Special Issue Article - Interventional Cardiology (2015) Volume 7, Issue 4
Potential of 3D-printed models in planning structural interventional procedures
- Corresponding Author:
- Israel Valverde
Division of Imaging Sciences & Biomedical Engineering,
King’s College London, The Rayne Institute, St. Thomas’ Hospital, London, UK
Tel: +34 955 923 000
Fax: +34 955 923 101
E-mail: ivalverde-ibis@us.es
Abstract
The interventional horizon in the field of structural heart disease is increasing as new indications can now be successfully treated for conditions that were previously left untreated or necessitated a surgical approach. The complexity of the interventional skills required to successfully treat these new indications are unprecedented. Interventional planning is therefore essential to identify in advance the existing anatomical issues, evaluate the best interventional strategy to avoid unexpected findings and optimize the procedure time to reduce radiation dose. Furthermore, since many of the cardiovascular anomalies targeted for catheter-based interventions are infrequent and because no two cases are alike, it is difficult to accumulate experience. All these new challenges demand better training and improved planning in the field of structural interventional catheterization. 3D cardiovascular models are an accurate representation of the patient’s anatomy and are a novel and appealing technique, which have a huge potential in the field of intervention in structural heart disease. Its applications range from training staff to simulation of complex and challenging cases. This review article will summarize the basic principles, the techniques and the current applications.Keywords
3D printing, congenital heart disease, rapid prototyping, stereolitography, structural cardiac interventions
The interventional horizon in the field of structural heart disease is increasing as new indications can now be successfully treated for conditions that were previously left untreated or necessitated a surgical approach. The complexity of the interventional skills required to successfully treat these new indications are unprecedented. Interventional planning is therefore essential to identify in advance the existing anatomical issues, evaluate the best interventional strategy to avoid unexpected findings and optimize the procedure time to reduce radiation dose. Furthermore, since many of the cardiovascular anomalies targeted for catheter-based interventions are infrequent and because no two cases are alike, it is difficult to accumulate experience. All these new challenges demand better training and improved planning in the field of structural interventional catheterization.
3D cardiovascular models are an accurate representation of the patient’s anatomy and are a novel and appealing technique, which have a huge potential in the field of intervention in structural heart disease. Its applications range from training staff to simulation of complex and challenging cases. This review article will summarize the basic principles, the techniques and the current applications.
3D printing technology
3D printing is a technology based in the use of virtual objects for producing 3D physical models. It is also referred to as rapid prototyping, computer-aided manufacturing or layered manufacturing, depending on the kind of production method used. The principle of rapid prototyping is to use 3D computer models for the reconstruction of a 3D physical model by the addition of material layers [1]. In other words, medical rapid prototyping use conventional 3D radiological images to generate solid replicas of the anatomical structures [2]. To print a 3D model of the heart, there are some sequential stages (Figure 1): image acquisition of the patientspecific cardiovascular anatomy, segmentation of the 3D images, manipulation of the images into printable pieces using computeraided design and, finally, rapid prototyping techniques to print the model.
Image acquisition
Image acquisition is the most important stage in the design process. A good quality acquisition guarantees a precise and accurate virtual model of the heart. Any medical image containing 3D information can be used, such as MRI, CT or echocardiography. MRI and CT are very useful for delineating the intracardiac and extracardiac vascular anatomy;
however, CT has the inconvenience of ionizing radiation. Echocardiography has the advantage of imaging the valves and atrial septal defects.
Segmentation
Segmentation is the process by which a 3D virtual model is generated with only the structures and areas of interest. It is based in the isolation from the medical images of the cardiac structures of interest such as the aorta and the ventricles and exclusion of other nonrelevant such as bones or pulmonary arteries (Figure 1B). For this task, there are several commercial software packages such as AYRA [3,4] or Mimics [5]. Usually, commercial software have a preprocessing phase in which the image is standardized and filtered to eliminate noise. Then, an enhancement of the contrast is applied to increase the intensity difference between the blood pool (white) and the myocardium and vessel wall (gray) (Figure 1A). After this preprocessing, the image is segmented using thresholding and regiongrowing methodologies [6]. There is normally a 2D DICOM viewer where the user can see the selected anatomical structures of interest (Figure 1B). Then, the software creates a 3D polygonal mesh that can be visualized from different distances and perspectives.
