Research Article - Clinical Practice (2018) Volume 15, Issue 2
Predicting exacerbation in young children with intermittent asthma
- Corresponding Author:
- Stefan Zielen
Children’s Hospital, Division of Allergology, Pulmonology and Cystic fibrosis
University Hospital Frankfurt, Frankfurt am Main, Germany
E-mail: Stefan.Zielen@kgu.de
Abstract
Background: To predict asthma exacerbations (AE) in young children with intermittent asthma is often difficult. Objective: To find predictive parameters for AE in young children with intermittent asthma by examining different biomarkers and anamnestic factors. Methods: 142 children with intermittent asthma aged 4-7 years were included in the study. AE was defined as increased need of Salbutamol (≥ 2 puffs per week or ≥ 5 puffs in two weeks) due to cough symptoms. We analyzed lung function, bronchial hyperreactivity by methacholine testing, exhaled NO (eNO), total IgE, specific IgE and former medical history of 142 children as possible predictive parameters for a further episode during a one year observation period. Of 142 children 63 (44.4%) had an AE during the study. An analysis of the basic parameters indicated that FEV1 (%), FEV1/VC (%) and PD20FEV1 were significantly decreased in children with consecutive AE (p <0.05). Analysis concluded cut off levels of 103% (FEV1), 89.7% (FEV1/VC) and 0.129 mg (PD20FEV1) as risk factors. In addition younger age indicated a trend towards AE (p=0.053). All other parameters (number of previous episodes, parental smoking, eNO and allergic sensitization) did not have an effect on the AE rate. The presence of two or three risk factors was associated with a significantly higher risk of AE. Conclusion: Levels of FEV1, FEV1/VC and PD20FEV1 below cut off levels are highly predictive for a consecutive AE. Easyto- evaluate the presence of two or three parameters justifies a risk factor based treatment strategy.
Keywords
childhood asthma, asthma exacerbation, prediction, biomarkers, spirometry, bronchial hyperreactivity
List of abbreviations
AE: Asthma Exacerbations; ATS: American Thoracic Society; AUC: Area Under The Curve; BHR: Bronchial hyperreactivity; BMI: Body Mass Index; eNO: Exhaled Nitric Oxide; FEV1: Forced Expiratory Volume in 1 Second; FVC: Forced Vital Capacity; ICS: Inhaled Steroids; IgE: Immunoglobulin E; LTRA: Leukotriene Receptor Antagonist; NO: Nitric Oxide; OR: Odds Ratio; P: Probability; PD20: Inhalation of a methacholine solution until a 20% fall in FEV1 (%) was observed (PD20); PEF: Peak Expiratory Flow; ppb: Parts Per Billion; ROC: Receiver-Operating Characteristic; SD: Standard Deviation.
Introduction
Asthma and wheezy bronchitis are the most common respiratory diseases in the western world. Both affect 20-30% of children aged 2-6 years, cause considerable morbidity, affect quality of life and are associated with significant mortality [1,2]. There are several triggers associated with asthma and asthma exacerbations (AE) in early childhood. It is known that children with a family history of asthma and atopy are more often affected by asthma than children without such history [3-5]. The most common triggers for wheezy episodes in early childhood are infections in sensitized children and infections [6]. Especially viral infections are associated with asthma exacerbations in this age group [6,7]. Of course not all of the children having a viral infection are at risk for an AE and not all of those with a history of wheezing will have further AE. Therefore it is difficult for the pediatrician to identify those children who need a protective anti-inflammatory therapy in order to avoid or to reduce severity of further AE [2,8]. Despite the identification of risk factors such as a family history of asthma or a personal history of atopy, low Vitamin D levels, passive smoke exposure, predictions which intermittent asthmatic remain symptomatic and need antiinflammatory treatment is difficult [1-5,8,9]. Interestingly, it was recently shown that about one third of children with infantile asthma aged 2-5 years did not experience a further episode during a one year observation period [10]. In this study no clinical parameter was helpful to distinguish between patients with and without further episodes. Thus the selection criteria and the optimal time to begin anti-inflammatory treatment are particularly unclear. To avoid over treatment with anti-inflammatory medication, great importance is placed on defining risk factors that can predict further AE, justifying costs and possible side effects of a seasonally adjusted treatment [2,4,8]. For physicians and parents, it is particularly important to discriminate between children at high risk for a consecutive AE from those at low risk in the subsequent weeks or months. Thus, the selection criteria and the optimal time to begin anti-inflammatory treatment are of special interest for the general physician who sees many healthy children, most likely in the summer, with previous wheezing episodes. Therefore we evaluated clinical parameters and biomarkers from 142 children with intermittent asthma in a symptom-free interval to identify risk factors that predict further AE in this age group
Marterials and methods
Study design
The risk parameter analysis of 142 children with intermittent asthma was in part a subanalysis of a previous trial (“Leukotriene receptor antagonist or inhaled steroid in young children with asthma”) (Clinical Trial Registration NCT00543686). Results of 102 patients were recently published [11]. The study was a monocentre, randomised, open and parallel-group study in young children with intermittent asthma conducted at the outpatient centre of the Frankfurt department for paediatric respiratory diseases. The study was approved by the medical ethics committee of the University Hospital, Frankfurt / Main, and all parents provided written informed consent.
