds, have great potential for use in the treatment and prevention of cardiovascular disease.
Pequi is a genuinely natural and cultural heritage of the Brazilian Cerrado. Furthermore, the pharmacological potential contained in native fruits from all over the world may constitute a natural alternative for treating and preventing cardiovascular disease and a sustainable option for economic exploration to low-income populations that contributes, at the same time, to preserve their autochthonous biome.
References
- Xu DP, Li Y, Meng X, et al. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int J Mol Sci. 18(1):96(2017).
[Crossref] [Google Scholar] [PubMed]
- Kaptoge S, Pennells L, de Bacquer D, et al. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health. 7(10):e1332- e1345 (2019).
[Crossref] [Google Scholar] [PubMed]
- Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 5(1):9-19 (2012).
[Crossref] [Google Scholar] [PubMed]
- Wu P, Li F, Zhang J, et al. Phytochemical compositions of extract from peel of hawthorn fruit, and its antioxidant capacity, cell growth inhibition, and acetylcholinesterase inhibitory activity. BMC Complement Altern Med. 17(1):1-7 (2017).
[Crossref] [Google Scholar] [PubMed]
- Beigrezaei S, Nasri H. Tempol as an antioxidant; An updated review on current knowledge. J Antioxid Act. 2(1) (2017).
- Godic A, Poljšak B, Adamic M, et al. The role of antioxidants in skin cancer prevention and treatment. Oxid Med Cell Longev. (2014).
[Crossref] [Google Scholar] [PubMed]
- Li Y, Zhang JJ, Xu DP, et al. Bioactivities and health benefits of wild fruits. Int J Mol Sci. 17(8):1258 (2016).
[Crossref] [Google Scholar] [PubMed]
- Düsman E, Berti AP, Mariucci RG, et al. Radioprotective effect of the Barbados Cherry (Malpighia glabra L.) against radiopharmaceutical Iodine-131 in Wistar rats in vivo. BMC Complement Altern Med. 14(1):1-9 (2014).
[Crossref] [Google Scholar] [PubMed]
- Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 54(4):287-293 (2018).
- Mattera R, Benvenuto M, Giganti MG, et al. Effects of polyphenols on oxidative stress-mediated injury in cardiomyocytes. Nutrients. 9(5):523 (2017).
[Crossref] [Google Scholar] [PubMed]
- Alam MN, Bristi NJ, Rafiquzzaman M, et al. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J. 21(2):143-52 (2013).
[Crossref] [Google Scholar] [PubMed]
- Cebova M, Pechanova O. Protective effects of polyphenols against ischemia/reperfusion injury. Molecules. 25(15):3469 (2020).
[Crossref] [Google Scholar] [PubMed]
- Incalza MA, D'Oria R, Natalicchio A, et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 100:1-9 (2018).
[Crossref] [Google Scholar] [PubMed]
- Tang GY, Meng X, Li Y, et al. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients. 9(8):857(2014).
[Crossref] [Google Scholar] [PubMed]
- Monteiro SS, da Silva RR, da S Martins SC, et al. Phenolic compounds and antioxidant activity of extracts of pequi peel (Caryocar brasiliense Camb.). Int Food Res J. 22(5) (2015).
- Baiano A, Del Nobile MA. Antioxidant compounds from vegetable matrices: Biosynthesis, occurrence, and extraction systems. Crit Rev Food Sci Nutr. 56(12):2053-68 (2016).
[Crossref] [Google Scholar] [PubMed]
- Bertelli A, Biagi M, Corsini M, et al. Polyphenols: From theory to practice. Foods. 10(11):2595 (2021).
[Crossref] [Google Scholar] [PubMed]
- Chaplin A, Carpéné C, Mercader J, et al. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 10(11):1651(2018).
[Crossref] [Google Scholar] [PubMed]
- Robich MP, Osipov RM, Nezafat R, et al. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 122(11_suppl_1):S142- S149 (2010).
[Crossref] [Google Scholar] [PubMed]
- Chu LM, Lassaletta AD, Robich MP, et al. Resveratrol in the prevention and treatment of coronary artery disease. Curr Atheroscler Rep. 13(6):439-46 (2011).