Computer-aided design
The segmented geometry (virtual model) is exported in a standard fabrication format as stereolitography file (.stl), widely used for rapid prototyping, computer aided design and manufacturing. It is necessary to manipulate the mesh created to transform it into a valid file for the rapid prototyping machine. Holes, roughness, incorrect mesh density and other problems of the mesh are corrected, and then the wall thickness of the model is defined to simulate, in as realistic way as possible, the vessel walls. Supports and reinforcements to assure the correct fabrication of the model are also included in the 3D geometry. The file information is processed by the 3D printer software transforming the 3D mesh file into a machine-code file. The machine code is a list of commands that indicate all the actions of the machine during the fabrication (heat printing head, move home, all axis moves, stop, pause, etc.) [7].
Rapid prototyping
Rapid prototyping is a wide concept that includes an important number of established and experimental technologies, some of them are: stereolithography uses photopolymers that can be cured by ultraviolet light; selective laser sintering is based on small particles of thermoplastic, metal, ceramic or glass powders that are fused by a high-power laser; inkjet printing techniques are based on different kinds of fine powders such as plaster or starch, dispensed by a piston and bonded by an adhesive liquid deposited by another piston. Inkjet printing techniques can also be used to generate a 3D scaffold with different types of tissue by printing living cells and biomaterials simultaneously [8].
An affordable technology that allows the sustainability of the research in this area is fused deposition modeling. Models are fabricated in 3D printers, technology whereby a filament is melted and deposited over a bed where it solidifies forming a solid layer of material. There are different polymers that can be used in this kind of machines, some of them are rigid (Polylactic acid, Acrylonitrile Butadiene Styrene, High-impact polystyrene, Nylon, etc.) and others are flexible (MakerBot Flexible Filament, NinjaFlex Filament, Recreus Filaflex). Printing temperature varies from 195 to 240°C and elongation at break from 160 to >300%. Both kind of polymers are processed melted in an extrusion nozzle that is moved under the machine code commands creating a 0.1–0.25 mm Z-layer. Each layer deposited over the previous solidifies building a 3D solid structure. Heart models walls can have different thicknesses, this range means that the models have different distensibility and behavior when they are simulated as a real heart in the catheterization room. The process needs about 600 layers to build a 15-cm height model (Figure 1E).
Clinical translation
As this technology evolves, it will become incorporated in the routine clinical practice as other imaging investigations. Dedicated hospital-based 3D printing units are necessary to address the clinical translation and allow collaboration between clinicians experts in imaging and engineers with knowledge in 3D printing. The overall process of segmentation, computer aided designing and printing requires approximately 15 h of work per case depending on the complexity: 2.5 h of segmentation done by a clinician, 2.5 h of CAD design done by an engineer and 10 h of overnight printing. There are basic Fused Deposition Modeling 3D printers from $400 and filament spools from $20 per kilogram. The overall cost of a 3D-printed heart may range from the $350 to $1500 depending on the type of material and complexity [9].
Clinical applications in interventional cardiology
The application of 3D models using rapid prototyping techniques is a novel technology in the medical field. This technology is the evolution of imaging techniques, providing a very close representation of the patient’s anatomy. The first models were applied to the bone reconstruction such as orthopedic and maxillofacial surgery [10], where its applicability and clinical potential were evident immediately showing reduced surgical time and minimizing exposure and blood loss in the surgical field. Surgeons can have and study in their own hands precise copies of the patient’s anatomy. Surgical strategy can be designed in the patient anatomy: see, touch and outline spot exactly where you will dissect what length and there is to mobilize the anatomical structures that can physically manipulate, etc. Several papers have illustrated its application in the surgical planning of complex congenital heart diseases [9,11–14]. Recently, this application has been moved to the field of interventional structural heart disease.