All children were examined at the initial visit (visit 1). Visit 1 included a detailed medical history, clinical examination, skin prick test, total and specific IgE, exhaled NO (eNO), spirometry and measurement of bronchial hyperreactivity (BHR) by methacholine bronchoprovocation. After visit 1, the parents were trained to keep a diary and to use salbutamol by the pressurised meter dose inhaler via a spacer on demand. The parents were also advised to make an appointment when symptoms increased and when salbutamol had to be given as frequently as indicated (frequent use of salbutamol, i.e., more than 2 puffs a week or 5 puffs in 14 days), which defines an acute AE. During the one year observation period, regular telephone conversations were held with the parents every two months.
Study population
The children participating in the trial were recruited from the out-patient department for paediatric respiratory disease. The centre cares for 1500 new patients a year with recurrent wheezing and suggested or chronic asthma. Children aged 4 - <7 years with the diagnosis of intermittent asthma were included in the study if they fulfilled the following criteria:
AE-free interval >4 weeks prior to visit 1
Written informed consent of parents after they received written and oral information about the aim, purpose and risks of the study.
The exclusion criteria were the following:
Chronic persistent asthma
Regular use of either inhaled steroids (ICS) or leukotriene receptor antagonist (LTRA) in the previous 3 months
Severe concomitant diseases
The patients received only inhaled salbutamol for respiratory symptoms (i.e., coughing and wheezing) during the observation period. No other treatment for allergic or asthma symptoms was allowed (e.g., LTRAs, antihistamines and ICS) until exacerbation. Children with AE were randomized either to LTRA or to ICS, results of this examination were published in 2010 [11].
Symptom diary
The diary was completed every day by caregivers during the study [12]. The following data were included as discrete variables in a paper diary: rhinitis and asthma symptoms, symptom free days, rescue medication, salbutamol use, systemic steroid use and fever. Symptom scores were calculated at the end of the study as applicable.
Lung function-spirometry
Pulmonary function tests were performed according to the American Thoracic Society (ATS) guidelines [13]. Predicted FEV1 (%) values were determined based on the reference values for children [14]. The following variables were utilised: Vital capacity (VC (L, %pred)), FEV1 (L, %pred), Forced Vital Capacity (FVC (L, %pred)), and FEV1/FVC.
Exhaled nitric oxide (eNO) and sputum leukotrienes
The levels of eNO were measured according to current guidelines at a defined expiratory flow rate of 50 mL/sec using the NIOX® (by Aerocrine, Solna, Sweden). The NIOX® measures NO in exhaled air according to the ATS guidelines [15].
Skin prick test
A skin prick test using standard allergens to a panel of aeroallergens including D. pteronyssinus, D. farinae, cat and dog, mould mix, grass-pollen mix and tree pollen mix (Allergopharma, Rheinbeck, Germany) was performed. A response of at least 3 mm greater than saline control was deemed positive.
Measurement of bronchial hyperreactivity
Bronchial hyperreactivity (BHR) was assessed using inhalation of a methacholine solution until a 20% fall in FEV1 (%) was observed (PD20) [16]. The doses of inhaled methacholine were increased every two minutes according to the following pattern from step 1 to 5: 0.01, 0.1, 0.4, 0.8 and 1.6 mg. Thus, the entire protocol delivered cumulative doses of 0.11, 0.51, 1.31 and 2.91 mg [16].