[Crossref] [Google Scholar] [PubMed]
- Chu LM, Robich MP, Lassaletta AD, et al. Resveratrol supplementation abrogates pro-arteriogenic effects of intramyocardial vascular endothelial growth factor in a hypercholesterolemic swine model of chronic ischemia. Surgery. 150(3): 390-399 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Robich MP, Osipov RM, Chu LM, et al. Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur J Pharmacol. 664(1-3): 45-53 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Lassaletta AD, Chu LM, Elmadhun NY, et al. Cardioprotective effects of red wine and vodka in a model of endothelial dysfunction. J Surg Res. 178(2): 586-592 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Robich MP, Chu LM, Burgess TA, et al. Resveratrol preserves myocardial function and perfusion in remote nonischemic myocardium in a swine model of metabolic syndrome. J Am Coll Surg. 215(5): 681-689 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Lassaletta AD, Chu LM, Sellke FW. Effects of alcohol on pericardial adhesion formation in hypercholesterolemic swine. J Thorac Cardiovasc Surg. 143(4): 953-959 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Elmadhun NY, Sabe AA, Robich MP, et al. The pig as a valuable model for testing the effect of resveratrol to prevent cardiovascular disease. Ann N Y Acad Sci. 1290(1): 130-135 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Sabe AA, Elmadhun NY, Dalal RS, et al. Resveratrol regulates autophagy signaling in chronically ischemic myocardium. J Thorac Cardiovasc Surg. 147(2): 792-799 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Sabe AA, Sadek AA, Elmadhun NY, et al. Investigating the effects of Resveratrol on chronically ischemic myocardium in a swine model of metabolic syndrome: A proteomics analysis. J Med Food 18(1): 60-66 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Robich MP, Chu LM, Chaudray M, et al. Anti-angiogenic effect of high-dose resveratrol in a swine model of metabolic syndrome. Surgery. 148(2): 453-462 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Burgess TA, Robich MP, Chu LM, et al. Improving glucose metabolism with resveratrol in a swine model of metabolic syndrome through alteration of signaling pathways in the liver and skeletal muscle. Arch Surg. 146(5): 556-564 (2011).
[CrossRef] [Google Scholar] [PubMed]
- Elmadhun NY, Lassaletta AD, Chu LM, et al. Vodka and wine consumption in a swine model of metabolic syndrome alters insulin signaling pathways in the liver and skeletal muscle. Surg. 152(3): 414-422 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Sabe AA, Elmadhun NY, Robich MP, et al. Does resveratrol improve insulin signaling in chronically ischemic myocardium? J Surg Res. 183(2): 531-536 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Potz BA, Lawandy IJ, Clements RT, et al. Alcohol modulates autophagy and apoptosis in pig liver tissue. J Surg Res. 203(1): 154-162 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Maria PVI, Hamerski L, Silva GW, et al. Antimicrobial activity of some medicinal plants from the Cerrado of the central-western region of Brazil. Brazilian J Microbiol. 43(4): 1302-1308 (2012).
[CrossRef] [Google Scholar] [PubMed]
- deCastro PJ, Neves BJ, Vasconcelos FG, et al. Flavonoids from brazilian cerrado: Biosynthesis, chemical and biological profile. Molecules. 24(16):2891(2019).
[CrossRef] [Google Scholar] [PubMed]
- Aubert L, Quinet M. Comparison of heat and drought stress responses among twelve tartary buckwheat (Fagopyrum tataricum) Varieties. Plants. 11(11): 1517 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Kowalczewski PŁ, Radzikowska D, Ivanišová E, et al. Influence of abiotic stress factors on the antioxidant properties and polyphenols profile composition of green barley (Hordeum vulgare L.). Int J Mol Sci. 21(2): 397 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Sarker U, Oba S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 18(1): 258 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Dutra RC, Campos MM, Santos ARS, et al. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol Res. 112: 4-29 (2016).
[CrossRef] [Google Scholar] [PubMed]
- Geocze KC, Barbosa LCA, Fidêncio PH, et al. Essential oils from pequi fruits from the Brazilian Cerrado ecosystem. Food Res Int. 54(1): 1-8 (2013).
- Oliveira LG, Moreno LG, Melo DS, et al. Caryocar brasiliense oil improves cardiac function by increasing Serca2a/PLB ratio despite no significant changes in cardiovascular risk factors in rats. Lipids Health Dis. 16(1): 1-8 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Braga KMS, Araujo EG, Sellke FW, et al. Pequi fruit extract increases antioxidant enzymes and reduces oxidants in human coronary artery endothelial cells. Antioxidants 11(3): 474 (2022).
[CrossRef] [Google Scholar] [PubMed]