Aortic pathology
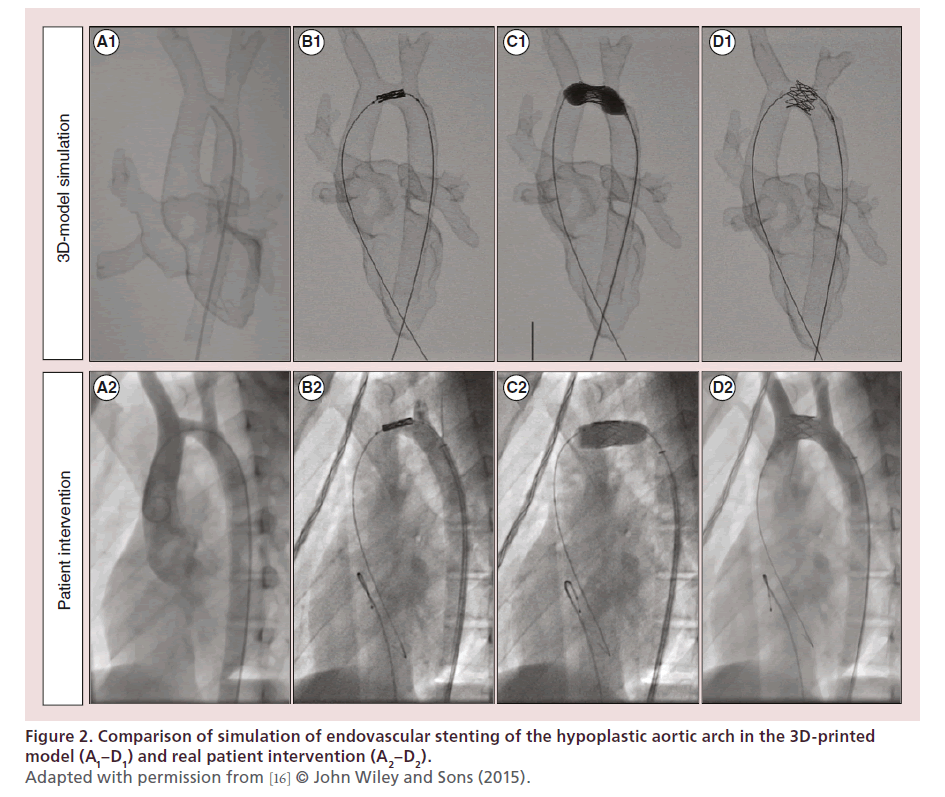
Stenting of aortic coarctation and aortic arch hypoplasia is technically challenging. Balloon specifications and the procedure have to be carefully planned in order to avoid overdistension due to the risk of rupture or complications during the placing or releasing of the stent. Stent parameters have to be well defined in order, for example, to be long enough to cover the whole segment but short enough to avoid overlapping of communicating vessels. Therefore, complications relating to stenting such as stent migration, stroke and neuropraxia are of great importance and have been reported [15]. As a proof of concept, Valverde et al. recently showed that 3D-printed models are an accurate replica of the patient’s anatomy and are helpful in planning endovascular stenting in transverse arch hypoplasia, specifically in determining balloon size and stent length and optimal position (Figure 2) [16]. Sodian et al. also demonstrated how 3D models could potentially guide the delivery of covered stent in the case of aortic pseudoaneurysm [17]. Based on the aortic 3D model, they fabricated a custom-made occluder device to simulate the intervention and successfully treated the patient.
Figure 2. Comparison of simulation of endovascular stenting of the hypoplastic aortic arch in the 3D-printed
model (A1–D1) and real patient intervention (A2–D2).
Adapted with permission from [16] © John Wiley and Sons (2015).