Measurement of total IgE and specific IgE
Blood collection was performed for measurement of total IgE and specific IgE levels (grass, rye, mugwort, birch, cat, horse, cladosporium, alternaria, house dust mite and egg) by specific and sensitive immunoassays (Immunolite, DPC Bad Nauheim, Germany) in our laboratory [17].
Statistics
The results are expressed as the means and standard deviation (SD) or, if not normally distributed or in the case of categorical variables, as the medians and range.
The collected data were analysed with Microsoft Excel (Office 2007) and the statistical programs BiAS for Windows, Version 9.04 (Epsilon Verlag 1989-2009), which is designed specifically for biostatistics by the biostatistics institute of the Goethe University Frankfurt/ Main, and SPSS, Version 19 (IBM).
The exacerbated group versus the nonexacerbated group was compared with the help of the non-parametric Wilcoxon-Mann-Whitney- U-Test with various numbers of biomarkers, including height, weight, eNO, lung function test results, metacholine test results, and blood test results. A statistically significant difference between the groups was defined by a p-value less than 0.05, and all tests were two-sided. Odds ratio, sensitivity and specificity of the score were also calculated.
To define the sensitivity and specificity of a predictor for detecting an AE, a receiveroperating characteristic (ROC) curve was plotted. The cut-off levels were optimized using the Youden index (sensitivity+specificity-1). The individual probability of an AE was calculated by logistic and log-logistic regression analyses. The accuracy was measured with area under the curve (AUC) analysis. Probability (p) values ≤ 0.05 were considered statistically significant.
Results
Study population
142 children previously diagnosed with intermittent asthma were enrolled in the trial (n=83 male, 58.5%). The mean age of the children was 4.92 years. During the observation period of one year, n=63 children (44.4%) had an acute exacerbation defined as increased demand of salbutamol due to asthma symptoms (i.e., 3 puffs a week or 5 puffs in 14 days). The baseline descriptive characteristics of all patients are shown in TABLE 1. FIGURE 1 shows the flow chart of the analysis. Medical history revealed that 30 children suffered from concomitant allergic rhinoconjuctivitis (21.1%) and 41 from atopic dermatitis (35.9%). 51 children (35.9%) were affected by parenteral smoking. In 17 of these cases (33.3%), both parents were smokers (TABLE 1).
| Total | Exacerbated | Non-Exacerbated | p-value | ||
|---|---|---|---|---|---|
| Total number | n | 142 | 79 | 63 | |
| Age (years) | Mean | 4.92 | 5.03 | 4.78 | 0.062 |
| SD | 0.79 | 0.78 | 0.79 | ||
| 4-5 years | 51 | 23 | 28 | 0.059 | |
| 5-6 years | 52 | 31 | 21 | 0.470 | |
| 6-7 years | 39 | 25 | 14 | 0.453 | |
| Male | n | 83 | 43 | 40 | 0.278 |
| % | 58.45 | 54.43 | 63.49 | ||
| Height (cm) | Mean | 114.81 | 115.02 | 114.65 | 0.507 |
| SD | 7.07 | 7.89 | 6.35 | ||
| Weight (kg) | Mean | 21.10 | 21.57 | 20.73 | 0.887 |
| SD | 4.17 | 5.21 | 3.05 | ||
| BMI (kg/m²) | Mean | 15.92 | 16.16 | 15.73 | 0.548 |
| SD | 2.15 | 2.59 | 1.71 | ||
| Passive smoking | n | 51 | 25 | 26 | 0.537 |
| % | 35.92 | 39.68 | 32.91 |
Table 1: Baseline descriptive characteristics of the study population.
Lung function-spirometry
Baseline lung function values were compared between exacerbated and non-exacerbated children (TABLE 2). Children with an acute AE showed significantly lower FEV1 (%) and FEV1/FVC values compared to symptom free children (p=0.006 and p=0.038). No difference was observed for the vital capacity (p=0.72). Discriminant analysis showed a cut-off level for FEV1 (%) of 103.2%. Lower values were associated with elevated risk for AE (OR 4.54).
| Total | Exacerbated | Non-Exacerbated | p-value | ||
|---|---|---|---|---|---|
| FEV1 pred. % | Mean | 101.12 | 97.34 | 104.22 | 0.006 |
| SD | 16.75 | 17.12 | 15.79 | ||
| Tiffeneau | Mean | 90.16 | 88.38 | 91.61 | 0.037 |
| SD | 8.92 | 9.33 | 8.29 | ||
| eNO (ppB) | Mean | 14.91 | 14.72 | 15.08 | 0.685 |
| SD | 15.70 | 14.42 | 16.71 | ||
| PD20FEV1 (mg) | Mean | 0.28 | 0.23 | 0.32 | 0.049 |
| SD | 0.47 | 0.44 | 0.48 |
Table 2: Lung function parameters, eNO and bronchial hyperreactivity.
The cut-off value for the FEV1/FVC was 89.7. Lower values had an increased risk for AE with an OR of 2.24.
Bronchial hyperreactivity
Significantly lower doses of methacholine were necessary for a drop in FEV1 of ≥ 20% from baseline in the bronchoprovocation test (PD20FEV1 0.23 vs. 0.32 mg methacholine, p=0.049). Discriminant analysis produced a cutoff level of 0.1285 mg methacholine; a higher risk for AE was associated with lower doses of methacholine (OR 2.12).
With the presence of multiple risk factors (values for FEV1, FEV1/FVC or PD20FEV1 below the cut-off levels), the risk for an asthma exacerbation was even higher (TABLE 3). With 2 out of these 3 risk factors, the OR for an AE increased to 5.25 and reached 11.3 with 3 risk factors. When comparing those children with 3 risk factors to children without risk factors, the chi-squared test revealed a sensitivity of 77.3% and a specificity of 76.9%. When comparing 2 or more risk factors to only 1 or less risk factor, sensitivity was 68.3% and specificity was 70.9%.
| Total | Exacerbated | Non-Exacerbated | ||
|---|---|---|---|---|
| Total IgE | Mean | 177.86 | 141.86 | 205.27 |
| SD | 334.60 | 235.23 | 391.56 | |
| Seasonal specific IgE cumulativ | Mean | 18.44 | 21.50 | 15.92 |
| - grass, birch, rye, mugwort | SD | 50.18 | 44.52 | 54.27 |
| All-season specific IgE cumulativ | Mean | 21.72 | 23.96 | 19.87 |
| - house dust mite, cat, cladosporium, alternaria | SD | 50.31 | 50.08 | 50.42 |
| House dust mite IgE | Mean | 8.47 | 8.81 | 9.50 |
| SD | 24.73 | 20.25 | 20.09 |
Table 3: Total IgE and specific IgE values in our patients (kU/L).
Allergy and RAST
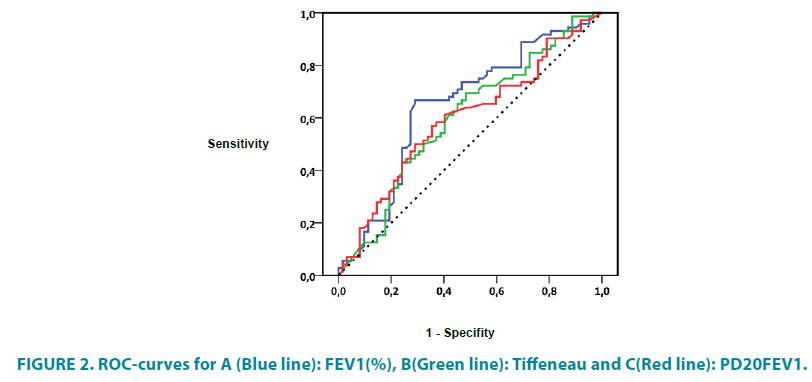
124 blood samples could be analyzed. In 18 patients, the parents did not allow a blood withdrawal in their children. Allergy was assumed in RAST-class ≥ 2. In the whole group, 49 (39.5%) of the 124 children had at least one allergy with a RAST-class ≥ 2, while 16 patients had at least 2 and 9 had 3 positive RAST tests ≥ 2. No differences between the exacerbated and non-exacerbated groups were found. Neither groups showed a difference in the summation of specific IgE values for all-season and seasonal allergens, although the values for specific IgE tended to be slightly higher in children with AE (TABLE 4 and FIGURE 2).
| Exacerbated | Non-Exacerbated | OR | ||
|---|---|---|---|---|
| 0 Risk Factors (n) | 5 | 21 | ||
| 1 Risk Factor (n) | FEV1 (%) | 44 | 27 | 4.54 |
| Tiffeneau | 33 | 25 | 2.24 | |
| PD20FEV1 | 41 | 35 | 2.12 | |
| 2 Risk Factors (n) | 26 | 17 | 5.25 | |
| 3 Risk Factors (n) | 17 | 6 | 11.3 |
Table 4: Risk Factors and risk for exacerbation.