Atrial septal defect closure
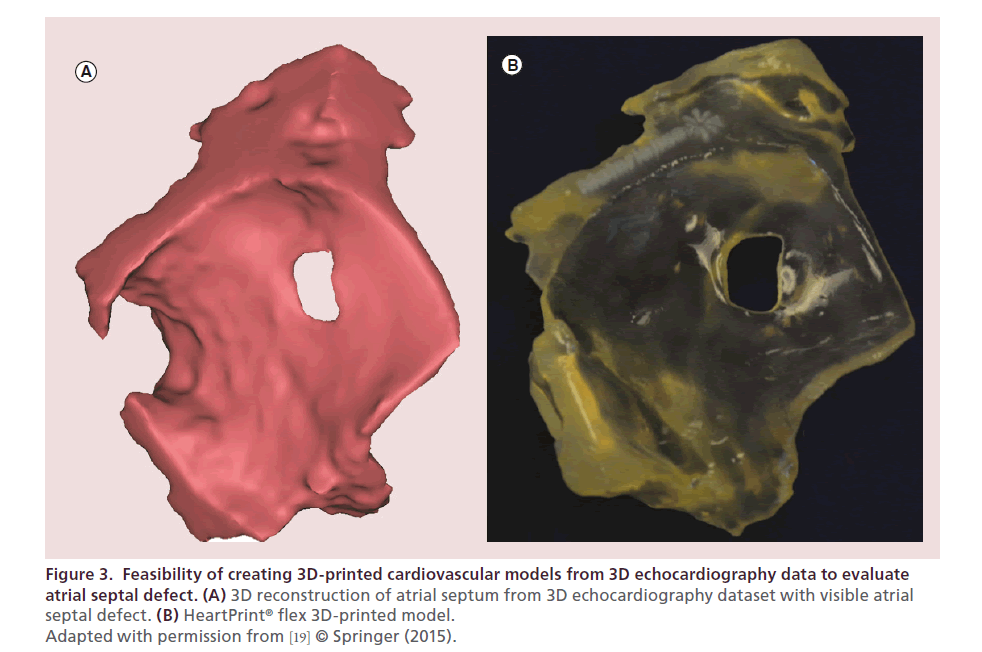
The wide variety of anatomic variability in the case of atrial septal defects and its potential for preclinical development of new medical devices offered by 3D models was initially showed by Kim et al. [18]. Later on, Samuel et al. demonstrated the feasibility of creating 3D-printed cardiovascular models from 3D echocardiography data to evaluate atrial septal defect (Figure 3) [19]. This technology may overcome the current limitations of CT and MRI to delineate the functional morphology of Ebstein’s anomaly, atrioventricular septal defects, development defect sizing in atrial septal defects and evaluation of mitral regurgitation. 3D models based on echocardiography may have the potential to improve our understanding of the morphology in these conditions before device placement or surgical repair.
Figure 3. Feasibility of creating 3D-printed cardiovascular models from 3D echocardiography data to evaluate
atrial septal defect. (A) 3D reconstruction of atrial septum from 3D echocardiography dataset with visible atrial
septal defect. (B) HeartPrint® flex 3D-printed model.
Adapted with permission from [19] © Springer (2015).
Stent angioplasty of pulmonary venous baffle obstruction
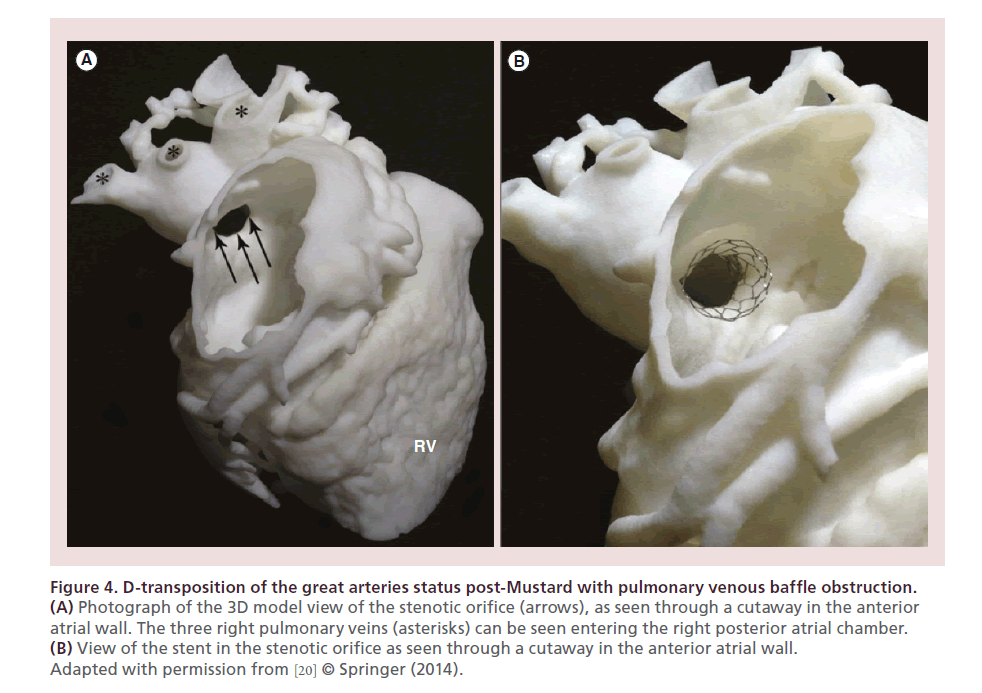
Utilization of 3D models to assist in planning for interventional procedure of a case of D-transposition of the great arteries status post-Mustard with pulmonary venous baffle obstruction was showed by Olivieri et al. (Figure 4) [20]. Multiple catheter approaches to the pulmonary venous atrium could be trialed in advance on the 3D model. The stent was deployed within the model to visualize its relationship to adjacent structures and its position within the stenosis. This case demonstrated how a printed model of structural heart disease may help planning the interventional approach by informing the selection of the appropriate catheter course and device.