Exhaled NO (eNO)
As shown in TABLE 2, levels of eNO did not differ between patients with and without AE. As expected, children with allergies had significantly higher levels of eNO in contrast to children without allergies (19.3 ppb vs. 12.3 ppb, p=0.016).
Discussion
Identifying biomarker of asthma exacerbations in young children is attractive but has proven elusive so far. Several studies have examined the long term outcome (i.e., which children will outgrow the problem around school age and which will not [3,18-20], but few studies have calculated the risk factors for AE for the next winter season in young children with intermittent asthma. Although some risk factors for asthma in young children have been well described as presence of allergy, eczema or a first-degree family history, low vitamin D-levels, passive smoke exposure and crowding most decisions to start preventive therapy rely solely on the individual judgment and expertise of the treating physician.
Most common triggers for AE of childhood asthma are viral infections [6,7]. However the knowledge that viral infections are the major trigger for an asthma exacerbation in this age group does not help to determine which child may benefit from preventive treatment. Additionally, protective anti-inflammatory therapy for bronchial asthma is associated with non-negligible side effects, especially in the case of inhaled corticosteroids [21,22]. Therefore, paediatricians would profit from well-defined predictors that help to decide which patient with intermittent asthma needs a protective therapy.
To answer this question, we analysed biomarkers collected in 142 healthy children, 4-6 years of age, with the diagnosis of intermittent asthma. With only 63 children having an AE during one year observation period, less than 50% of children with previous asthmatic symptoms became symptomatic again. This is in keeping with earlier studies and shows the heterogeneity in the group of wheezing in young children and the urgency to identify only those who will profit from anti-inflammatory treatment [10].
First, we analyzed the gender, age and body mass index (BMI) of our patients and we could not find significant differences between the exacerbated and non-exacerbated group, even though there was a trend for AE with younger age. Our results are similar to those of Caudri et al [20] and Covar et al. [23] and support the theory that smaller airways in younger children are a risk factor of exacerbations. Interestingly Mahut et al. [24] showed that each additional year of life reduces the risk for an AE by 15% underlying that age i.e. lung growth is an important factor.
Second we could not find an effect of allergic sensitization, passive smoking, and eNO, a marker of eosinophilic airway inflammation, as predictors. Our findings for eNO are similar to those found in other studies where the measurement in symptomatic patients had no predictive value for the occurrence and severity of AE [25,26]. eNO might have better predictive power in optimising and controlling anti-inflammatory treatment rather than predicting AE, particularly when ICS are used [27]. In addition eNO has been a more effective parameters of AE in adults [28] than in children [25,29].
Surprisingly, we could not find an effect of allergic sensitization and passive smoking on the rate of AE. However, in contrast to other studies where an effect of these parameters on asthma development was observed [18,30-32], we did not examine the long-term outcome of disease but tried to predict the risk of exacerbation during the next winter period. Rabinovitch et al. [33] recently showed an influence of tobacco smoke via a Cysteinyl-leukotriene-mediated pathway on asthmatic symptoms and the risk of AE [34]. Although the influence of smoking on AE remains controversial, its influence on the course and the control of the disease is evident, as multiple studies have demonstrated [30,35,36].
The influence of allergic sensitization and IgE levels had no impact on the risk of AE our observation. Although we observed a trend of higher cumulative specific IgE values in children with AE, these results were not significant, in contrast to previous results [31,37]. We assume that because of the high influence of viral infections as a major cause for AE in young children, the presence of early sensitization is more relevant for the long-term outcome [17,32]. In deed it is well known that nonatopic children with recurrent wheeze are more likely to outgrow this condition compared to atopic children [32].