Figure 4. D-transposition of the great arteries status post-Mustard with pulmonary venous baffle obstruction.
(A) Photograph of the 3D model view of the stenotic orifice (arrows), as seen through a cutaway in the anterior
atrial wall. The three right pulmonary veins (asterisks) can be seen entering the right posterior atrial chamber.
(B) View of the stent in the stenotic orifice as seen through a cutaway in the anterior atrial wall.
Adapted with permission from [20] © Springer (2014).
Percutaneous mitral annuloplasty
Despite the well-established role of cardiac surgery in mitral valve repair, several percutaneous techniques are being developed to treat mitral regurgitation. However, they are highly dependent on intraoperative imaging techniques. Despite the advantages of 3D echocardiography, the result remains a 2D representation of a 3D anatomy, which is not tangible and does not allow for the preoperative adjustment of tools to be used during the procedure. Dankowski et al. created a 3D-printed replica of the patient’s mitral valve to plan and carry out a procedure of percutaneous mitral annuloplasty [21]. Based on the 3D model simulation, several potential observations were noted that improved the success of the intervention such as the additional torque required by the guide catheter. In summary, they showed that the creation of an individual 3D model can be used to optimize the implantation of the Mitralign percutaneous annuloplasty system.
Pulmonary valve implantation
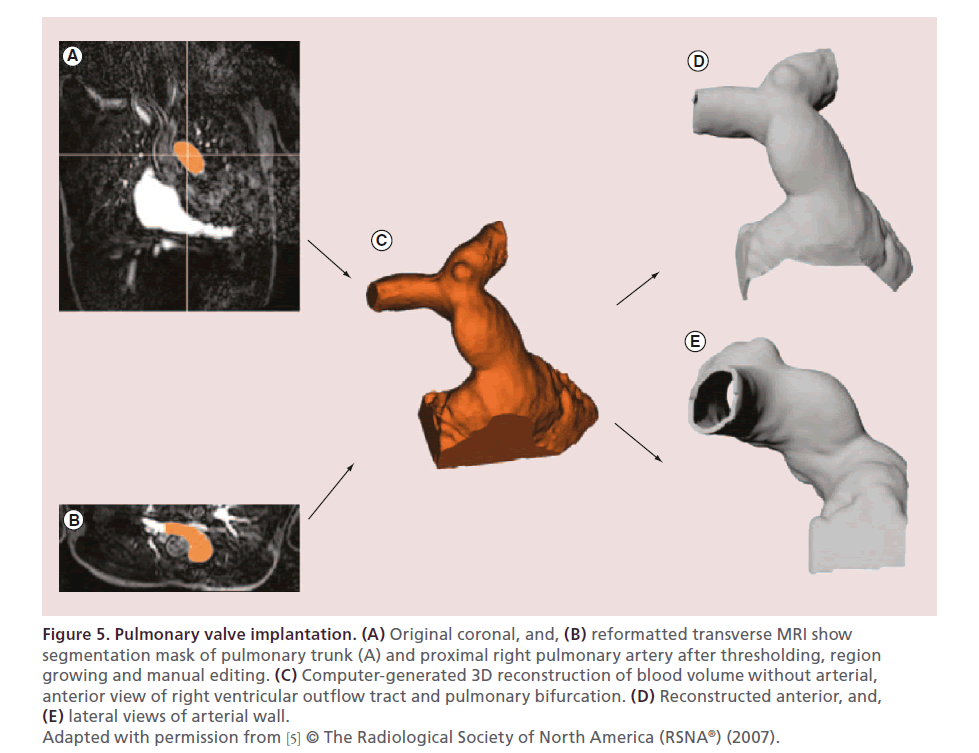
Schievano et al. described the use of 3D models of the right ventricular outflow tract and pulmonary artery for accurate selection of patients undergoing percutaneous pulmonary valve implantation (Figure 5) [5]. They elegantly showed how such models could be used to select patients for pulmonary valve implantation more accurately compared with the patient selection performed solely by using MRI. They even envisaged that 3D models might have the potential to aid in the design of future devices in this field.
Figure 5. Pulmonary valve implantation. (A) Original coronal, and, (B) reformatted transverse MRI show
segmentation mask of pulmonary trunk (A) and proximal right pulmonary artery after thresholding, region
growing and manual editing. (C) Computer-generated 3D reconstruction of blood volume without arterial,
anterior view of right ventricular outflow tract and pulmonary bifurcation. (D) Reconstructed anterior, and, (E) lateral views of arterial wall.
Adapted with permission from [5] © The Radiological Society of North America (RSNA®) (2007).
Transcatheter aortic valve implantation
Schmauss et al. [22] demonstrated the use of 3D printing models for preoperative planning of transcatheter valve replacement in a patient with a severe porcelain aorta. They stated that being able to know the exact position of the critical structures and to anticipate difficulties may reduce the perioperative risk.
Structural interventional cardiology training: board certification & revalidation
Interventional cardiology is a perfectly suited field for simulation, particularly in congenital heart disease where anatomic variability is the rule. The potential application is unlimited and may be extended to include fellow training, board certification and its revalidation [23]. Far from being a utopia, it is likely that 3D model simulations might be incorporated into clinical practice for interventional cardiology in a near future. Being aware of this potential, several societies including the Cardiovascular and Interventional Radiological Society of Europe (CIRSE), the Society for Cardiovascular Angiography and Interventions (SCAI), the Society of Interventional Radiology (SIR) and the Radiological Society of North America (RSNA) have started joint task forces with the objective of advising on the use of simulators for training in interventional catheterization [23,24]. Compared with currently available simulation platforms which cost from $90,000 to $250,000 per simulator [23], 3D-printed models fare very well costing less than $1000 [9]. Expired catheters and stents can be reused in the models with no additional cost. But more importantly, 3D-printed models allow patient-specific planning compared with generic simulation platforms.
Conclusion
3D cardiovascular models are potentially very useful in interventional cardiology. Simulations in a high-fidelity environment allow repetitive practice of tasks, thus improving the probability of success and safety, as has been widely demonstrated in the reviewed selected cases.
Future perspective
3D cardiovascular models are the evolution of advanced imaging. In the field of interventional cardiology, we envisage that in a close future 3D models will be incorporated to the daily clinical practice, ranging from board certification and revalidation to simulation of the most complex cases. Soon, 3D printers will be incorporated as part of the conventional MRI and CT scanners, and a replica of the patient’s specific anatomy will be printed as part of the conventional dictated imaging report. In the years to come, new and exciting clinical applications of 3D-printed models will emerge in the field of structural interventional cardiology.
Financial & competing interests disclosure
G Gómez-Ciriza works for Digitalica Salud. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
3D printing technology
• How 3D printing works.
• From medical images to 3D models.
• Current printing technologies and materials.
Clinical applications in interventional cardiology
• Structural interventions in congenital heart disease.
• Intracardiac and vascular interventions.
Structural interventional cardiology training: board certification & revalidation
• Fellow training and board certification.
• Revalidation of training and appraisals.
References
Papers of special note have been highlighted as: •• of considerable interest
- Peltola SM, Melchels FP, Grijpma DW, Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 40(4), 268–280 (2008).
- Petzold R, Zeilhofer HF, Kalender WA. Rapid protyping technology in medicine-basics and applications. Comput. Med. Imaging Graph. 23(5), 277–284 (1999).
- Gacto-Sanchez P, Sicilia-Castro D, Gomez-Cia T et al. Computed tomographic angiography with VirSSPA three-dimensional software for perforator navigation improves perioperative outcomes in DIEP flap breast reconstruction. Plast. Reconstr. Surg. 125(1), 24–31 (2010).
- Fernandez-Alvarez JA, Infante-Cossio P, Barrera-Pulido F et al. Virtual reality AYRA software for preoperative planningin facial allotransplantation. J. Craniofac. Surg. 25(5), 1805–1809 (2014).
- Schievano S, Migliavacca F, Coats L et al. Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology 242(2), 490–497 (2007).