Interestingly, lung function parameters had a high potential to predict future AE in this age group. Our results suggest that the uses of standard spirometry as well as the measurement of bronchial hyperactivity are excellent predictors for future AE. We determined cut-off levels for FEV1, FEV1/FVC and PD20FEV1, to identify those children for whom an anti-inflammatory therapy is not necessary. When combining the results of spirometry and methacholine bronchoprovocation, a strong prediction of an AE is possible. Two out of 3 risk factors were already associated with a 5-fold higher risk for exacerbation (OR 5.25). If all 3 risk factors are fulfilled, the risk was more than 10-times higher (OR 11.3) (TABLE 3).
Previous studies examining the influence of FEV1 on AE rate showed different results. Two studies found a significant higher risk for AE in children with lower FEV1 supporting the use of spirometry as an objective measure of lung function in assessment of risk for AE [38,39] whereas one study in children with moderate asthma could not find an influence at all [23].
Studies investigating the influence of bronchial hyperactivity in AE are rare. Covar et al. [23] did not find an influence of methacholine bronchoprovocation on the rate of AE. In contrast to our study, they examined older children (6-14 years of age) treated with anti-inflammatory therapy. In most of the other studies, exacerbations are defined as the need of systemic steroids and/or hospitalisation [24,40,41]. Therefore, our results cannot be compared to these studies concerning AE in early childhood.
Conclusion
Our results demonstrate that levels of FEV1, FEV1/FVC and PD20FEV1 below the cut-off levels are highly predictive for a consecutive AE. When 2 or more risk factors are above cut-off values, we suggest not starting preventive antiinfammatory therapy in this patient group in order to avoid over-treatment.
References
- Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N. Engl. J. Med. 332(3), 133-138 (1995).
- Miller EK, Avila PC2, Khan YW, et al. Wheezing exacerbations in early childhood: evaluation, treatment, and recent advances relevant to the genesis of asthma. J. Allergy Clin. Immunol. Pract. 2(5), 537-543 (2014).
- Kurukulaaratchy RJ, Matthews S, Holgate ST, Arshad SH. Predicting persistent disease among children who wheeze during early life. Eur. Respir. J. 22(5), 767-771 (2003).
- Bacharier LB, Guilbert TW. Diagnosis and management of early asthma in preschool-aged children. J. Allergy Clin. Immunol. 130(2), 287-296 (2012).
- Dick S, Doust E, Cowie H, Ayres JG, Turner S. Associations between environmental exposures and asthma control and exacerbations in young children: a systematic review. BMJ Open. 4(2), e003827 (2014).
- Beigelman A, Bacharier LB. Infection-induced wheezing in young children. J. Allergy Clin. Immunol. 133(2), 603-604 (2014).
- Busse WW, Lemanske RF, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 376(9743), 826-834 (2010).
- Castro-Rodríguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am. J. Respir. Crit. Care Med. 162(4 Pt 1), 1403-1406 (2000).
- Stenberg Hammar K, Hedlin G, Konradsen JR, et al. Subnormal levels of vitamin D are associated with acute wheeze in young children. Acta Paediatr. 103(8), 856-861 (2014).
- Bisgaard H, Zielen S, Garcia-Garcia ML, et al. Montelukast reduces asthma exacerbations in 2- to 5-year-old children with intermittent asthma. Am. J. Respir. Crit. Care Med. 171(4), 315-322 (2005).
- Zielen S, Christmann M, Kloska M, et al. Predicting short term response to anti-inflammatory therapy in young children with asthma. Curr. Med. Res. Opin. 26(2), 483-492 (2010).
- Santanello NC, Demuro-Mercon C, Davies G, et al. Validation of a pediatric asthma caregiver diary. J. Allergy Clin. Immunol. 106(5), 861-866 (2000).
- Beydon N, Davis SD, Lombardi E, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am. J. Respir. Crit. Care Med. 175(12), 1304-1345 (2007).
- Quanjer PH, Borsboom GJ, Brunekreef B, et al. Spirometric reference values for white European children and adolescents: Polgar revisited. Pediatr. Pulmonol. 19(2), 135-142 (1995).
- ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am. J. Respir. Crit. Care Med. 171(8), 912-930 (2005).
- Schulze J, Rosewich M, Riemer C, et al. Methacholine challenge-comparison of an ATS protocol to a new rapid single concentration technique. Respir. Med. 103(12), 1898-1903 (2009).
- Rose MA, Stieglitz F, Köksal A, et al. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin. Exp. Allergy. 40(9), 1398-1405 (2010).