- Suarez C, Acha B, Serrano C, Parra C, Gomez T. VirSSPA: a virtual reality tool for surgical planning workflow. Int. J. Comput. Assist. Radiol. Surg. 4(2), 133–139 (2009).
- Mallepree T, Bergers D. Accuracy of medical RP models. Rapid Prototyping J. 15(5), 325–332 (2009).
- Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert Opin. Biol. Ther. 7(8), 1123–1127 (2007).
- Valverde I, Gomez G, Gonzalez A et al. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol. Young 25(4), 698–704 (2015).
- Kernan BT, Wimsatt JA, 3rd. Use of a stereolithography model for accurate, preoperative adaptation of a reconstruction plate. J. Oral Maxillofac. Surg. 58(3), 349–351 (2000).
- Shiraishi I, Yamagishi M, Hamaoka K, Fukuzawa M, Yagihara T. Simulative operation on congenital heart disease using rubber-like urethane stereolithographic biomodels based on 3D datasets of multislice computed tomography. Eur. J. Cardiothorac. Surg. 37(2), 302–306 (2010).
- Torres K, Staskiewicz G, Sniezynski M, Drop A, Maciejewski R. Application of rapid prototyping techniques for modelling of anatomical structures in medical training and education. Folia Morphol. 70(1), 1–4 (2011).
- Jacobs S, Grunert R, Mohr FW, Falk V. 3D-imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study. Interactive Cardiovasc. Thorac. Surg. 7(1), 6–9 (2008).
- Markert M, Weber S, Lueth TC. A beating heart model 3D printed from specific patient data. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 4472–4475 (2007). www.researchgate.net/profile/Stefan_Weber5/publication/262723330_A_ beating_heart_model_3D_printed_from_specific_patient_ data/links/0f317538893a34b2b6000000.pdf
- Pushparajah K, Sadiq M, Brzezińska Rajszys G, Thomson J, Rosenthal E, Qureshi SA. Endovascular stenting in transverse aortic arch hypoplasia. Catheter. Cardiovasc. Interv. 82(4), E491–E499 (2013).
- Valverde I, Gomez G, Coserria JF et al. 3D printed models for planning endovascular stenting in transverse aortic arch hypoplasia. Catheter. Cardiovasc. Interv. 85(6), 1006–1012 (2015).
- Sodian R, Schmauss D, Schmitz C et al. 3-Dimensional printing of models to create custom-made devices for coil embolization of an anastomotic leak after aortic arch replacement. Ann. Thorac. Surg. 88(3), 974–978 (2009).
- Kim MS, Hansgen AR, Carroll JD. Use of rapid prototyping in the care of patients with structural heart disease. Trends Cardiovasc. Med. 18(6), 210–216 (2008).
- Samuel BP, Pinto C, Pietila T, Vettukattil JJ. Ultrasound-derived three-dimensional printing in congenital heart disease. J. Digit. Imaging doi:10.1007/s10278-014-9761-5 (2014) (Epub ahead of print).
- Olivieri L, Krieger A, Chen MY, Kim P, Kanter JP. 3D heart model guides complex stent angioplasty of pulmonary venous baffle obstruction in a Mustard repair of D-TGA. Int. J. Cardiol. 172(2), e297–e298 (2014).
- Dankowski R, Baszko A, Sutherland M et al. 3D heart model printing for preparation of percutaneous structural interventions: description of the technology and case report. Kardiol. Pol. 72(6), 546–551 (2014).
- Schmauss D, Schmitz C, Bigdeli AK et al. Three-dimensional printing of models for preoperative planning and simulation of transcatheter valve replacement. Ann. Thorac. Surg. 93(2), e31–e333 (2012).
- Green SM, Klein AJ, Pancholy S et al. The current state of medical simulation in interventional cardiology: a clinical document from the Society for Cardiovascular Angiography and Intervention’s (SCAI) Simulation Committee. Catheter. Cardiovasc. Interv. 83(1), 37–46 (2013).
- Gould DA, Reekers JA, Kessel DO et al. Simulation devices in interventional radiology: validation pending. J. Vasc. Interv. Radiol. 17(2 Pt 1), 215–216 (2006).
•• Overview of 3D models as the next evolution in advanced imaging in patients with congenital heart disease.
•• First study to demonstrate that 3D models resulted in more accurate selection of patients for percutaneous pulmonary valve implantation than imaging alone.