- Civelek E, Cakir B, Orhan F, et al. Risk factors for current wheezing and its phenotypes among elementary school children. Pediatr. Pulmonol. 46(2), 166-174 (2011).
- Brand PLP, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur. Respir. J. 32(4), 1096-1110 (2008).
- Caudri D, Wijga A, A Schipper CM, et al. Predicting the long-term prognosis of children with symptoms suggestive of asthma at preschool age. J. Allergy Clin. Immunol. 124(5), 903-910.e1-7 (2009).
- Allen DB. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Adv. Pediatr. 53, 101-110 (2006).
- Aalderen WMC, Sprikkelman AB. Inhaled corticosteroids in childhood asthma: the story continues. Eur. J. Pediatr. 170(6), 709-718 (2011).
- Covar RA, Szefler SJ, Zeiger RS, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J. Allergy Clin. Immunol. 122(4), 741-747 (2008).
- Mahut B, Trinquart L, Delclaux C. Influence of Age on the Risk of Severe Exacerbation and Asthma Control in Childhood. J. Asthma. 48(1), 65-68 (2011).
- Cabral ALB, Vollmer WM, Barbirotto RM, Martins MA. Exhaled nitric oxide as a predictor of exacerbation in children with moderate-to-severe asthma: a prospective, 5-month study. Ann. Allergy Asthma Immunol. 103(3), 206-211 (2009).
- Gill M, Walker S, Khan A, et al. Exhaled nitric oxide levels during acute asthma exacerbation. Acad. Emerg. Med. 12(7), 579-586 (2005).
- Strunk RC, Szefler SJ, Phillips BR, et al. Relationship of exhaled nitric oxide to clinical and inflammatory markers of persistent asthma in children. J. Allergy Clin. Immunol. 112(5), 883-892 (2003).
- Dweik RA, Sorkness RL, Wenzel S, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am. J. Respir. Crit. Care Med. 181(10), 1033-1041 (2010).
- Stern G, de Jongste J, van der Valk R, et al. Fluctuation phenotyping based on daily fraction of exhaled nitric oxide values in asthmatic children. J. Allergy Clin. Immunol. 128(2), 293-300 (2011).
- Ehrlich RI, Du Toit D, Jordaan E, et al. Risk factors for childhood asthma and wheezing. Importance of maternal and household smoking. Am. J. Respir. Crit. Care Med. 154(3 Pt 1), 681-688 (1996).
- Wever-Hess J, Kouwenberg JM, Duiverman EJ, Hermans J, Wever AM. Risk factors for exacerbations and hospital admissions in asthma of early childhood. Pediatr. Pulmonol. 29(4), 250-256 (2000).
- Ili S, von Mutius E, Lau S, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 368(9537), 763-770 (2006).
- Rabinovitch N, Strand M, Stuhlman K, Gelfand EW. Exposure to tobacco smoke increases leukotriene E4-related albuterol usage and response to montelukast. J. Allergy Clin. Immunol. 121(6), 1365-1371 (2008).
- Rabinovitch N, Graber NJ, Chinchilli VM, et al. Urinary leukotriene E4/exhaled nitric oxide ratio and montelukast response in childhood asthma. J. Allergy Clin. Immunol. 126(3), 545-551 (2010).
- Goodwin RD, Cowles RA. Household smoking and childhood asthma in the United States: a state-level analysis. J. Asthma. 45(7), 607-610 (2008).
- Rosewich M, Adler S, Zielen S. Effects of active and passive smoking on the health of children and adolescents. Pneumologie. 62(7), 423-429 (2008).
- Simpson A, Soderstrom L, Ahlstedt S, et al. IgE antibody quantification and the probability of wheeze in preschool children. J. Allergy Clin. Immunol. 116(4), 744-749 (2005).
- Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV1 is associated with risk of asthma attacks in a pediatric population. J. Allergy Clin. Immunol. 107, 61-67 (2001).
- Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J. Allergy Clin. Immunol. 118(6), 1218-1225 (2006).
- Blais L, Kettani FZ, Lemière C, et al. Inhaled corticosteroids vs. leukotriene-receptor antagonists and asthma exacerbations in children. Respir. Med. 105(6), 846-855 (2011).
- Forno E, Fuhlbrigge A, Soto-Quirós ME, et al. Risk factors and predictive clinical scores for asthma exacerbations in childhood. Chest. 138(5), 1156-1165 (2010).